Potential of Scots Pine for a Push Strategy against the European Spruce Bark Beetle Ips typographus
Abstract
:1. Introduction
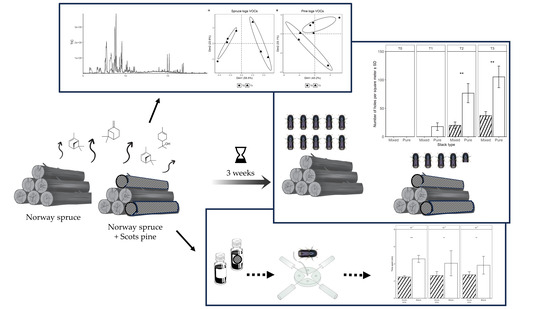
2. Materials and Methods
2.1. Field Trials
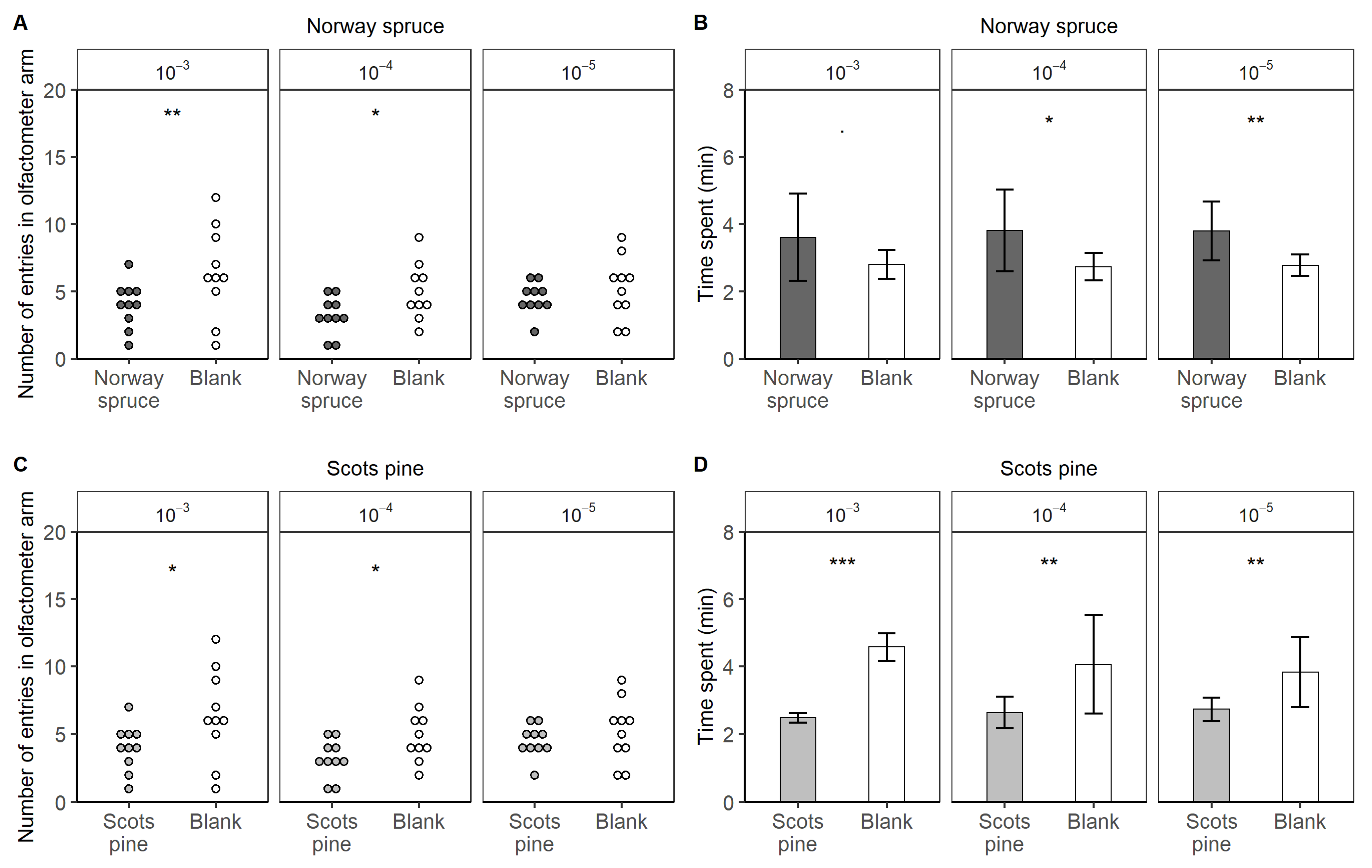
2.2. Behavioral Trials
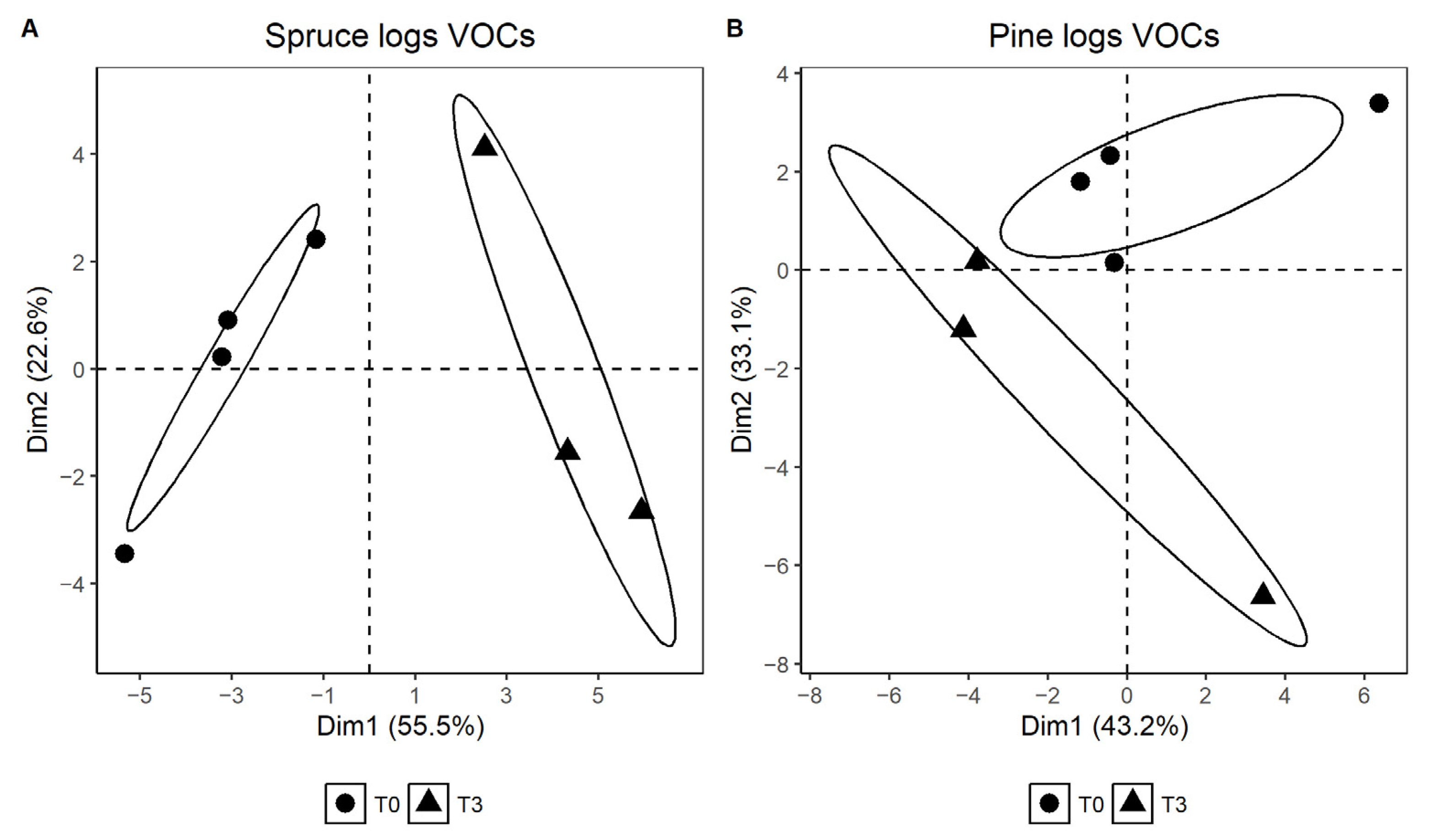
2.3. Bark VOCs Collection and Analysis
2.4. Statistical Analysis
- Stack volumes were compared with an analysis of variance test (ANOVA).
