Relationships between Climate Variability and Radial Growth of Larix potaninii at the Upper Altitudinal Limit in Central Hengduan Mountain, Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling
2.3. Tree-Ring Chronology Development
2.4. Climate Data
2.5. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoch, G.; Korner, C. Growth, demography and carbon relations of Polylepis trees at the world’s highest treeline. Funct. Ecol. 2005, 19, 941–951. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Thomas, A.; Li, J.B.; Cao, K.F. Spatial and temporal temperature trends on the Yunnan Plateau (southwest China) during 1961–2004. Int. J. Climatol. 2011, 31, 2078–2090. [Google Scholar] [CrossRef]
- Li, Z.S.; Zhang, Q.B.; Ma, K.P. Tree-ring reconstruction of summer temperature for A.D. 1475–2003 in the central Hengduan Mountains, Northwestern Yunnan, China. Clim. Chang. 2012, 110, 455–467. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F. Annual temperature reconstruction in the central Hengduan Mountains, China, as deduced from tree rings. Dendrochronologia 2008, 26, 97–107. [Google Scholar] [CrossRef]
- Bi, Y.F.; Xu, J.C.; Gebrekirstos, A.; Guo, L.; Zhao, M.X.; Liang, E.Y.; Yang, X.F. Assessing drought variability since 1650 AD from tree-rings on the Jade Dragon Snow Mountain, southwest China. Int. J. Climatol. 2015, 35, 4057–4065. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tansley review no. 123. Tree and forest functioning in response to global warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Martnez-Vilalta, J.; López, B.C.; Adell, N.; Badiella, L.; Ninyerola, M. Twentieth century increase of scots pine radial growth in NE Spain shows strong climate interactions. Glob. Chang. Biol. 2010, 14, 2868–2881. [Google Scholar] [CrossRef]
- Fang, O.Y.; Zhang, Q.B.; Vitasse, Y.; Zweifel, R.; Cherubini, P. The frequency and severity of past droughts shape the drought sensitivity of juniper trees on the Tibetan Plateau. Forest. Ecol. Manag. 2022, 486, 118968. [Google Scholar] [CrossRef]
- Yao, Y.B.; Xiao, G.J.; Wang, R.Y. Climatic changes of semi-arid region over the northwest China in recent 50a. Arid. Land. Geogr. 2009, 32, 159–165. [Google Scholar]
- Song, L.N.; Zhu, J.J.; Zhang, J.X.; Wang, K.; Lü, L.Y.; Wang, F.B.; Wang, G.C. Divergent growth responses to warming and drying climates between native and non-native tree species in Northeast China. Trees 2019, 33, 1143–1155. [Google Scholar] [CrossRef]
- Chen, L.; Wu, S.; Pan, T. Variability of climate–growth relationships along an elevation gradient in the Changbai Mountain, northeastern China. Trees 2011, 25, 1133–1139. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.; Chang, Y.; Chen, Z. Increased sensitivity of Dahurian larch radial growth to summer temperature with the rapid warming in Northeast China. Trees 2016, 30, 1799–1806. [Google Scholar] [CrossRef]
- Gou, X.X.; Zhang, T.W.; Yuan, Y.J.; Yu, S.L.; Zhang, R.B.; Jiang, S.X.; Guo, Y.L. Radial growth of dominant coniferous species and their responses to climate changes in the Altay Mountains, China. China J. Appl. Ecol. 2021, 32, 3594–3608. [Google Scholar]
- Zhou, P.; Huang, J.G.; Liang, H.X.; Rossi, S.; Bergeron, Y.; Shishov, V.V.; Jiang, S.W.; Kang, J.; Zhu, H.X.; Dong, Z.C. Radial growth of Larix sibirica was more sensitive to climate at low than high altitudes in the Altai Mountains, China. Agric. For. Meteorol. 2021, 304–305, 108392. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, R.; Yin, J.; Tian, K.; Yin, D. Radial growth response of major conifers to climate change on Haba Snow Mountain, southwestern China. Dendrochronologia 2020, 60, 125682. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, D.C.; Sun, M.; Wang, H.; Tian, K.; Xiao, D.R.; Zhang, W.G. Variations of climate-growth response of major conifers at upper distributional limits in Shika Snow Mountain, Northwestern Yunnan Plateau, China. Forests 2017, 8, 377. [Google Scholar] [CrossRef]
- Panthi, S.; Bräuning, A.; Zhou, Z.K.; Fan, Z.X. Growth response of Abies georgei to climate increases with elevation in the central Hengduan Mountains, southwestern China. Dendrochronologia 2018, 47, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, P.L.; Lin, Y.X.; Ge, S.; Yang, J.Q.; Gerong, Q.Z.; Fan, Z.X. Intra-annual radial growth of Abies georgei and Larix potaninii and its responses to environmental factors in the Baima Snow Mountain, Northwest Yunnan, China. J. Appl. Ecol. 2022, 33, 2881–2888. [Google Scholar]
- Cui, J.; Qin, J.; Sun, H. Population spatial dynamics of Larix potaninii in alpine treeline ecotone in the eastern margin of the Tibetan plateau, China. Forests 2017, 8, 356. [Google Scholar] [CrossRef]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Larsson. CooRecorder and CDendro Programs of the CooRecorder/CDendro Package Version 9.3. Available online: http://www.cybis.se/forfun/dendro (accessed on 14 October 2022).
