The Range Potential of North American Tree Species in Europe
Abstract
:1. Introduction
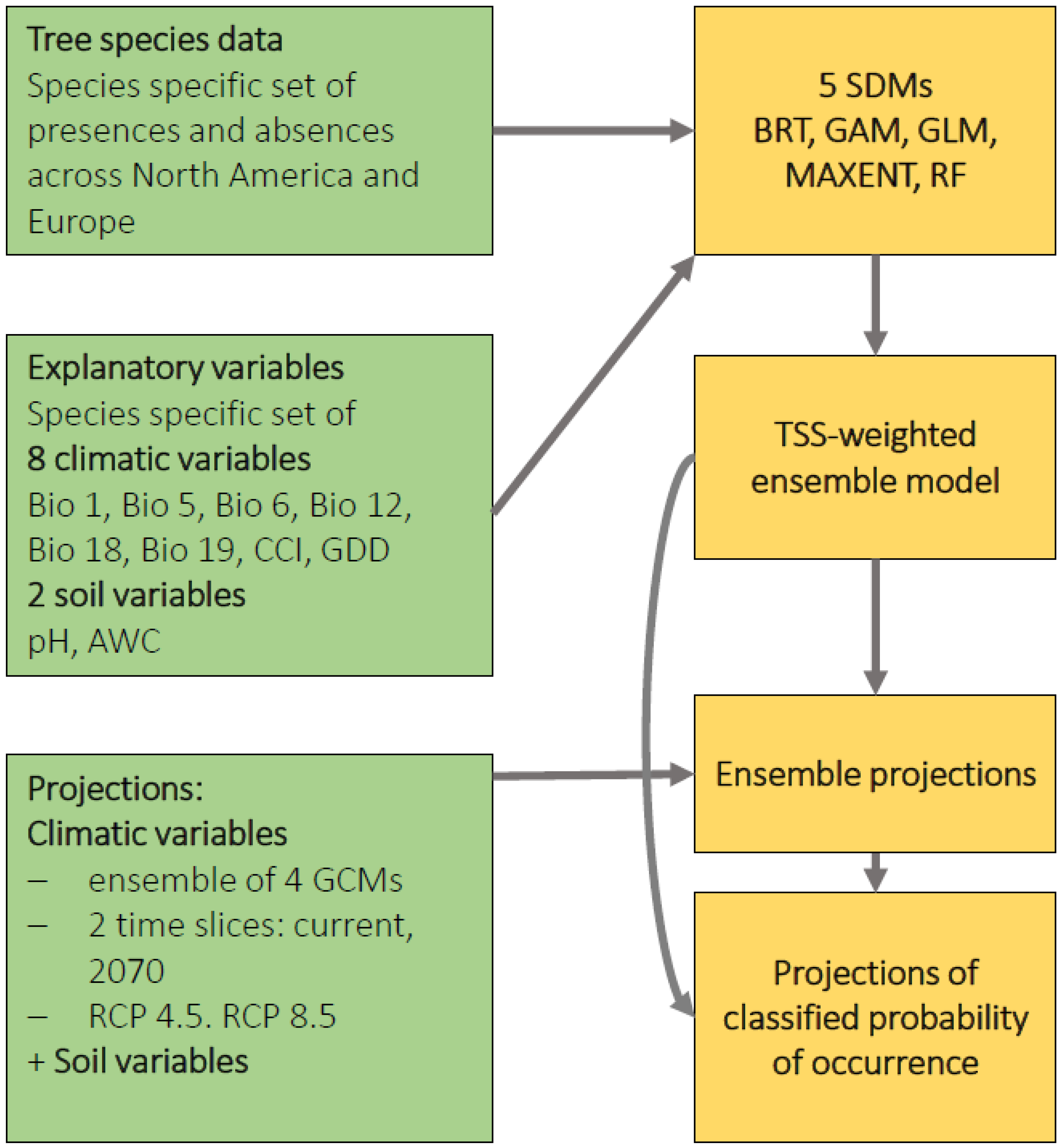
2. Materials and Methods
2.1. Climate Data
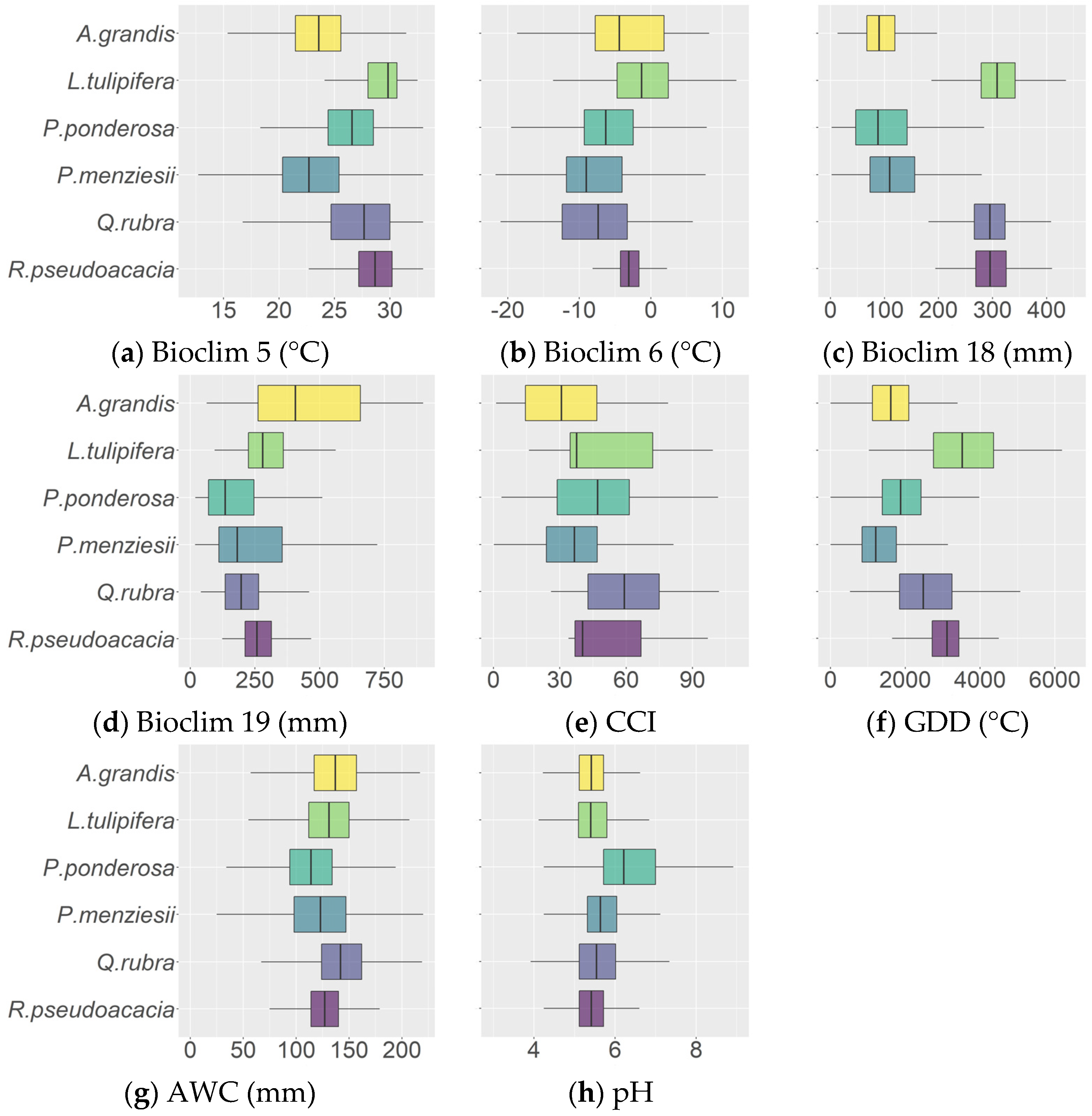
2.2. Tree Species Data
2.3. Soil Data
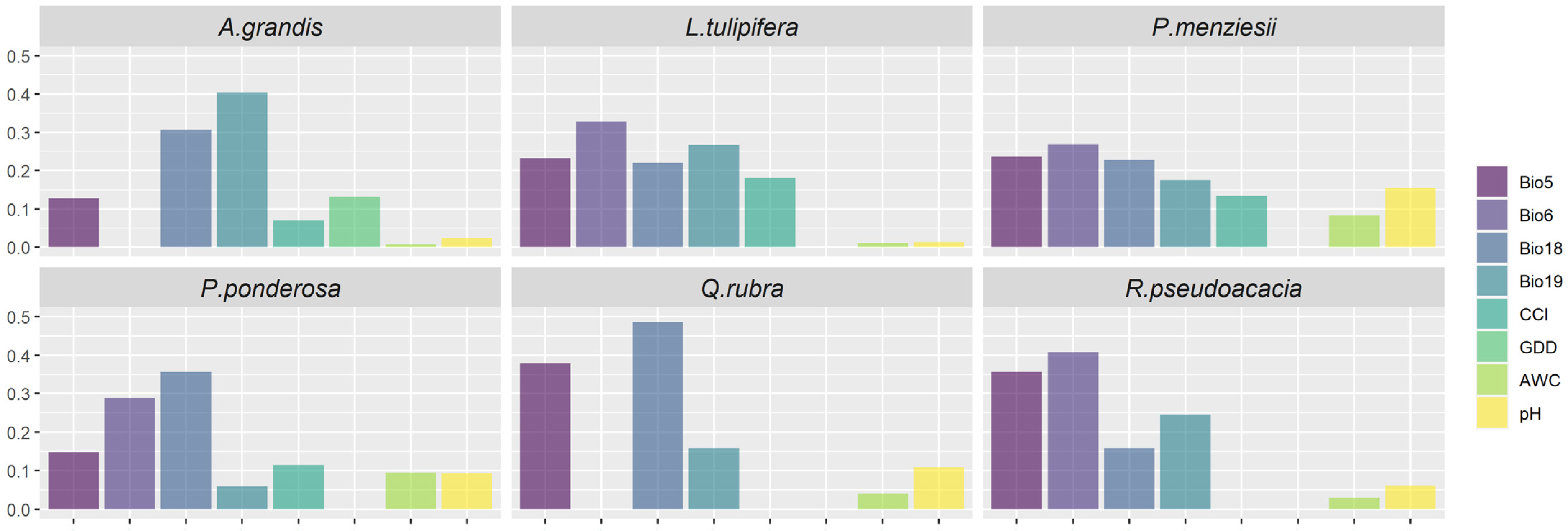
2.4. Explanatory Variables
2.5. Statistical Modeling
2.5.1. Niche Transferability
2.5.2. Species Distribution Models
3. Results
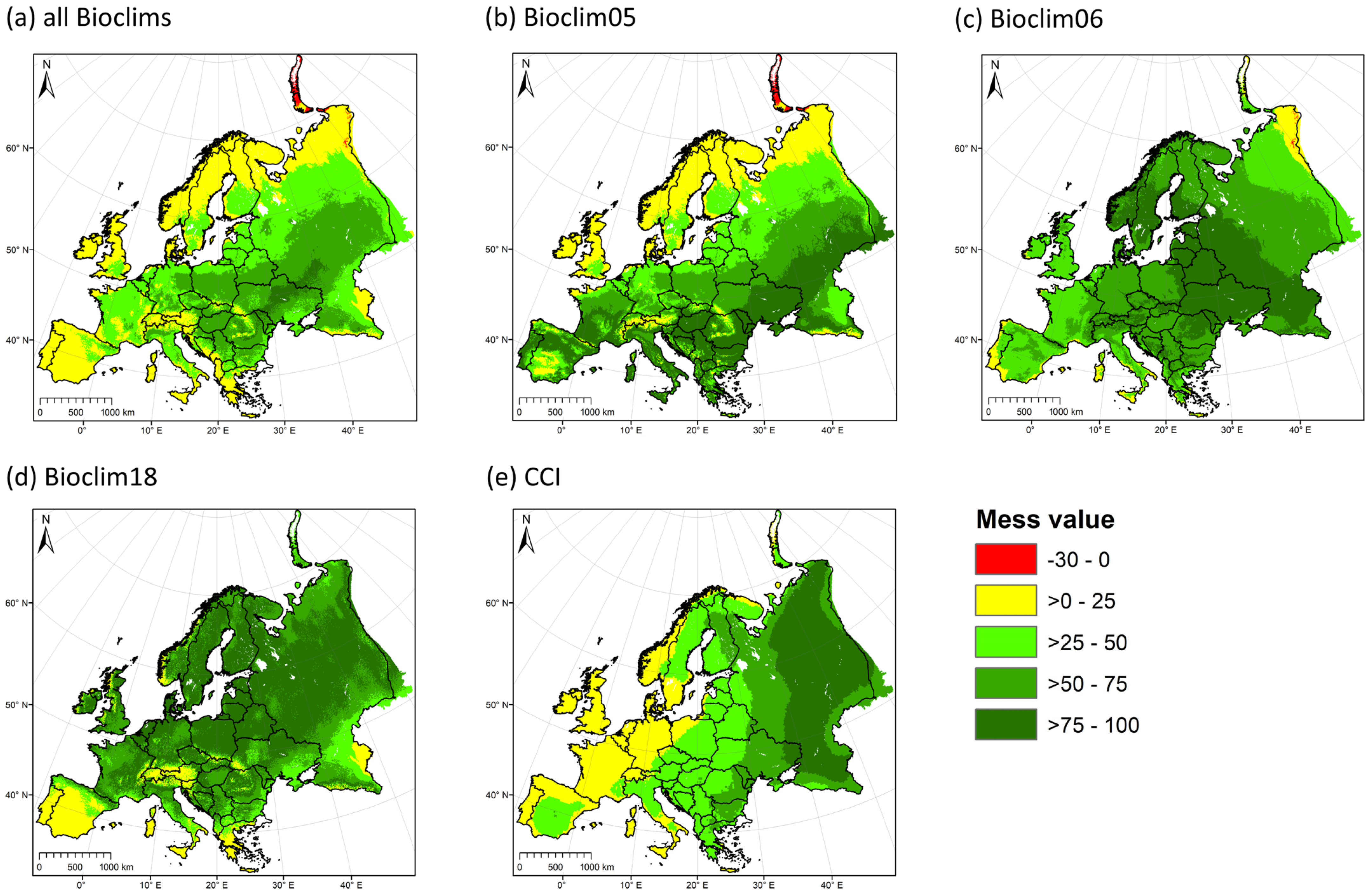
3.1. High Climatic Similarity between the Native and Introduced Ranges
3.2. Ensemble Model Performance
3.3. Model Uncertainty
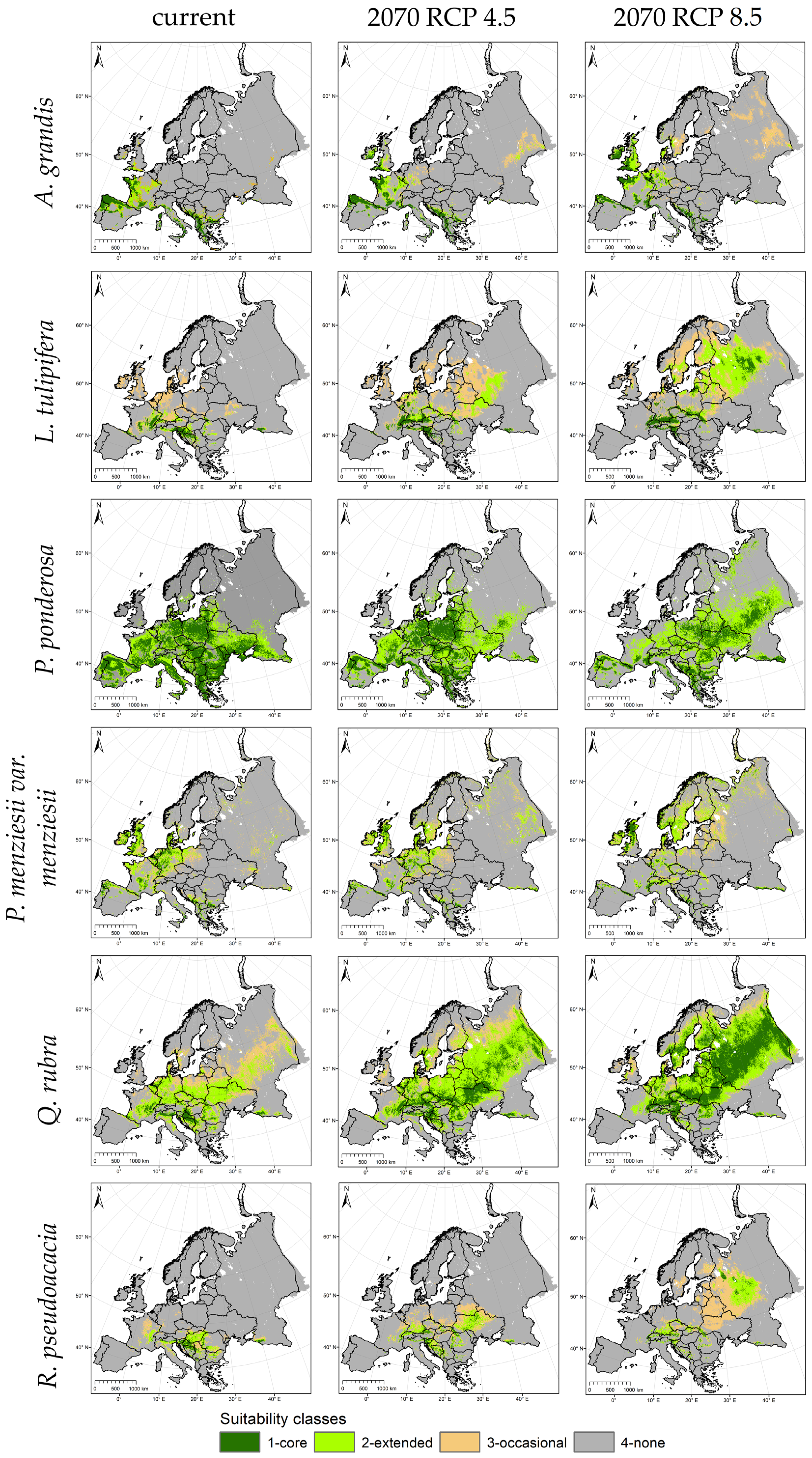
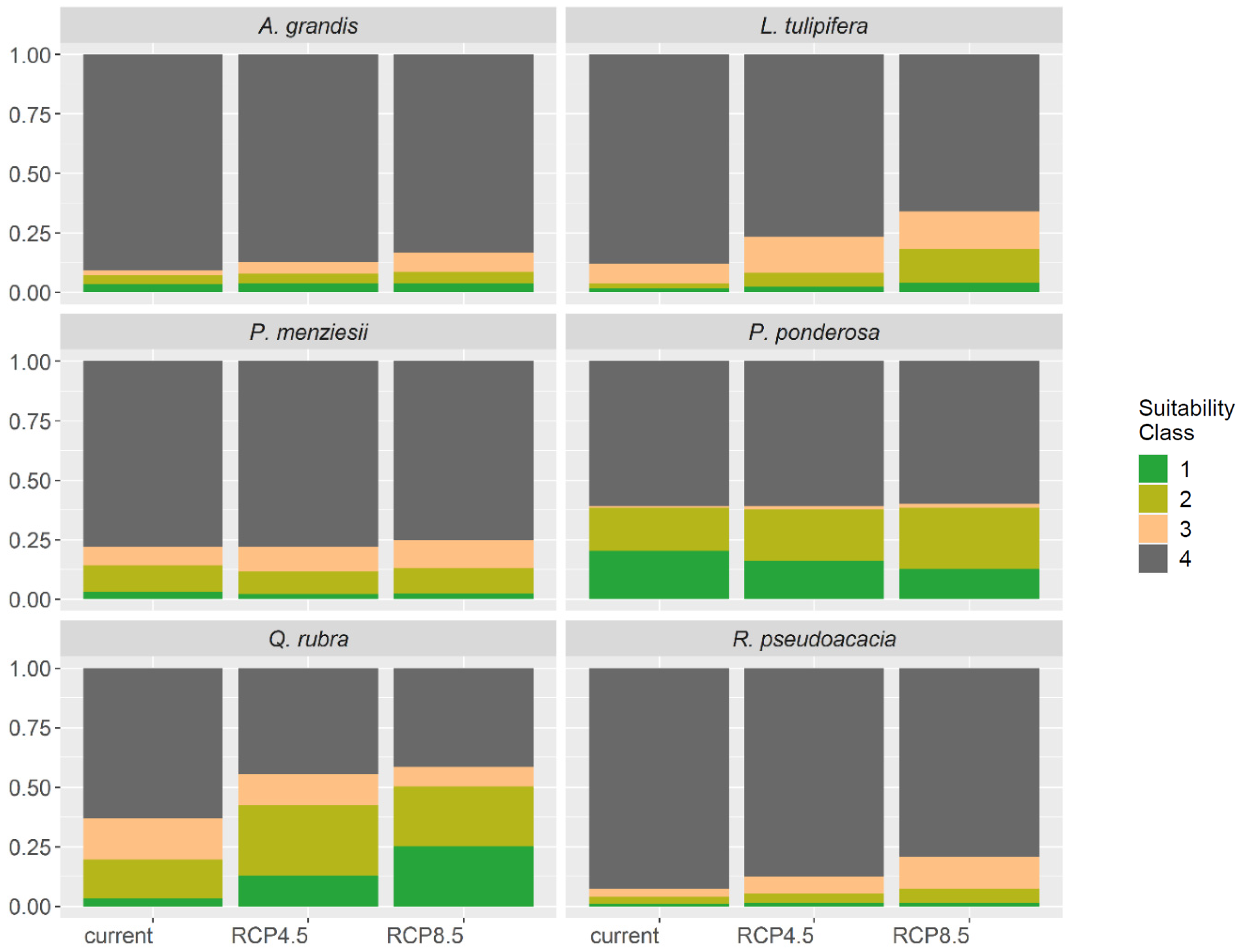
3.4. Range Shifts and Predicting Future Species Distribution in Europe
| Suitability (%) | Area Gain and Loss (%) | ||||
|---|---|---|---|---|---|
| Species | Current | 2070 RCP 4.5 | 2070 RCP 8.5 | 2070 RCP 4.5 | 2070 RCP 8.5 |
| A. grandis | 7.0 | 7.8 | 8.6 | 11.6 | 22.7 |
