Influences of Wood Decomposition Associated with Tree Types on Soil Nutrient Concentrations and Enzyme Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Sampling and Measurements
2.4. Data Analysis
3. Results
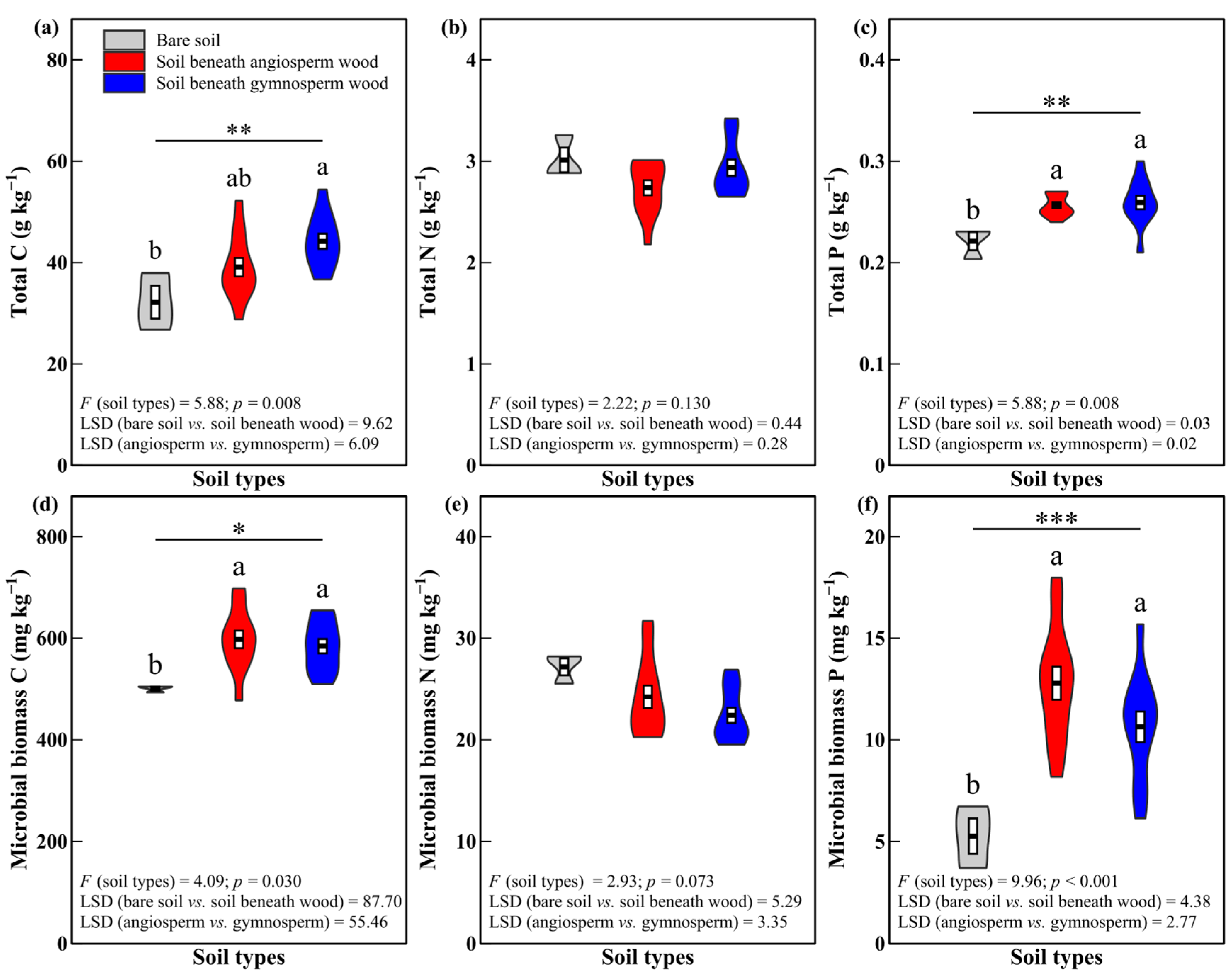
3.1. Soil Total C and Nutrient Concentrations
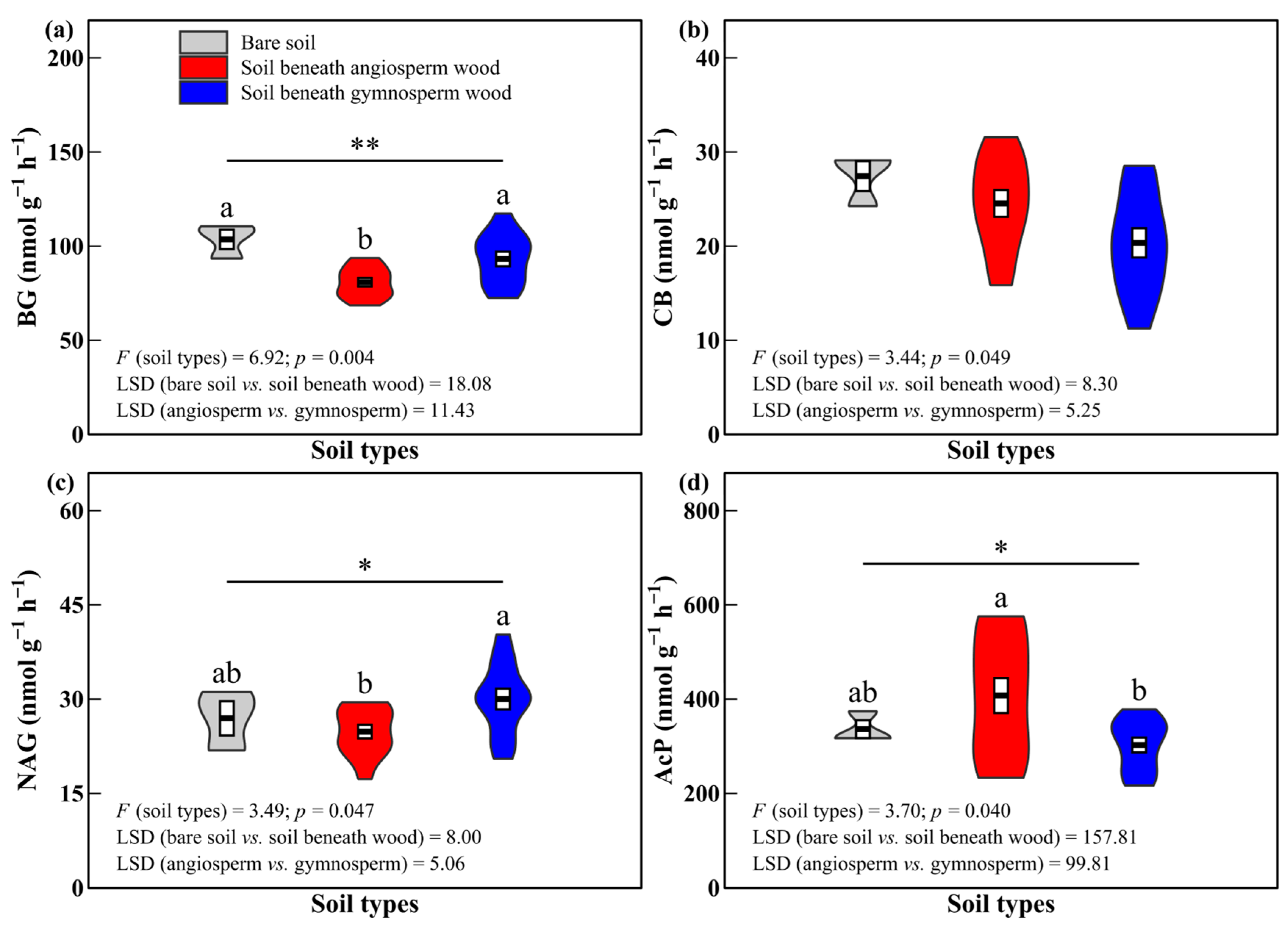
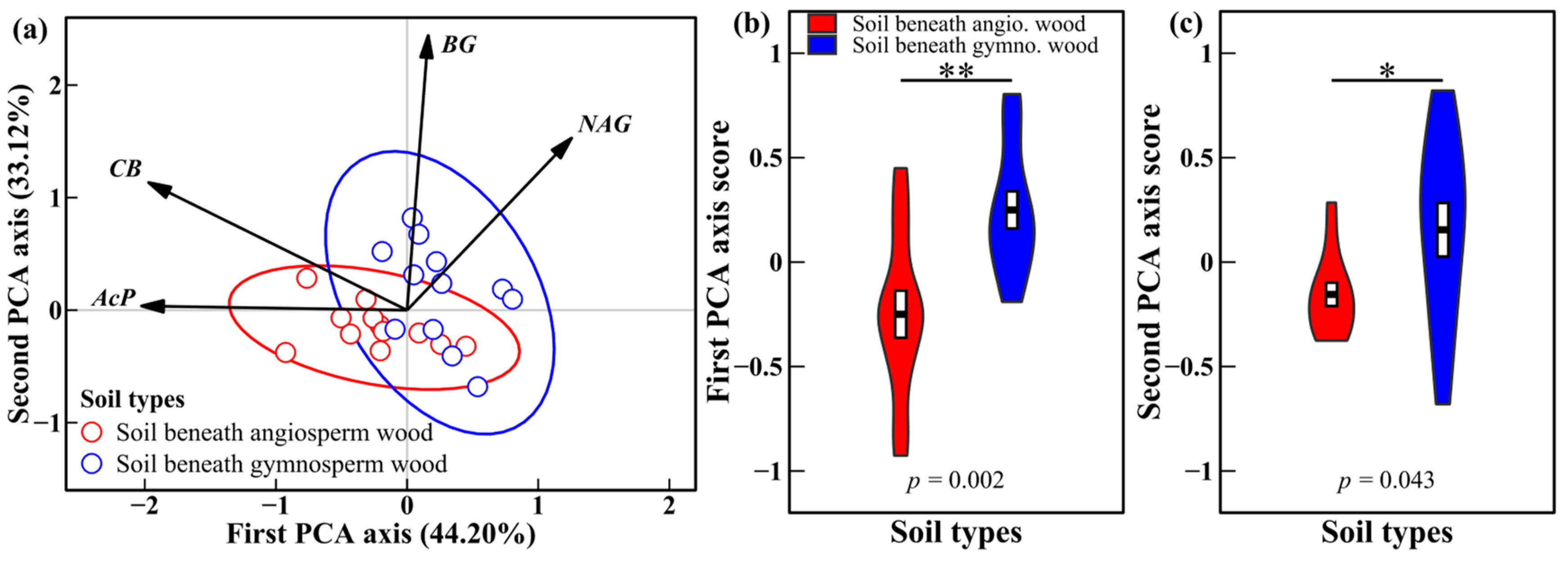
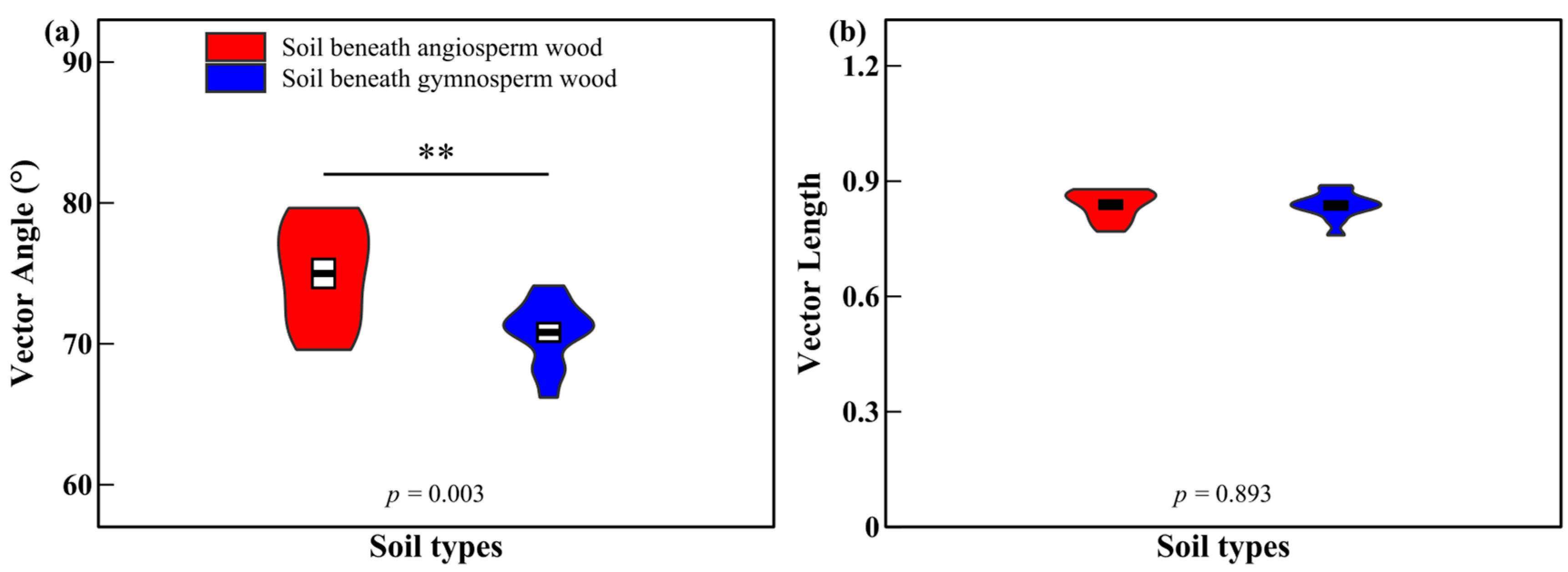
3.2. Soil Enzyme Activities and Enzyme Characteristics
4. Discussion
4.1. Effects of Decomposing Wood on Soil Total C and Nutrient Concentrations
4.2. Effects of Decomposing Wood on Soil Enzyme Activities and Enzyme Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Bantle, A.; Borken, W.; Matzner, E. Dissolved nitrogen release from coarse woody debris of different tree species in the early phase of decomposition. For. Ecol. Manag. 2014, 334, 277–283. [Google Scholar] [CrossRef]
- Stutz, K.P.; Dann, D.; Wambsganss, J.; Scherer-Lorenzen, M.; Lang, F. Phenolic matter from deadwood can impact forest soil properties. Geoderma 2017, 288, 204–212. [Google Scholar] [CrossRef]
- Gonzalez-Polo, M.; Fernández-Souto, A.; Austin, A.T. Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an old-growth temperate forest of Patagonia, Argentina. Ecosystems 2013, 16, 1025–1038. [Google Scholar] [CrossRef]
- Wojciech, P.; Ewa, B.; Jarosław, L. Soil biochemical properties and stabilisation of soil organic matter in relation to deadwood of different species. FEMS Microbiol. Ecol. 2019, 95, fiz011. [Google Scholar] [CrossRef]
- Goldin, S.R.; Hutchinson, M.F. Coarse woody debris modifies surface soils of degraded temperate eucalypt woodlands. Plant Soil 2013, 370, 461–469. [Google Scholar] [CrossRef]