- Holmes, R.L. Computer-assisted quality control in tree-ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Users Manual for Program ARSTAN: Laboratory of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 1986. [Google Scholar]
- Kendall, M.G.; Gibbons, J.D. Rank Correlation Methods, 5th ed.; Edward Arnold: London, UK, 1990. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical community ordination. Part I: Basic theory and linear methods. Ecoscience 1994, 1, 127–140. [Google Scholar] [CrossRef]
- Drobyshev, I.; Gewehr, S.; Berninger, F.; Bergeron, Y. Species specifific growth responses of black spruce and trembling aspen may enhance resilience of boreal forest to climate change. J. Ecol. 2013, 101, 231–242. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Cai, Q.F.; Liu, Y. Altitudinal difference of growth-climate response models in the north subtropical forests of China. Dendrochronologia 2022, 72, 125935. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows ser’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Thaca, NY, USA, 2002. [Google Scholar]
- Sergio, R.; Annie, D.; Jozica, G.; Jeong-Wook, S.; Cyrille, B.R.; Tommaso, A.; Hubert, M.; Tom, L.; Primoz, O.; Risto, J. Critical temperatures for xylogenesis in conifers of cold climates. Global. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar]
- Zhang, J.; Gou, X.; Manzanedo, R.D.; Zhang, F.; Pederson, N. Cambial phenology and xylogenesis of Juniperus przewalskii over a climatic gradient is influenced by both temperature and drought. Agric. For. Meteorol. 2018, 260–261, 165–175. [Google Scholar] [CrossRef]