| L. tulipifera | 4.0 | 8.2 | 18.3 | 107.9 | 362.1 |
| P. menziesii var. menziesii | 14.3 | 11.5 | 13.1 | −19.8 | −8.6 |
| P. ponderosa | 38.3 | 37.7 | 38.4 | −1.6 | 0.3 |
| Q. rubra | 19.6 | 42.6 | 50.3 | 217.5 | 257.0 |
| R. pseudoacacia | 3.8 | 5.5 | 7.1 | 47.7 | 89.6 |
| Mean | 14.5 | 18.9 | 22.6 | 60.6 | 120.5 |
4. Discussion
4.1. Hypotheses
4.1.1. Limitations in Niche Transferability (H1) and Ensemble Model Performance
4.1.2. North and East Range Shifts of All North American Tree Species (H2)
4.1.3. Changes of the Distribution Potential (H3)
4.1.4. Species with Potential Area Gains (H4)
4.2. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.; Hauck, M.; Hajek, P. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; del Río, M.; Arcangeli, C.; Bielak, K.; Dudzinska, M.; Forrester, D.I.; Klädtke, J.; Kohnle, U.; Ledermann, T.; Matthews, R.; et al. Forest growth in Europe shows diverging large regional trends. Sci. Rep. 2023, 13, 15373. [Google Scholar] [CrossRef] [PubMed]