- Nazari, M.; Pausch, J.; Bickel, S.; Bilyera, N.; Rashtbari, M.; Razavi, B.S.; Zamanian, K.; Sharififar, A.; Shi, L.; Dippold, M.A.; et al. Keeping thinning-derived deadwood logs on forest floor improves soil organic carbon, microbial biomass, and enzyme activity in a temperate spruce forest. Eur. J. For. Res. 2023, 142, 287–300. [Google Scholar] [CrossRef]
- Błońska, E.; Prażuch, W.; Lasota, J. Deadwood affects the soil organic matter fractions and enzyme activity of soils in altitude gradient of temperate forests. For. Ecosyst. 2023, 10, 100115. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.; Weedon, J.T.; Wirth, C.; Zanne, A.E. Plant traits and wood fates across the globe: Rotted, burned, or consumed? Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.C.; Zanne, A.E.; Wirth, C.; Coomes, D.A. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, H.Y.H.; Yue, C.; Gong, X.Y.; Shao, J.; Zhou, G.; Wang, J.; Wang, M.; Xia, J.; Li, Y.; et al. Traits mediate drought effects on wood carbon fluxes. Glob. Chang. Biol. 2020, 26, 3429–3442. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Krüger, D.; Buscot, F. Molecular evidence strongly supports deadwood-inhabiting fungi exhibiting unexpected tree species preferences in temperate forests. ISME J. 2018, 12, 289–295. [Google Scholar] [CrossRef]
- van der Wal, A.; Klein Gunnewiek, P.J.A.; Cornelissen, J.H.C.; Crowther, T.W.; de Boer, W. Patterns of natural fungal community assembly during initial decay of coniferous and broadleaf tree logs. Ecosphere 2016, 7, e01393. [Google Scholar] [CrossRef]
- Mueller, K.E.; Eissenstat, D.M.; Hobbie, S.E.; Oleksyn, J.; Jagodzinski, A.M.; Reich, P.B.; Chadwick, O.A.; Chorover, J. Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 2012, 111, 601–614. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.; Olsson, B.A.; Olsson, M.; Johansson, U.; Kleja, D.B. Differences in soil properties in adjacent stands of Scots pine, Norway spruce and silver birch in SW Sweden. For. Ecol. Manag. 2011, 262, 522–530. [Google Scholar] [CrossRef]