- Shi, C.M.; Shen, M.G.; Wu, X.C.; Cheng, X.; Li, X.Y.; Fan, T.Y.; Li, Z.S.; Zhang, Y.D.; Fan, Z.X.; Shi, F.Z.; et al. Growth response of alpine treeline forests to a warmer and drier climate on the southeastern Tibetan Plateau. Agr. Forest. Meteorol. 2018, 264, 73–79. [Google Scholar] [CrossRef]
- Aryal, S.; Grießinger, J.; Arsalani, M.; Meier, W.J.H.; Fu, P.l.; Fan, Z.X.; Bräuning, A. Insect infestations have an impact on the quality of climate reconstructions using Larix ring-width chronologies from the Tibetan Plateau. Ecol. Indic. 2023, 148, 110124. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.L.; Chen, J.; Duan, J.P.; Shao, X.M.; Chen, K.L. Growth characteristics and response to climate change of Larix Miller tree-ring in China. Sci. China Earth Sci. 2010, 40, 645–653. [Google Scholar] [CrossRef]
- Bai, X.P.; Zhang, X.L.; Li, J.X.; Duan, X.Y.; Jin, Y.T.; Chen, Z.J. Altitudinal disparity in growth of Dahurian larch (Larix gmelinii Rupr.) in response to recent climate change in northeast China. Sci. Total Environ. 2019, 670, 466–477. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F.; Zhu, S.D. Growth-climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. Forest. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Bazzoffifi, P.; Nieddu, S. Effects of water logging on the soil structure of some Italian soils in relation to the GAEC cross-compliance standard maintenance of farm channel networks and fifield convexity. Ital. J. Agron. 2011, 6, e9. [Google Scholar] [CrossRef]