- Salomón, R.L.; Peters, R.L.; Zweifel, R.; Sass-Klaassen, U.G.; Stegehuis, A.I.; Smiljanic, M.; Poyatos, R.; Babst, F.; Cienciala, E.; Fonti, P. The 2018 European heatwave led to stem dehydration but not to consistent growth reductions in forests. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Ruiz-Labourdette, D.; Schmitz, M.F.; Pineda, F.D. Changes in tree species composition in Mediterranean mountains under climate change: Indicators for conservation planning. Ecol. Indic. 2013, 24, 310–323. [Google Scholar] [CrossRef]
- De Avila, A.; Häring, B.; Rheinbay, B.; Brüchert, F.; Hirsch, M.; Albrecht, A. Artensteckbriefe 2.0—Alternative Baumarten im Klimawandel; Self-published; Forest Research Institute Baden-Wuerttemberg: Freiburg, Germany, 2021; 246p. [Google Scholar]
- Araujo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Pearson, R.G.; Thuiller, W.; Araújo, M.B.; Martinez-Meyer, E.; Brotons, L.; McClean, C.; Miles, L.; Segurado, P.; Dawson, T.P.; Lees, D.C. Model-based uncertainty in species range prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Early, R.; Sax, D.F. Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob. Ecol. Biogeogr. 2014, 23, 1356–1365. [Google Scholar] [CrossRef]
- Wiens, J.J.; Ackerly, D.D.; Allen, A.P.; Anacker, B.L.; Buckley, L.B.; Cornell, H.V.; Damschen, E.I.; Jonathan Davies, T.; Grytnes, J.A.; Harrison, S.P. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010, 13, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Sax, D.F.; Early, R.; Bellemare, J. Niche syndromes, species extinction risks, and management under climate change. Trends Ecol. Evol. 2013, 28, 517–523. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Edwards, T.C., Jr.; Graham, C.H.; Pearman, P.B.; Svenning, J.C. New trends in species distribution modelling. Ecography 2010, 33, 985–989. [Google Scholar] [CrossRef]
- Puchałka, R.; Paź-Dyderska, S.; Jagodziński, A.M.; Sádlo, J.; Vítková, M.; Klisz, M.; Koniakin, S.; Prokopuk, Y.; Netsvetov, M.; Nicolescu, V.-N.; et al. Predicted range shifts of alien tree species in Europe. Agric. For. Meteorol. 2023, 341, 109650. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Zurell, D.; Elith, J.; Schröder, B. Predicting to new environments: Tools for visualizing model behaviour and impacts on mapped distributions. Divers. Distrib. 2012, 18, 628–634. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and uncertainties of species’ range shifts under climate change. Glob. Chang. Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Bohner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Conrad, V. Usual formulas of continentality and their limits of validity. Eos Trans. Am. Geophys. Union 1946, 27, 663–664. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014.