- Cremer, M.; Prietzel, J. Soil acidity and exchangeable base cation stocks under pure and mixed stands of European beech, Douglas fir and Norway spruce. Plant Soil 2017, 415, 393–405. [Google Scholar] [CrossRef]
- Cremer, M.; Kern, N.V.; Prietzel, J. Soil organic carbon and nitrogen stocks under pure and mixed stands of European beech, Douglas fir and Norway spruce. For. Ecol. Manag. 2016, 367, 30–40. [Google Scholar] [CrossRef]
- Shiau, Y.-J.; Chang, E.-H.; Tian, G.; Chen, T.-H.; Chiu, C.-Y. Improvements in soil C and N compositions after 40 and 80 years of reforestation in subtropical low mountain forests. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005598. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Piaszczyk, W. Dissolved carbon and nitrogen release from deadwood of different tree species in various stages of decomposition. Soil Sci. Plant Nutr. 2019, 65, 100–107. [Google Scholar] [CrossRef]
- Piaszczyk, W.; Błońska, E.; Lasota, J.; Lukac, M. A comparison of C:N:P stoichiometry in soil and deadwood at an advanced decomposition stage. CATENA 2019, 179, 1–5. [Google Scholar] [CrossRef]
- Minnich, C.; Peršoh, D.; Poll, C.; Borken, W. Changes in chemical and microbial soil parameters following 8 years of deadwood decay: An experiment with logs of 13 tree species in 30 forests. Ecosystems 2021, 24, 955–967. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Shaw, A.N.; Cleveland, C.C. The effects of temperature on soil phosphorus availability and phosphatase enzyme activities: A cross-ecosystem study from the tropics to the Arctic. Biogeochemistry 2020, 151, 113–125. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Wallenstein, M.D. Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol. Biochem. 2012, 55, 85–92. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Zuccarini, P.; Sardans, J.; Asensio, L.; Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Chang. Biol. 2023, 29, 2067–2091. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B.G.; Hawkes, C.V. Historical precipitation predictably alters the shape and magnitude of microbial functional response to soil moisture. Glob. Chang. Biol. 2016, 22, 1957–1964. [Google Scholar] [CrossRef]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S.; Malik, A.; Jones, D.L.; Clark, I.M.; Hirsch, P.R.; et al. The pH optimum of soil exoenzymes adapt to long term changes in soil pH. Soil Biol. Biochem. 2019, 138, 107601. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Liu, M.; Gan, B.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen and phosphorus addition on soil extracellular enzyme activity and stoichiometry in Chinese Fir (Cunninghamia lanceolata) forests. Front. Plant Sci. 2022, 13, 834184. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, H.; Zhao, M.; Monaco, T.A.; Rong, Y.; Huang, D.; Song, Q.; Zhao, K.; Wang, D. Soil extracellular enzyme activities and the abundance of nitrogen-cycling functional genes responded more to N addition than P addition in an Inner Mongolian meadow steppe. Sci. Total Environ. 2021, 759, 143541. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Yokoyama, D.; Imai, N.; Kitayama, K. Effects of nitrogen and phosphorus fertilization on the activities of four different classes of fine-root and soil phosphatases in Bornean tropical rain forests. Plant Soil 2017, 416, 463–476. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, S.; Ni, X.; Ni, X.; Guo, Z.; Tan, X.; Zhong, M.; Abu Hanif, M.; Zhu, J.; Ji, C.; et al. Long-term phosphorus addition inhibits phosphorus transformations involved in soil arbuscular mycorrhizal fungi and acid phosphatase in two tropical rainforests. Geoderma 2022, 425, 116076. [Google Scholar] [CrossRef]