- Huang, J.G.; Tardif, J.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Chang. Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Prislan, P.; Rossi, S.; Čufar, K. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree. Physiol. 2013, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Wang, Y.; Xu, Y.; Liu, B.; Shao, X. Growth variation in Abies georgei var. smithii along altitudinal gradients in the Sygera Mountains southeastern Tibetan Plateau. Trees 2010, 24, 363–373. [Google Scholar] [CrossRef]
- Hazeleger, W.; Severijns, C.; Semmler, T.; Ştefănescu, S.; Yang, S.; Wang, X.; Wyser, K.; Dutra, E.; Baldasano, J.M.; Bintanja, R.; et al. EC-Earth: A seamless earth-system prediction approach in action. Bull. Am. Meteorol. Soc. 2010, 91, 1357–1364. [Google Scholar] [CrossRef]
- Korner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef]
- Zou, F.; Tu, C.; Liu, D.; Yang, C.; Wang, W.; Zhang, Z. Alpine Treeline Dynamics and the Special Exposure Effect in the Hengduan Mountains. Front. Plant. Sci. 2022, 13, 861231. [Google Scholar] [CrossRef]
- Mamet, S.D.; Brown, C.D.; Trant, A.J.; Laroque, C.P. Shifting global Larix distributions: Northern expansion and southern retraction as species respond to changing climate. J. Biogeogr. 2019, 46, 30–44. [Google Scholar] [CrossRef]

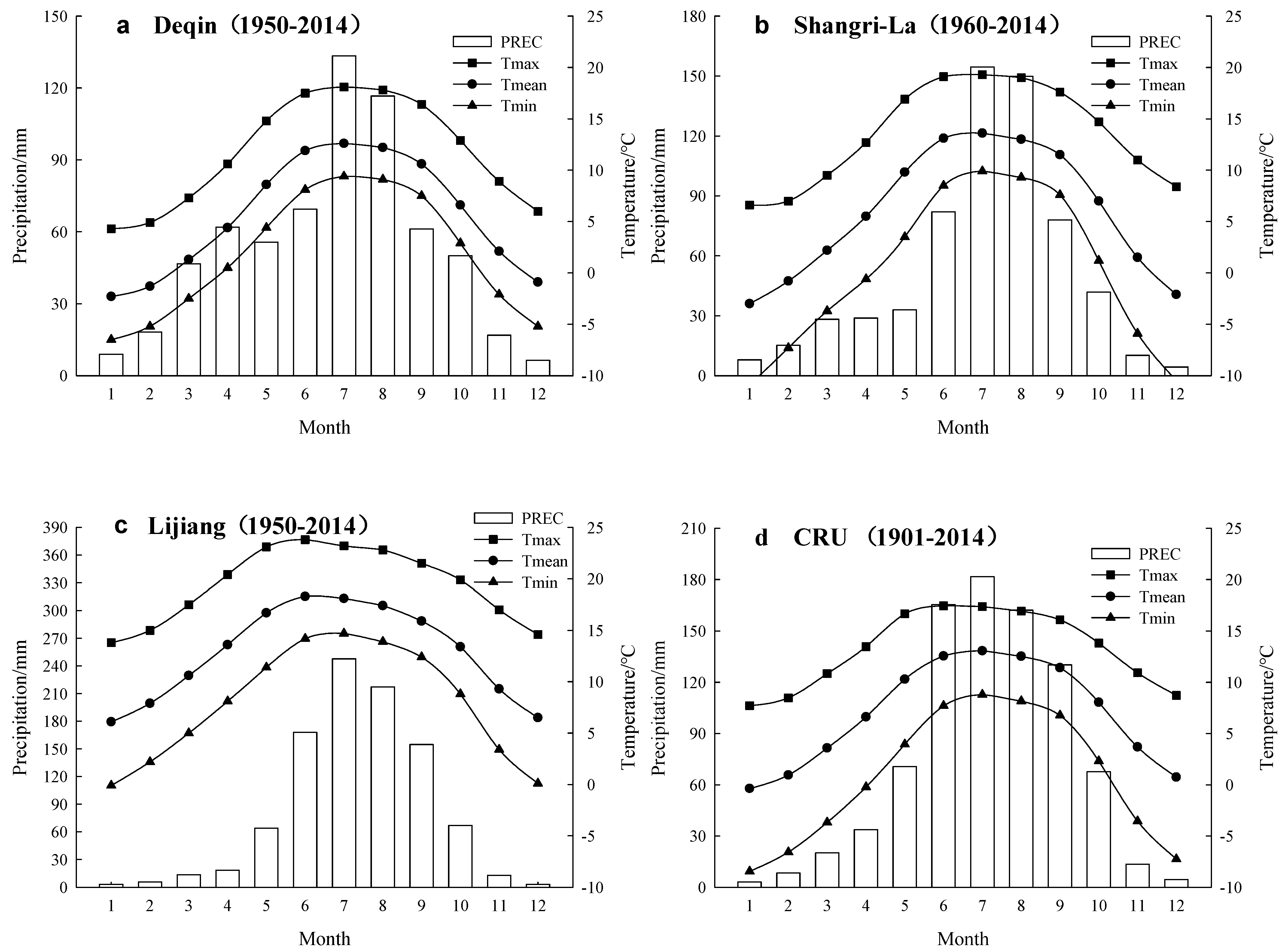

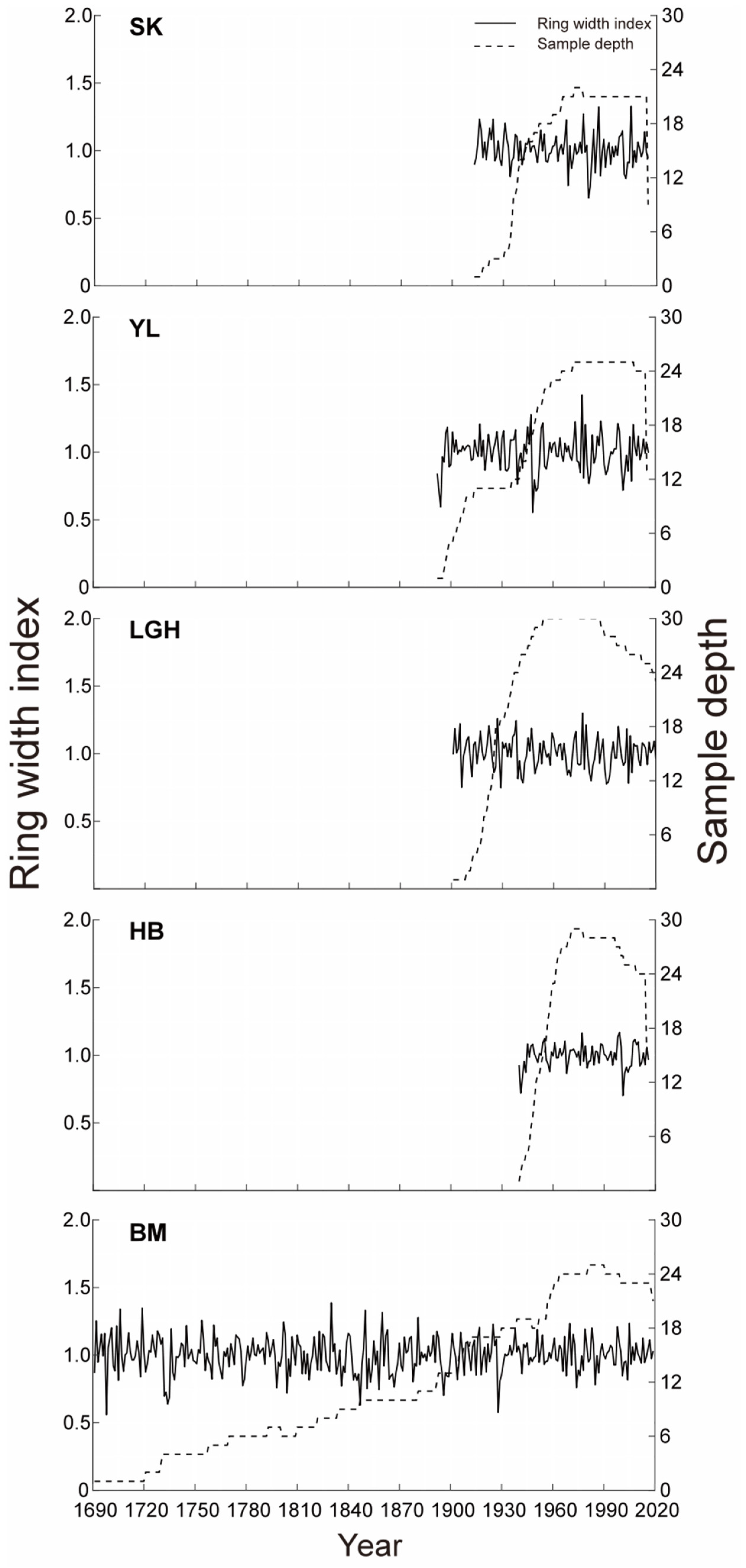
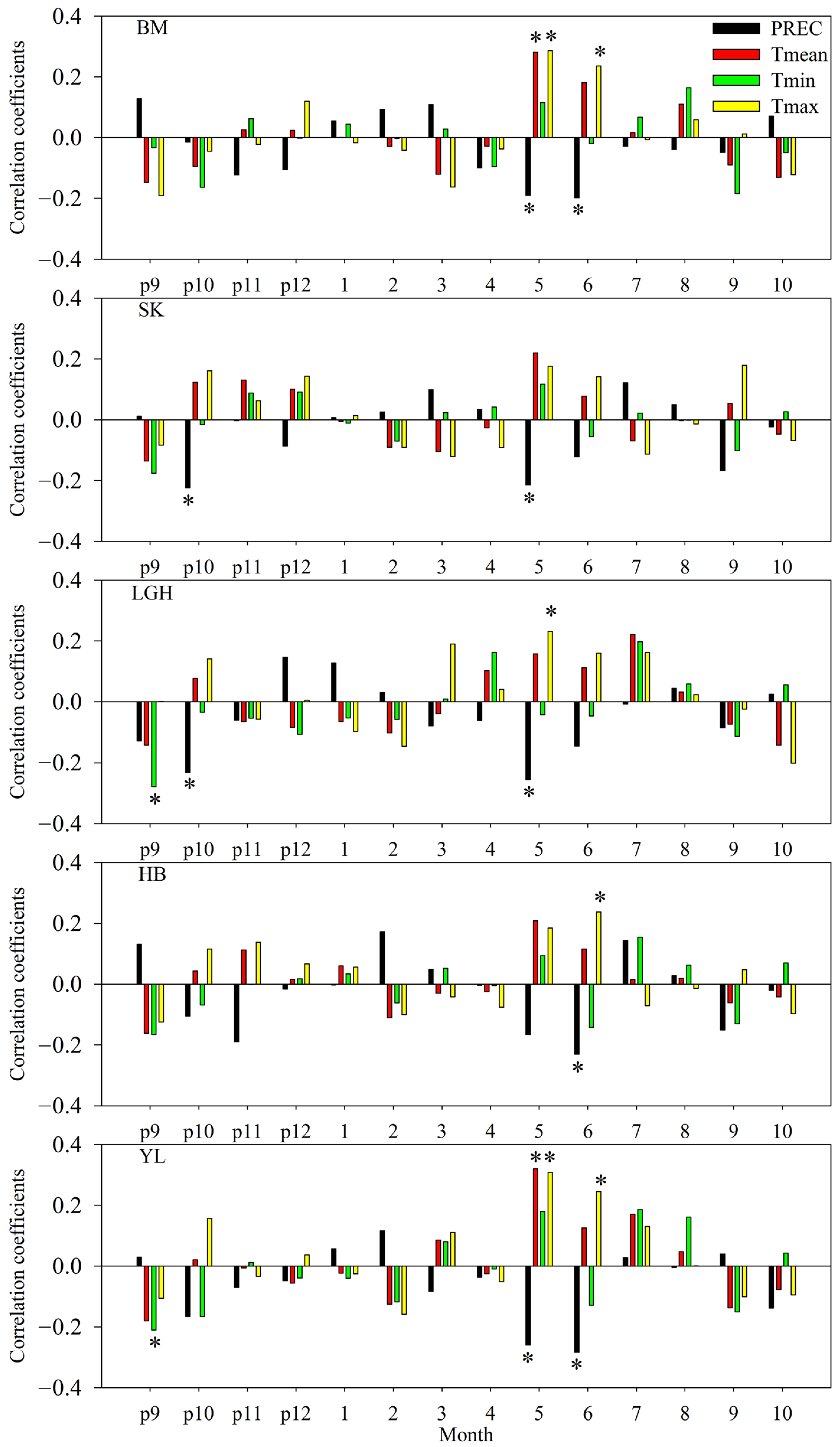

| Sites | Altitude/M | Longitude/E | Latitude/N | No.(Tree/Core) |