- Pletterbauer, F.; Graf, W.; Schmutz, S. Effect of biotic dependencies in species distribution models: The future distribution of Thymallus thymallus under consideration of Allogamus auricollis. Ecol. Model. 2016, 327, 95–104. [Google Scholar] [CrossRef]
- Kaini, S.; Nepal, S.; Pradhananga, S.; Gardner, T.; Sharma, A.K. Representative general circulation models selection and downscaling of climate data for the transboundary Koshi river basin in China and Nepal. Int. J. Climatol. 2020, 40, 4131–4149. [Google Scholar] [CrossRef]
- Fajardo, J.; Corcoran, D.; Roehrdanz, P.R.; Hannah, L.; Marquet, P.A. GCM compareR: A web application to assess differences and assist in the selection of general circulation models for climate change research. Methods Ecol. Evol. 2020, 11, 656–663. [Google Scholar] [CrossRef]
- Giorgetta, M.A.; Jungclaus, J.; Reick, C.H.; Legutke, S.; Bader, J.; Böttinger, M.; Brovkin, V.; Crueger, T.; Esch, M.; Fieg, K. Climate and carbon cycle changes from 1850 to 2100 in MPI-ESM simulations for the Coupled Model Intercomparison Project phase 5. J. Adv. Model. Earth Syst. 2013, 5, 572–597. [Google Scholar] [CrossRef]
- Schmidt, G.A.; Kelley, M.; Nazarenko, L.; Ruedy, R.; Russell, G.L.; Aleinov, I.; Bauer, M.; Bauer, S.E.; Bhat, M.K.; Bleck, R. Configuration and assessment of the GISS ModelE2 contributions to the CMIP5 archive. J. Adv. Model. Earth Syst. 2014, 6, 141–184. [Google Scholar] [CrossRef]
- Dufresne, J.-L.; Foujols, M.-A.; Denvil, S.; Caubel, A.; Marti, O.; Aumont, O.; Balkanski, Y.; Bekki, S.; Bellenger, H.; Benshila, R. Climate change projections using the IPSL-CM5 Earth System Model: From CMIP3 to CMIP5. Clim. Dyn. 2013, 40, 2123–2165. [Google Scholar] [CrossRef]
- Osprey, S.; Hardiman, S. HadGEM2-CC Model Output Prepared for CMIP5 RCP4.5, Served by ESGF; University of Oxford: Oxford, UK, 2014. [Google Scholar]
- Albrecht, A.T.; De Avila, A.L. Ein Vorschlag zur literaturbasierten Ermittlung möglicher Alternativbaumarten im Klimawandel am Beispiel der Artensteckbriefe in Baden-Württemberg. Allg. Forst Jagdztg. 2019, 189, 129–143. [Google Scholar]
- de Avila, A.L.; Albrecht, A. Alternative Baumarten im Klimawandel: Artensteckbriefe: Eine Stoffsammlung; Forstliche Versuchs-und Forschungsanstalt Baden-Württemberg (FVA): Freiburg, Germany, 2017. [Google Scholar]
- Spellmann, H.; Weller, A.; Brang, P.; Michiels, H.-G.; Bolte, A. Douglasie (Pseudotsuga menziesii (Mirb.) Franco); Vor, T., Spellmann, H., Bolt, A.U., Ammer, C., Eds.; Potenziale und Risiken eingeführter Baumarten; Universitätsverlag Göttingen: Göttingen, Germany, 2015; pp. 187–217. [Google Scholar]
- Koch, O.; de Avila, A.L.; Heinen, H.; Albrecht, A.T. Retreat of Major European Tree Species Distribution under Climate Change—Minor Natives to the Rescue? Sustainability 2022, 14, 5213. [Google Scholar]
- Thompson, R.S.; Anderson, K.H.; Pelltier, R.T.; Strickland, L.E.; Shafer, S.L.; Bartlein, P.J.; McFadden, A.K. Atlas of Relations between Climatic Parameters and Distributions of Important Trees and Shrubs in North America: Revisions for All Taxa from The United States and Canada and New Taxa from the Western United States; US Department of the Interior, US Geological Survey: Washington, DC, USA, 2015.
- Hengl, T.; Wheeler, I.; MacMillan, R.A. LandGIS: Global Soil and Vegetation Mapping Using Legacy Field Observations and State-of-the-Art Machine Learning; OpenGeoHub: Wageningen, The Netherlands, 2019. [Google Scholar]
- Little, E.L.; Viereck, L.A. Atlas of United States Trees: (no. 1146). Conifers and Important Hardwoods; EL, L., Jr., Ed.; US Government Printing Office: Washington, DC, USA, 1971; Volume 1.