- Wang, C.; Mori, T.; Mao, Q.; Zhou, K.; Wang, Z.; Zhang, Y.; Mo, H.; Lu, X.; Mo, J. Long-term phosphorus addition downregulates microbial investments on enzyme productions in a mature tropical forest. J. Soils Sediments 2020, 20, 921–930. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Shu, C.; Mo, Q.; Wang, H.; Kong, F.; Zhang, Y.; Geoff Wang, G.; Liu, Y. The response of coarse woody debris decomposition and microbial community to nutrient additions in a subtropical forest. For. Ecol. Manag. 2020, 460, 117799. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Chang. Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef]
- Moorhead, D.; Rinkes, Z.; Sinsabaugh, R.; Weintraub, M. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Guo, X.; Peng, S.; Wang, Y.; Zhang, X.; Fang, L. Stoichiometric models of microbial metabolic limitation in soil systems. Glob. Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef]
- Teste, F.P.; Lambers, H.; Enowashu, E.E.; Laliberté, E.; Marhan, S.; Kandeler, E. Soil microbial communities are driven by the declining availability of cations and phosphorus during ecosystem retrogression. Soil Biol. Biochem. 2021, 163, 108430. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, Y.; Guo, L.; Yang, L.; Wang, B.; Wang, X.; Liu, W.; Su, Y.; Wu, J.; Liu, L. Foliar nutrient resorption stoichiometry and microbial phosphatase catalytic efficiency together alleviate the relative phosphorus limitation in forest ecosystems. New Phytol. 2023, 238, 1033–1044. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Wang, X.-H.; Kent, M.; Fang, X.-F. Evergreen broad-leaved forest in Eastern China: Its ecology and conservation and the importance of resprouting in forest restoration. For. Ecol. Manag. 2007, 245, 76–87. [Google Scholar] [CrossRef]