|---|---|---|---|---|
| BM | 4150 | 99°07′18″ | 28°20′02″ | 29/58 |
| SK | 3819 | 99°36′35″ | 27°53′51″ | 23/46 |
| LGH | 3747 | 100°46′56″ | 27°38′10″ | 34/68 |
| HB | 3675 | 100°04′55″ | 27°20′20″ | 31/62 |
| YL | 3647 | 100°12′46″ | 27°06′14″ | 26/52 |
| Shangri-La | Deqin | Lijiang | |
|---|---|---|---|
| CRU | |||
| Precipitation | 0.462 ** | 0.495 ** | 0.727 ** |
| Tmean | 0.856 ** | 0.830 ** | 0.872 ** |
| Residual Chronologies | BM | SK | LGH | HB | YL |
|---|---|---|---|---|---|
| No.(tree/radii) | 29/52 | 23/44 | 34/61 | 31/58 | 26/50 |
| Chronology length | 1687–2019 | 1911–2015 | 1899–2020 | 1937–2016 | 1888–2016 |
| Mean sensitivity | 0.23 | 0.21 | 0.21 | 0.15 | 0.21 |
| Statistics of common interval analysis (1942–2014) | |||||
| Variance in first eigenvector/% | 40.87% | 47.03% | 41.98% | 37.29% | 44.52% |
| Standard deviation | 0.21 | 0.18 | 0.19 | 0.13 | 0.19 |
| Signal-to-noise ratio | 16.63 | 22.48 | 26.80 | 27.11 | 29.03 |
| Expressed population signal | 0.94 | 0.96 | 0.96 | 0.96 | 0.97 |
| Rbar | 0.41 | 0.54 | 0.43 | 0.39 | 0.43 |
| HB | BM | YL | SK | |
|---|---|---|---|---|
| BM | 0.467 ** | - | 0.445 ** | 0.436 ** |
| YL | 0.593 ** | 0.445 ** | - | 0.427 ** |
| SK | 0.481 ** | 0.436 ** | 0.427 ** | - |
| LGH | 0.442 ** | 0.331 ** | 0.543 ** | 0.393 ** |
| Site | Tmax | Tmean | Tmin | Precipitation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAU | CSP | CSU | CAU | PAU | CSP | CSU | CAU | PAU | CSP | CSU | CAU | PAU | CSP | CSU | CAU | |