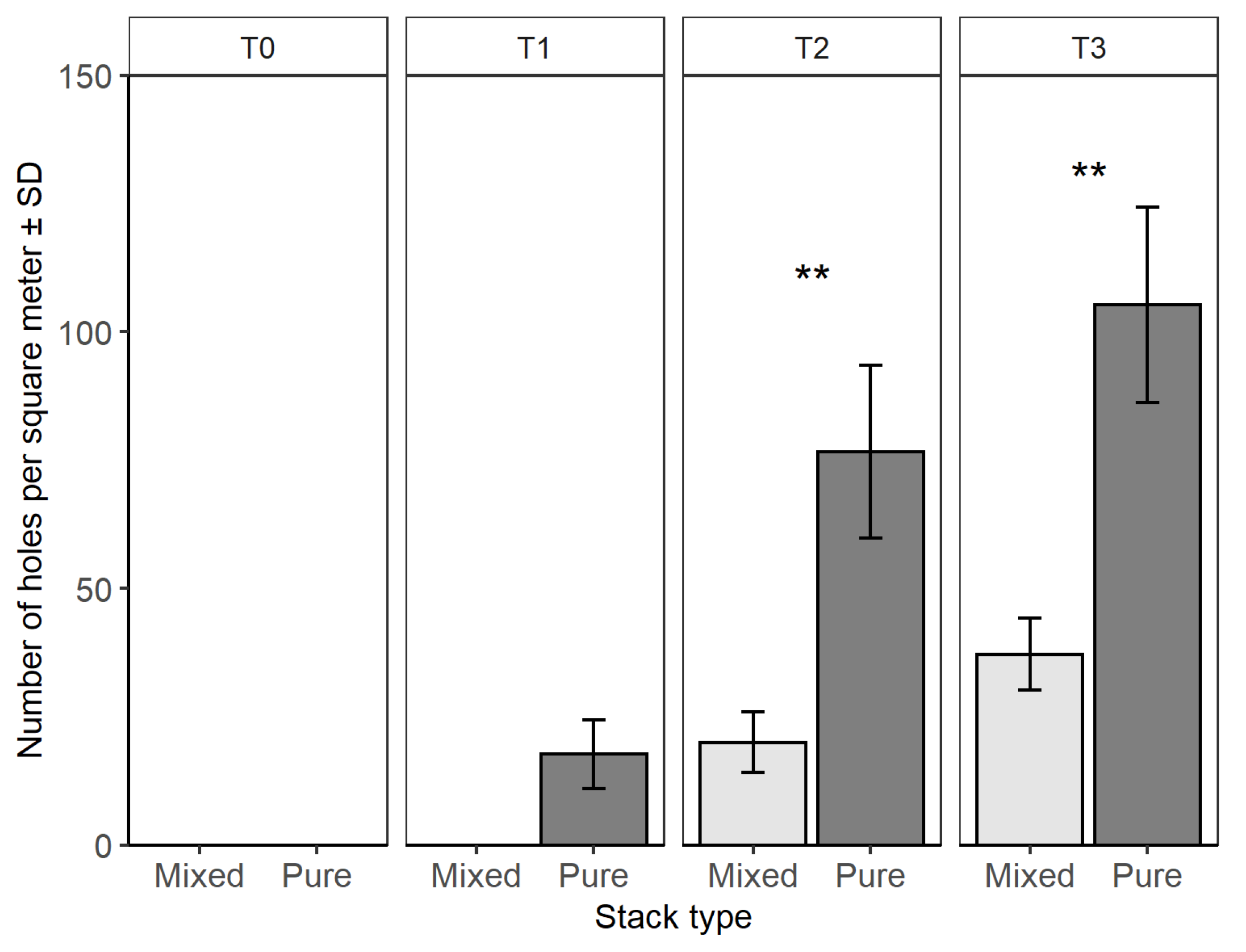
- The number of holes in the logs between pure and mixed stacks were analyzed for each time with an ANOVA test.
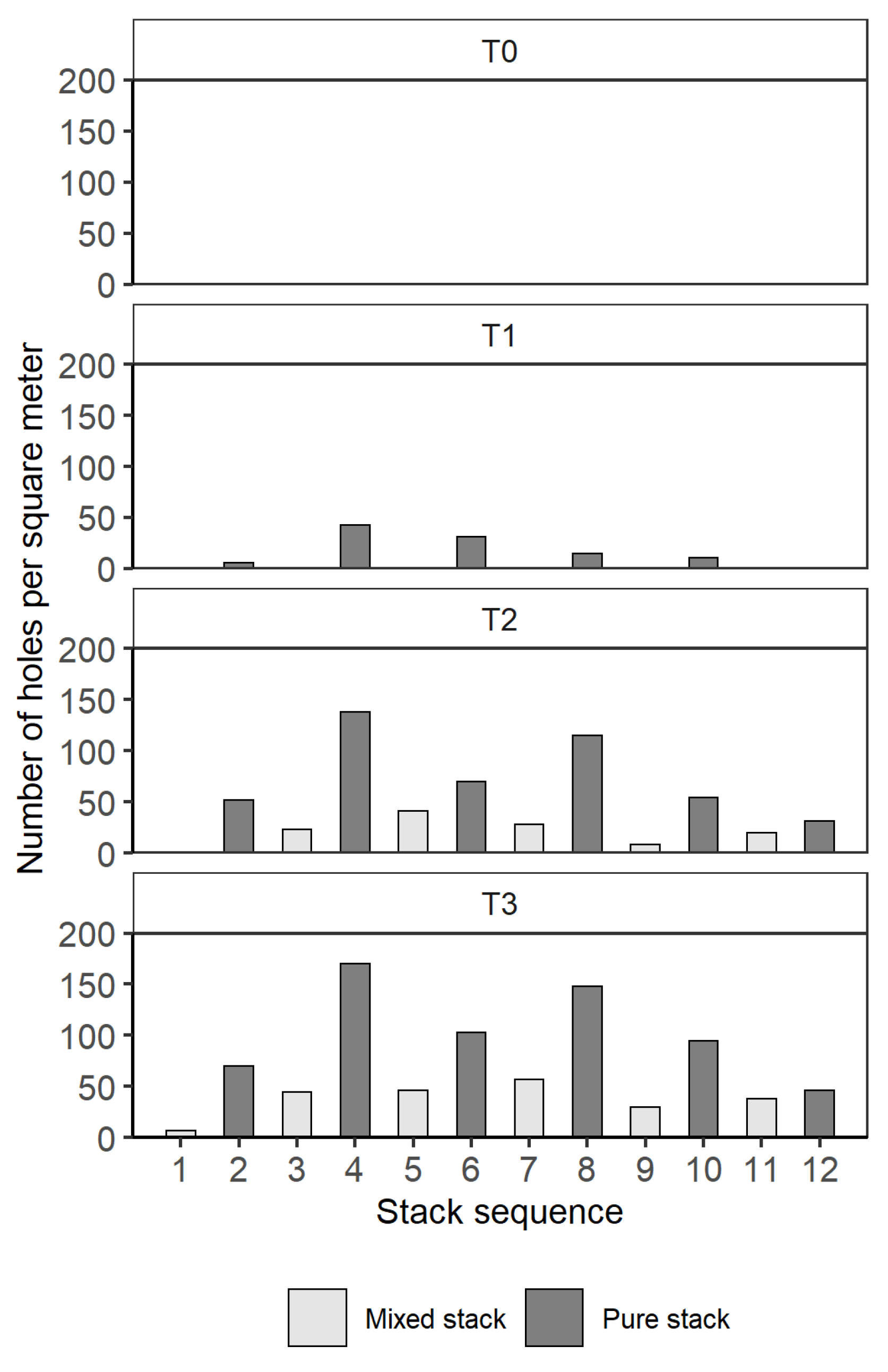
- The effect of stack position was analyzed with a generalized linear mixed effect model (glmer) from the lme4 package [31]. The number of holes was considered as response variable, while stack type, time and position were addressed as explanatory variables. The stack and the time were accounted as random variables to control for the variability.
- To test the attraction toward spruce essential oil, one arm was treated and tested against three blanks. The mean of the values from the three blank arms was considered for the statistical analysis. Reversely, the mean of the three treated arms was considered in the repellence from pine oil trials. Each concentration was tested separately. The number of entries in the treated arm vs. the control one was analyzed with a logistic regression. The time spent in the arms was tested with an ANOVA test.
- A dimension reduction principal component analysis (PCA) was performed using the R package “factoextra” [32]. The PCA calculated the combination of the compound area data by extracting eigenvalues and eigenvectors of a correlation matrix and then highlighting principal components. A two-dimensional score plot was created to compare the bark volatile profiles of spruce and pine logs at T0 and T3 of the field trial. Comparison of the point distances was achieved by analysis of variance using distance matrices (package Vegan [33]).
3. Results
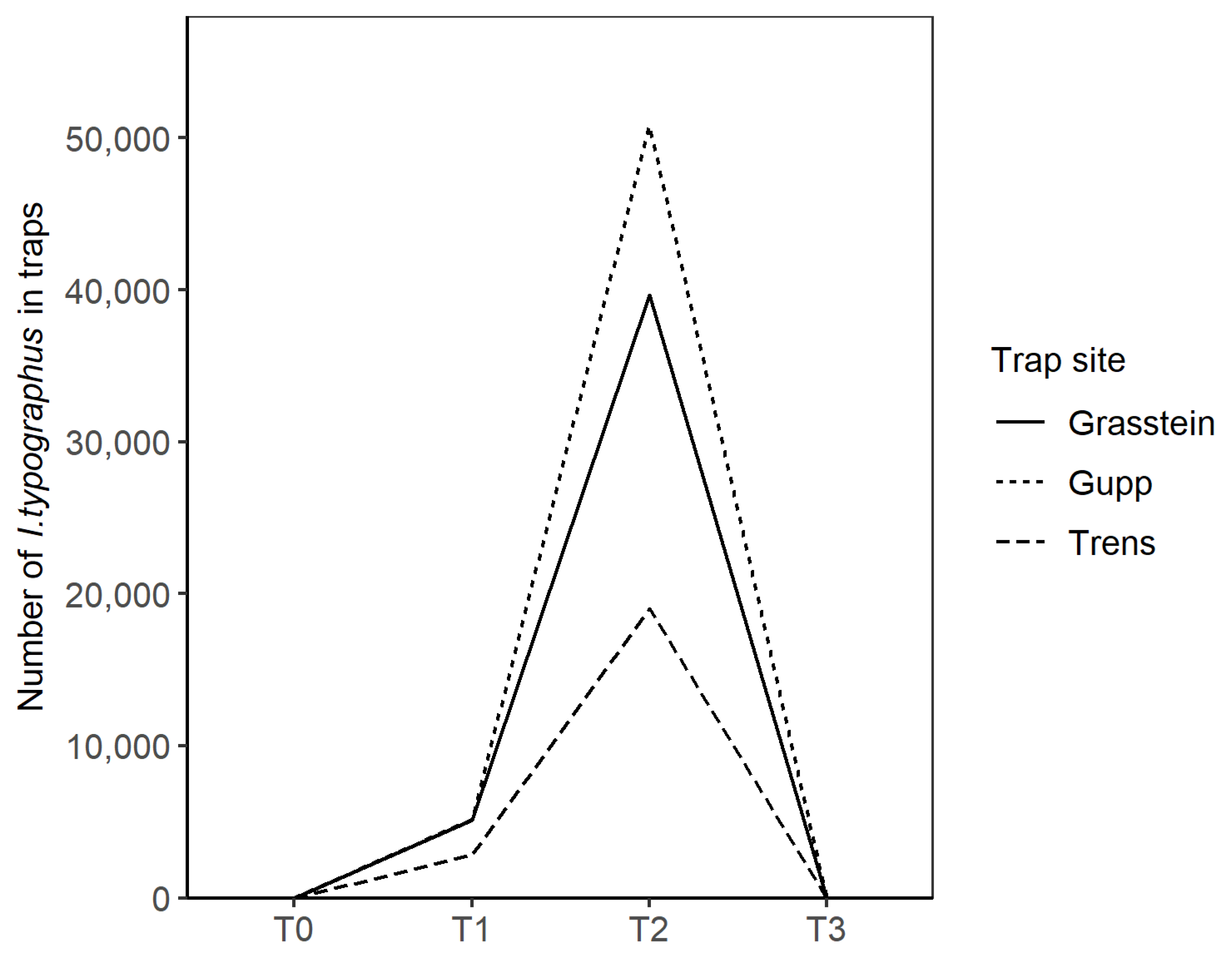
3.1. Field Trials
3.2. Behavioural Trials
3.3. VOCs Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Schlyter, F.; Stadelmann, G.; Brehm, M.; Rogell, H. Ecology of the spruce bark beetle Ips typographus in a climatic gradient: Norway spruce forests at high and low elevations. Insect Ecol. Evol. 2012, 2, 77–88. [Google Scholar]
- Lieutier, F.; Day, K.R.; Battisti, A.; Grégoire, J.C.; Evans, H.F. Bark and Wood Boring Insects in Living Trees in Europe: A Synthesis Kluwer; Academic Publishers: Dordrecht, The Netherlands, 2004; p. 569. [Google Scholar]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Seidl, R.; Müller, J.; Hothorn, T.; Bässler, C. Assessing risks and uncertainties before implementing forest management strategies for bark beetle control. J. Appl. Ecol. 2018, 55, 1698–1708. [Google Scholar]