- IUSS-Working-Group-WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Gao, Q.; Hasselquist, N.J.; Palmroth, S.; Zheng, Z.; You, W. Short-term response of soil respiration to nitrogen fertilization in a subtropical evergreen forest. Soil Biol. Biochem. 2014, 76, 297–300. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing; 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, 1.3.5; 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 6 September 2023).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, 2.6.4; 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 6 September 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; 3.4.1; Springer: New York, NY, USA, 2016. [Google Scholar]
- Peršoh, D.; Borken, W. Impact of woody debris of different tree species on the microbial activity and community of an underlying organic horizon. Soil Biol. Biochem. 2017, 115, 516–525. [Google Scholar] [CrossRef]
- Hu, Z.; Michaletz, S.T.; Johnson, D.J.; McDowell, N.G.; Huang, Z.; Zhou, X.; Xu, C. Traits drive global wood decomposition rates more than climate. Glob. Chang. Biol. 2018, 24, 5259–5269. [Google Scholar] [CrossRef]
- Kahl, T.; Arnstadt, T.; Baber, K.; Bässler, C.; Bauhus, J.; Borken, W.; Buscot, F.; Floren, A.; Heibl, C.; Hessenmöller, D.; et al. Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities. For. Ecol. Manag. 2017, 391, 86–95. [Google Scholar] [CrossRef]
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Mori, T. Does ecoenzymatic stoichiometry really determine microbial nutrient limitations? Soil Biol. Biochem. 2020, 146, 107816. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R.; Kitayama, K.; Mo, J. Does the ratio of β-1,4-glucosidase to β-1,4-N-acetylglucosaminidase indicate the relative resource allocation of soil microbes to C and N acquisition? Soil Biol. Biochem. 2021, 160, 108363. [Google Scholar] [CrossRef]
- Mori, T.; Rosinger, C.; Margenot, A.J. Enzymatic C:N:P stoichiometry: Questionable assumptions and inconsistencies to infer soil microbial nutrient limitation. Geoderma 2023, 429, 116242. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef]