| BM | 0.16 | 0.05 | 0.36 * | −0.06 | −0.06 | 0.02 | 0.23 * | −0.06 | −0.27 | −0.11 | 0.07 | −0.06 | 0.01 | −0.05 | −0.05 | 0.02 |
| SK | 0.11 | 0.06 | 0.28 | −0.13 | 0.00 | 0.01 | 0.11 | −0.08 | −0.11 | −0.07 | −0.03 | −0.03 | −0.07 | −0.08 * | −0.03 | 0.02 |
| LGH | 0.09 | 0.17 | 0.01 | −0.22 | −0.11 | 0.16 * | 0.01 | −0.05 | −0.27 | 0.15 | 0.04 | 0.14 | −0.05 | −0.09 | 0.06 | 0.08 |
| HB | 0.16 | 0.08 | 0.07 | −0.12 | −0.06 | 0.07 | 0.02 | −0.03 | −0.24 | 0.05 | 0.01 | 0.06 | −0.05 | −0.03 | 0.01 | 0.04 |
| YL | 0.26 | 0.08 | 0.13 | −0.27 | −0.11 | 0.08 | 0.06 | −0.05 | −0.43 * | 0.06 | 0.06 | 0.17 | −0.08 | −0.06 | −0.02 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, H.; Li, J.; Xie, S.; Chen, H.; Tian, K.; Sun, M.; Zhang, D.; Zhang, Y. Relationships between Climate Variability and Radial Growth of Larix potaninii at the Upper Altitudinal Limit in Central Hengduan Mountain, Southwestern China. Forests 2023, 14, 1790. https://doi.org/10.3390/f14091790
Yue H, Li J, Xie S, Chen H, Tian K, Sun M, Zhang D, Zhang Y. Relationships between Climate Variability and Radial Growth of Larix potaninii at the Upper Altitudinal Limit in Central Hengduan Mountain, Southwestern China. Forests. 2023; 14(9):1790. https://doi.org/10.3390/f14091790
Chicago/Turabian StyleYue, Haitao, Jianing Li, Siyu Xie, Hai Chen, Kun Tian, Mei Sun, Dacai Zhang, and Yun Zhang. 2023. "Relationships between Climate Variability and Radial Growth of Larix potaninii at the Upper Altitudinal Limit in Central Hengduan Mountain, Southwestern China" Forests 14, no. 9: 1790. https://doi.org/10.3390/f14091790