- McNichol, B.H.; Clarke, S.R.; Faccoli, M.; Montes, C.R.; Nowak, J.T.; Reeve, J.D.; Gandhi, K.J. Relationships between drought, coniferous tree physiology, and Ips bark beetles under climatic changes. In Bark Beetle Management, Ecology, and Climate Change; Academic Press: Cambridge, UK, 2022; pp. 153–194. [Google Scholar]
- Netherer, S.; Matthews, B.; Katzensteiner, K. Impact of climatic variation on infestation trends of the spruce bark beetle Ips typographus (Coleoptera: Curculionidae: Scolytinae) in the Alpine region. For. Ecol. Manag. 2020, 462, 118024. [Google Scholar]
- Lehmanski, L.M.; Kandasamy, D.; Andersson, M.N.; Netherer, S.; Alves, E.G.; Huang, J.; Hartmann, H. Addressing a century-old hypothesis–do pioneer beetles of Ips typographus use volatile cues to find suitable host trees? New Phytol. 2023, 238, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A.; Austara, O.; Pettersen, H. Seasonal flight activity and attack pattern of Ips typographus in Norway under epidemic condition. Meddelelser Fra Nor. Inst. Skogforsk. 1977, 33, 257–268. [Google Scholar]
- Keeling, C.I.; Tittiger, C.; MacLean, M.; Blomquist, G.J. Pheromone production in bark beetles. In Insect Pheromone Biochemistry and Molecular Biology, 2nd ed.; Blomquist, G.J., Vogt, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 123–162. [Google Scholar]
- Schiebe, C.; Hammerbacher, A.; Birgersson, G.; Witzell, J.; Brodelius, P.E.; Gershenzon, J.; Hansson, B.S.; Krokene, P.; Schlyter, F. Inducibility of chemical defenses in Norway spruce bark is correlated with unsuccessful mass attacks by the spruce bark beetle. Oecologia 2012, 170, 183–198. [Google Scholar] [CrossRef]
- Faccoli, M.; Jankowiak, R.; Schlyter, F. Bark beetles and their associated microbes. In Bark Beetles; Academic Press: Cambridge, UK, 2018; pp. 309–354. [Google Scholar]
- Netherer, S.; Schebeck, M.; Morgante, G.; Rentsch, V.; Kirisits, T. European spruce bark beetle, Ips typographus (L.) males are attracted to bark cores of drought-stressed Norway spruce trees with impaired defenses in Petri dish choice experiments. Forests 2022, 13, 537. [Google Scholar] [CrossRef]
- Stadelmann, G.; Bugmann, H.; Meier, F.; Wermelinger, B.; Bigler, C. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. For. Ecol. Manag. 2013, 305, 273–281. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Schlyter, F. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. For. Èntomol. 2004, 6, 1–20. [Google Scholar] [CrossRef]
- Frühbrodt, T.; Schebeck, M.; Andersson, M.N.; Holighaus, G.; Kreuzwieser, J.; Burzlaff, T.; Biedermann, P.H. Verbenone—The universal bark beetle repellent? Its origin, effects, and ecological roles. J. Pest Sci. 2023, 1–37. [Google Scholar] [CrossRef]