- Mauri, A.; Strona, G.; San-Miguel-Ayanz, J. EU-Forest, a high-resolution tree occurrence dataset for Europe. Sci. Data 2017, 4, 160123. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Austin, M.P.; Van Niel, K.P. Improving Species Distribution Models for Climate Change Studies: Variable Selection and Scale; Wiley Online Library: New York, NY, USA, 2011. [Google Scholar]
- Mod, H.K.; Scherrer, D.; Luoto, M.; Guisan, A. What we use is not what we know: Environmental predictors in plant distribution models. J. Veg. Sci. 2016, 27, 1308–1322. [Google Scholar] [CrossRef]
- Walentowski, H.; Falk, W.; Mette, T.; Kunz, J.; Bräuning, A.; Meinardus, C.; Zang, C.; Sutcliffe, L.M.E.; Leuschner, C. Assessing future suitability of tree species under climate change by multiple methods: A case study in southern Germany. Ann. For. Res. 2017, 60, 101–126. [Google Scholar] [CrossRef]
- Mauri, A.; Girardello, M.; Strona, G.; Beck, P.S.; Forzieri, G.; Caudullo, G.; Manca, F.; Cescatti, A. EU-Trees4F, a dataset on the future distribution of European tree species. Sci. Data 2022, 9, 37. [Google Scholar] [CrossRef]
- Boiffin, J.; Badeau, V.; Bréda, N. Species distribution models may misdirect assisted migration: Insights from the introduction of Douglas-fir to Europe. Ecol. Appl. 2017, 27, 446–457. [Google Scholar] [CrossRef]
- Thurm, E.A. Mixed Stands of Douglas-Fir and European Beech Compared to Pure Stands; Technische Universität München: München, Germany, 2017. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 5 December 2023).
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R. biomod2: Ensemble Platform for Species Distribution Modeling, R Package Version 4.2-4. 2023. Available online: https://rdrr.io/cran/biomod2/(accessed on 5 December 2023).
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Hargrove, W.W. The projection of species distribution models and the problem of non-analog climate. Biodivers. Conserv. 2009, 18, 2255–2261. [Google Scholar] [CrossRef]
- Camenen, E.; Porté, A.J.; Benito Garzón, M. American trees shift their niches when invading Western Europe: Evaluating invasion risks in a changing climate. Ecol. Evol. 2016, 6, 7263–7275. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Gallien, L.; Douzet, R.; Pratte, S.; Zimmermann, N.E.; Thuiller, W. Invasive species distribution models–how violating the equilibrium assumption can create new insights. Glob. Ecol. Biogeogr. 2012, 21, 1126–1136. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.-A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Meier, E.S.; Edwards, T.C., Jr.; Kienast, F.; Dobbertin, M.; Zimmermann, N.E. Co-occurrence patterns of trees along macro-climatic gradients and their potential influence on the present and future distribution of Fagus sylvatica L. J. Biogeogr. 2011, 38, 371–382. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Michiels, H.G.; Kandler, G. Converting probabilistic tree species range shift projections into meaningful classes for management. J. Environ. Manag. 2014, 134, 153–165. [Google Scholar] [CrossRef]
- Lee, S.; Jung, H.; Choi, J. Projecting the Impact of Climate Change on the Spatial Distribution of Six Subalpine Tree Species in South Korea Using a Multi-Model Ensemble Approach. Forests 2021, 12, 37. [Google Scholar] [CrossRef]
- Veloz, S.D.; Williams, J.W.; Blois, J.L.; He, F.; Otto-Bliesner, B.; Liu, Z. No-analog climates and shifting realized niches during the late quaternary: Implications for 21st-century predictions by species distribution models. Glob. Chang. Biol. 2012, 18, 1698–1713. [Google Scholar] [CrossRef]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef]
- McKenney, D.W.; Pedlar, J.H.; Lawrence, K.; Campbell, K.; Hutchinson, M.F. Potential impacts of climate change on the distribution of North American trees. BioScience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Sittaro, F.; Paquette, A.; Messier, C.; Nock, C.A. Tree range expansion in eastern North America fails to keep pace with climate warming at northern range limits. Glob. Chang. Biol. 2017, 23, 3292–3301. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Móricz, N.; Rasztovits, E.; Dobor, L.; Schueler, S. Provisioning forest and conservation science with high-resolution maps of potential distribution of major European tree species under climate change. Ann. For. Sci. 2021, 78, 26. [Google Scholar] [CrossRef]
- Liang, Y.; Duveneck, M.J.; Gustafson, E.J.; Serra-Diaz, J.M.; Thompson, J.R. How disturbance, competition, and dispersal interact to prevent tree range boundaries from keeping pace with climate change. Glob. Chang. Biol. 2018, 24, e335–e351. [Google Scholar] [CrossRef] [PubMed]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Schüler, S.; Chakraborty, D. Limitierende Faktoren für den Douglasienanbau in Mitteleuropa im Klimawandel. Schweiz. Z. Forstwes. 2021, 172, 84–93. [Google Scholar] [CrossRef]