- Kunito, T.; Isomura, I.; Sumi, H.; Park, H.-D.; Toda, H.; Otsuka, S.; Nagaoka, K.; Saeki, K.; Senoo, K. Aluminum and acidity suppress microbial activity and biomass in acidic forest soils. Soil Biol. Biochem. 2016, 97, 23–30. [Google Scholar] [CrossRef]
- Min, K.; Lehmeier, C.A.; Ballantyne, F.; Tatarko, A.; Billings, S.A. Differential effects of pH on temperature sensitivity of organic carbon and nitrogen decay. Soil Biol. Biochem. 2014, 76, 193–200. [Google Scholar] [CrossRef]
| Nutrient Content (g kg−1) | Tree Type | p Value | |
|---|---|---|---|
| Angiosperm | Gymnosperm | ||
| Wood C | 327 (28) b | 403 (17) a | 0.033 |
| Wood N | 3.6 (0.2) a | 2.3 (0.2) b | <0.001 |
| Wood P | 0.35 (0.05) a | 0.16 (0.01) b | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.-Y.; Xu, Q.; Zhao, Z.-Q.; Zheng, Y.-X.; Deng, L.; Hu, Z.-H. Influences of Wood Decomposition Associated with Tree Types on Soil Nutrient Concentrations and Enzyme Activities. Forests 2023, 14, 1846. https://doi.org/10.3390/f14091846
Ji X-Y, Xu Q, Zhao Z-Q, Zheng Y-X, Deng L, Hu Z-H. Influences of Wood Decomposition Associated with Tree Types on Soil Nutrient Concentrations and Enzyme Activities. Forests. 2023; 14(9):1846. https://doi.org/10.3390/f14091846
Chicago/Turabian StyleJi, Xiang-Yu, Qian Xu, Zhu-Qi Zhao, Yu-Xiong Zheng, Lei Deng, and Zhen-Hong Hu. 2023. "Influences of Wood Decomposition Associated with Tree Types on Soil Nutrient Concentrations and Enzyme Activities" Forests 14, no. 9: 1846. https://doi.org/10.3390/f14091846
APA StyleJi, X.-Y., Xu, Q., Zhao, Z.-Q., Zheng, Y.-X., Deng, L., & Hu, Z.-H. (2023). Influences of Wood Decomposition Associated with Tree Types on Soil Nutrient Concentrations and Enzyme Activities. Forests, 14(9), 1846. https://doi.org/10.3390/f14091846