- Xu, L.; Lou, Q.; Cheng, C.; Lu, M.; Sun, J. Gut-Associated Bacteria of Dendroctonus valens and their Involvement in Verbenone Production. Microb. Ecol. 2015, 70, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.A. Chemical ecology of bark beetles. Cell. Mol. Life Sci. 1989, 45, 271–283. [Google Scholar] [CrossRef]
- Berthelot, S.; Frühbrodt, T.; Hajek, P.; Nock, C.A.; Dormann, C.F.; Bauhus, J.; Fründ, J. Tree diversity reduces the risk of bark beetle infestation for preferred conifer species, but increases the risk for less preferred hosts. J. Ecol. 2021, 109, 2649–2661. [Google Scholar] [CrossRef]
- Jirošová, A.; Kalinová, B.; Modlinger, R.; Jakuš, R.; Unelius, C.R.; Blaženec, M.; Schlyter, F. Anti-attractant activity of (+)-trans-4-thujanol for Eurasian spruce bark beetle Ips typographus: Novel potency for females. Pest Manag. Sci. 2022, 78, 1992–1999. [Google Scholar] [CrossRef]
- Zhang, Q.-H. Interruption of aggregation pheromone in Ips typographus (L.) (Col. Scolytidae) by non-host bark volatiles. Agric. For. Èntomol. 2003, 5, 145–153. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schlyter, F.; Birgersson, G. (Bark volatiles from nonhost angiosperm trees of spruce bark beetle, Ips typographus (L.) (Coleoptera: Scolytidae): Chemical and electrophysiological analysis. Chemoecology 2000, 10, 69–80. [Google Scholar] [CrossRef]
- Unelius, C.R.; Schiebe, C.; Bohman, B.; Andersson, M.N.; Schlyter, F. Non-Host Volatile Blend Optimization for Forest Protection against the European Spruce Bark Beetle, Ips typographus. PLoS ONE 2014, 9, e85381. [Google Scholar] [CrossRef]
- Lindmark, M.; Wallin, E.A.; Jonsson, B.-G. Protecting forest edges using trap logs—Limited effects of associated push-pull strategies targeting Ips typographus. For. Ecol. Manag. 2022, 505, 119886. [Google Scholar] [CrossRef]
- Jakuš, R.; Schlyter, F.; Zhang, Q.H.; Blaženec, M.; Vaverčák, R.; Grodzki, W.; Brutovský, D.; Lajzová, E.; Turčáni, M.; Bengtsson, M.; et al. Overview of development of an anti-attractant based technology for spruce protection against Ips typographus: From past failures to future success. Anz. Schädlingskunde = J. Pest Sci. 2003, 76, 89–99. [Google Scholar] [CrossRef]
- Schiebe, C.; Blaženec, M.; Jakuš, R.; Unelius, C.R.; Schlyter, F. Semiochemical diversity diverts bark beetle attacks from Norway spruce edges. J. Appl. Entomol. 2011, 135, 726–737. [Google Scholar] [CrossRef]
- Cavaleri, L.; Bajo, M.; Barbariol, F.; Bastianini, M.; Benetazzo, A.; Bertotti, L.; Umgiesser, G. The October 29, 2018 storm in Northern Italy–an exceptional event and its modelling. Prog. Oceanogr. 2019, 178, 102178. [Google Scholar] [CrossRef]
- Bozzini, A.; Francini, S.; Chirici, G.; Battisti, A.; Faccoli, M. Spruce Bark Beetle Outbreak Prediction through Automatic Classification of Sentinel-2 Imagery. Forests 2023, 14, 1116. [Google Scholar] [CrossRef]
- Pettersson, J. An aphid sex attractant, insect systematics & evolution. Entomol. Scand 1970, 1, 63–73. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 15 March 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 15 March 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R. Vegan: Community Ecology Package R Package Version 2, 5-7. 2020. Available online: http://CRAN.R-project.org/package=vegan (accessed on 15 March 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Andersson, M.N.; Larsson, M.C.; Schlyter, F. Specificity and redundancy in the olfactory system of the bark beetle Ips typographus: Single-cell responses to ecologically relevant odors. J. Insect Physiol. 2009, 55, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Blažytė-Čereškienė, L.; Apšegaitė, V.; Radžiutė, S.; Mozūraitis, R.; Būda, V.; Pečiulytė, D. Electrophysiological and behavioural responses of Ips typographus (L.) to trans-4-thujanol—A host tree volatile compound. Ann. For. Sci. 2016, 73, 247–256. [Google Scholar] [CrossRef]
- Kalinová, B.; Břízová, R.; Knížek, M.; Turčáni, M.; Hoskovec, M. Volatiles from spruce trap-trees detected by Ips typographus bark beetles: Chemical and electrophysiological analyses. Arthropod-Plant Interact. 2014, 8, 305–316. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schlyter, F.; Anderson, P. Green leaf volatiles interrupt pheromone response of spruce bark beetle, Ips typographus. J. Chem. Ecol. 1999, 25, 2847–2861. [Google Scholar] [CrossRef]
- Schlyter, F. Semiochemical Diversity in Practice: Antiattractant Semiochemicals Reduce Bark Beetle Attacks on Standing Trees—A First Meta-Analysis. Psyche A J. Èntomol. 2012, 2012, 268621. [Google Scholar] [CrossRef]
- Birgersson, G.; Bergström, G. Volatiles released from individual spruce bark beetle entrance holes quantitative variations during the first week of attack. J. Chem. Ecol. 1989, 15, 2465–2483. [Google Scholar] [CrossRef]
- Schlyter, F.; Löfqvist, J.; Jakus, R. Green leaf volatiles and verbenone modify attraction of European Tomicus, Hylurgops, and Ips bark beetles. In Behavior, Population Dynamics, and Control of Forest Insects. In Proceedings of the a Joint IUFRO Working Party Conference-February, Maui, HI, USA, 6–11 February 1994; pp. 29–44. [Google Scholar]
- Lindgren, B.S.; Raffa, K.F. Evolution of tree killing in bark beetles (Coleoptera: Curculionidae): Trade-offs between the maddening crowds and a sticky situation. Can. Entomol. 2013, 145, 471–495. [Google Scholar] [CrossRef]
- Netherer, S.; Kandasamy, D.; Jirosová, A.; Kalinová, B.; Schebeck, M.; Schlyter, F. Interactions among Norway spruce, the bark beetle Ips typographus and its fungal symbionts in times of drought. J. Pest Sci. 2021, 94, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Pick, J.A. Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
| Compound | LRIa | LRIb | Norway Spruce | Scots Pine | ||
|---|---|---|---|---|---|---|
| T0 | T3 | T0 | T3 | |||
| Aromatic hydrocarbons | ||||||
| toluene | <800 | 770 1 | 6.3 ± 0.9 (3) | 107 ± 165.2 (4) | ||
| 1,3,5-trimethyl-benzene | 998 | 996 1 | 15.9 ± 3.4 (3) | 32.4 ± 10.8 (3) * | 28.1 ± 18 (4) | 37.1 ± 51.8 (3) |
| 1,2,4-trimethyl-benzene | 1001 | 1001 2 | 17.2 ± 2.6 (3) | 21.3 ± 18.7 (3) | ||
| 1,2,3-trimethyl-benzene | 1029 | 1029 2 | 76.8 ± 17.7 (4) | |||
| 1-methyl-3-propyl-benzene | 1058 | 1059 2 | 74.3 ± 20.1 (4) * | 15.1 ± 7.1 (3) | 47.2 ± 41.2 (4) | 11.9 ± 14.1 (2) |
| 1-ethyl-2,5-dimethylbenzene | 1062 | 1063 2 | 68.7 ± 30.7 (4) * | 19.9 ± 9.9 (3) | 48.6 ± 41.3 (4) | 14.6 ± 17.1 (2) |
| 1-methyl-2-propyl-benzene | 1072 | 1073 2 | 36.2 ± 13.9 (4) * | 9.9 ± 5.8 (3) | 17.6 ± 13.8 (4) | 7.1 ± 8.4 (2) |
| 2-ethyl-1,3-dimethylbenzene | 1083 | 1082 2 | 165.2 ± 69.7 (4) * | 44.5 ± 25 (3) | 79 ± 61.8 (4) | 22.4 ± 32 (3) |
| 1-ethyl-2,4-dimethylbenzene | 1090 | 1089 2 | 169.3 ± 64.4 (4) * | 47 ± 25.4 (3) | 85.4 ± 65.7 (4) | 28.3 ± 42.6 (3) |
| 2-ethyl-1,4-dimethylbenzene | 1097 | 1090 2 | 18.9 ± 8.8 (4) * | 8.2 ± 5.2 (3) | 7.7 ± 4.6 (4) * | 2 ± 0.9 (2) |
| 1,2,3,4-tetraisopropyl-5-methylene-1,3cyclopentadiene ‡ | 1111 | 48.5 ± 26.2 (4) | 16.1 ± 10 (3) | 21.6 ± 15.1 (4) | 9.9 ± 14.1 (3) | |
| 1,2,4,5-tetramethylbenzene | 1121 | 1121 2 | 50 ± 29.6 (4) | 18.4 ± 11.4 (3) | 19.1 ± 12.7 (4) | 15 ± 18 (2) |
| 1,2,3,5-tetramethylbenzene | 1125 | 1125 2 | 73 ± 44.9 (4) | 26.2 ± 15.8 (3) | 29.2 ± 20.5 (4) | 14 ± 20.1 (3) |
| 4-methylindan | 1144 | 1144 2 | 14.9 ± 8.6 (4) | 11 ± 5.8 (3) | 7.4 ± 5.7 (4) | 6 ± 7.3 (3) |
| 1,2,3,4-tetramethylbenzene | 1156 | 1155 2 | 29.1 ± 18.6 (4) | 12 ± 7.2 (3) | 11.6 ± 7.1 (4) | 10.3 ± 12.5 (2) |
| 2,6,6-trimethyl-bicyclo-heptan-3-one ‡ | 1166 | 12.1 ± 6.9 (4) | 7.3 ± 4.2 (3) | |||
| Monoterpenoids | ||||||
| α-pinene | 941 | 941 2 | 82.1 ± 145.6 (4) | 165.6 ± 150.4 (3) | 77.8 ± 47.8 (4) | 801.6 ± 626.2 (3) * |
| β-pinene | 979 | 983 † | 113.9 ± 203.1 (4) | 170.3 ± 153.9 (3) | 123.5 ± 97.6 (3) | 126.7 ± 163.8 (3) |
| β-myrcene | 993 | 996 † | 43.4 ± 74.2 (4) * | 8 ± 1.3 (3) | ||
| β-terpinene | 1036 | 1036 2 | 193.9 ± 40.4 (3) | 100.6 ± 65.1 (4) | 32.1 ± 24.6 (3) | |
| isopinocamphone | 1180 | 1180 1 | 6.2 ± 3.1 (3) | 11.3 ± 5.9 (3) | 3.2 ± 1.8 (2) | |
| α-terpineol | 1215 | 1218 1 | 7 ± 4.3 (3) | |||
| verbenone | 1292 | 1285 1 | 1.7 ± 0.3 (3) | |||
| Sesquiterpenoids | ||||||
| α-cubebene | 1360 | 1360 2 | 4.7 ± 4 (2) | |||
| cyclosativene | 1379 | 1380 4 | 8.2 ± 11 (3) | |||
| α-copaene | 1388 | 1388 2 | 1.8 ± 0.6 (2) | 20 ± 26.3 (3) * | ||
| 2-epi-α-funebrene | 1395 | 1396 2 | 9.8 ± 2.8 (2) | |||
| β-bourbonene | 1398 | 1391 4 | 2.8 ± 2.1 (2) | 10.4 ± 15.6 (3) | ||
| α-cedrene | 1426 | 1423 2 | 3.8 ± 2 (2) | |||
| β-copaene | 1443 | 1444 2 | 18.5 ± 25.1 (3) | |||
| γ-muurolene | 1458 | 1457 4 | 7.7 ± 3.9 (2) | |||
| zonarene | 1511 | 1518 2 | 3 ± 2.4 (2) | |||
| γ-cadinene | 1525 | 1525 2 | 6 ± 6.2 (2) | |||
| δ-cadinene | 1533 | 1539 3 | 6.9 ± 7.4 (2) | |||
| Esters | ||||||
| bornyl acetate ‡ | 1362 | 5.7 ± 2.6 (3) | ||||
| Alcohols | ||||||
| benzyl alcohol | 1040 | 1039 2 | 9.2 ± 2.4 (3) | |||
| Alkenes | ||||||
| 1-tetradecene | 1420 | 1418 2 | 7.1 ± 3.8 (2) | |||
| 3-pentadecene | 1494 | 1491 2 | 2.7 ± 1.3 (2) | |||
| Alkanes | ||||||
| 2,2,4,6,6-pentamethyl-heptane | 993 | 994 2 | 14.8 ± 9.6 (3) | 16.8 ± 18.6 (2) | ||
| 4-methyl-undecane | 1167 | 1167 2 | 5.5 ± 2.4 (4) | 5.5 ± 6.3 (3) | ||
| 3,5-dimethylundecane | 1215 | 1211 2 | 1.4 ± 0.3 (3) | |||
| nC15 | 1502 | 1500 † | 1.7 ± 0 (2) | |||
| nC16 | 1601 | 1600 † | 1.4 ± 0.3 (2) | |||
| nC17 | 1702 | 1700 † | 2.1 ± 0.4 (2) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favaro, R.; Andriolo, A.; Sieder, C.; Angeli, S. Potential of Scots Pine for a Push Strategy against the European Spruce Bark Beetle Ips typographus. Forests 2023, 14, 1727. https://doi.org/10.3390/f14091727
Favaro R, Andriolo A, Sieder C, Angeli S. Potential of Scots Pine for a Push Strategy against the European Spruce Bark Beetle Ips typographus. Forests. 2023; 14(9):1727. https://doi.org/10.3390/f14091727
Chicago/Turabian StyleFavaro, Riccardo, Alessandro Andriolo, Cinthia Sieder, and Sergio Angeli. 2023. "Potential of Scots Pine for a Push Strategy against the European Spruce Bark Beetle Ips typographus" Forests 14, no. 9: 1727. https://doi.org/10.3390/f14091727