- Lockow, K.-W.; Lockow, J. Die Robinie (Robinia pseudoacacia L.): Eine Schnellwachsende Baumart Mit Wertvollen Holzeigenschaften; Gesellschaft zur Förderung Schnellwachsender Baumarten in Norddeutschland eV; cw Nordwest Media Verlagsgesellschaft: Grevesmühlen, Germany, 2013. [Google Scholar]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Mountain landscapes offer few opportunities for high-elevation tree species migration. Glob. Chang. Biol. 2014, 20, 1441–1451. [Google Scholar] [CrossRef]
- Peters, M.P. Assessing Ponderosa Pine (Pinus ponderosa) Suitable Habitat throughout Arizona in Response to Future Climate Models; Arizona State University: Maricopa, AZ, USA, 2011. [Google Scholar]
- Cao, Y.; Feng, J.; Hwarari, D.; Ahmad, B.; Wu, H.; Chen, J.; Yang, L. Alterations in Population Distribution of Liriodendron chinense (Hemsl.) Sarg. and Liriodendron tulipifera Linn. Caused by Climate Change. Forests 2022, 13, 488. [Google Scholar] [CrossRef]
- Thuiller, W.; Gueguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Iverson, L.R.; McKenzie, D. Tree-species range shifts in a changing climate: Detecting, modeling, assisting. Landsc. Ecol. 2013, 28, 879–889. [Google Scholar] [CrossRef]
- Liu, Q.; Piao, S.; Janssens, I.A.; Fu, Y.; Peng, S.; Lian, X.; Ciais, P.; Myneni, R.B.; Peñuelas, J.; Wang, T. Extension of the growing season increases vegetation exposure to frost. Nat. Commun. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Hellmann, J.J.; McLachlan, J.M.; Sax, D.F.; Borevitz, J.O.; Brennan, J.; Camacho, A.E.; Ceballos, G.; Clark, J.R.; Doremus, H. Managed relocation: Integrating the scientific, regulatory, and ethical challenges. BioScience 2012, 62, 732–743. [Google Scholar] [CrossRef]
- Feurdean, A.; Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Lischke, H.; Hickler, T. Tree Migration-Rates: Narrowing the Gap between Inferred Post-Glacial Rates and Projected Rates. PLoS ONE 2013, 8, e71797. [Google Scholar] [CrossRef]
- Pretzsch, H.; Poschenrieder, W.; Uhl, E.; Brazaitis, G.; Makrickiene, E.; Calama, R. Silvicultural prescriptions for mixed-species forest stands. A European review and perspective. Eur. J. For. Res. 2021, 140, 1267–1294. [Google Scholar] [CrossRef]
- Sow, M.D.; Allona, I.; Ambroise, C.; Conde, D.; Fichot, R.; Gribkova, S.; Jorge, V.; Le-Provost, G.; Pâques, L.; Plomion, C.; et al. Chapter Twelve—Epigenetics in Forest Trees: State of the Art and Potential Implications for Breeding and Management in a Context of Climate Change. In Advances in Botanical Research; Mirouze, M., Bucher, E., Gallusci, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 88, pp. 387–453. [Google Scholar]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Jactel, H.; Scherer-Lorenzen, M.; Garcia-Valdes, R.; Bugmann, H. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci. Rep. 2018, 8, 5627. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Seidel, D.; Annighöfer, P.; Kreft, H.; Köhler, M.; Zemp, D.C.; Puettmann, K.; Nilus, R.; Babweteera, F.; Willim, K.; et al. Global patterns and climatic controls of forest structural complexity. Nat. Commun. 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, L.S.; Panetta, F.D.; Gooden, B. Identifying thresholds in the impacts of an invasive groundcover on native vegetation. Sci. Rep. 2021, 11, 20512. [Google Scholar] [CrossRef] [PubMed]
- Kumar Rai, P.; Singh, J.S. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Jagodziński, A.M. Impact of invasive tree species on natural regeneration species composition, diversity, and density. Forests 2020, 11, 456. [Google Scholar] [CrossRef]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]


| Species | Presence NA [41] | Prevalence NA (%) | Presence EUR [44] | Prevalence EUR (%) |
|---|---|---|---|---|
| A. grandis | 25,013 | 2.5 | 430 | 0.2 |
| L. tulipifera | 142,252 | 14.4 | 1 | 0.0 |
| P. menziesii var. menziesii | 114,847 | 11.6 | 5223 | 2.1 |
| P. ponderosa | 70,189 | 7.1 | 4 | 0.0 |
| Q. rubra | 280,782 | 28.4 | 1725 | 0.7 |
| R. pseudoacacia | 40,219 | 4.1 | 4788 | 1.9 |
| Species | Model | SE 1 | SP 1 | TSS | AUC |
|---|---|---|---|---|---|
| A. grandis | BRT | 0.98 | 0.95 | 0.93 | 0.99 |
| A. grandis | GAM | 0.98 | 0.96 | 0.94 | 0.99 |
| A. grandis | GLM | 0.98 | 0.95 | 0.93 | 0.99 |
| A. grandis | MAXENT | 0.95 | 0.95 | 0.90 | 0.98 |
| A. grandis | RF | 0.98 | 0.97 | 0.95 | 1 |
| L. tulipifera | BRT | 0.99 | 0.97 | 0.96 | 1 |
| L. tulipifera | GAM | 0.99 | 0.98 | 0.97 | 1 |
| L. tulipifera | GLM | 0.99 | 0.96 | 0.95 | 0.99 |
| L. tulipifera | MAXENT | 0.97 | 0.95 | 0.92 | 0.98 |
| L. tulipifera | RF | 0.99 | 0.99 | 1 | 1 |
| P. ponderosa | BRT | 0.87 | 0.87 | 0.74 | 0.94 |
| P. ponderosa | GAM | 0.91 | 0.85 | 0.75 | 0.94 |
| P. ponderosa | GLM | 0.91 | 0.85 | 0.76 | 0.95 |
| P. ponderosa | MAXENT | 0.89 | 0.85 | 0.74 | 0.95 |
| P. ponderosa | RF | 0.94 | 0.94 | 0.99 | 1 |
| P. menziesii var. menziesii | BRT | 0.87 | 0.80 | 0.66 | 0.90 |
| P. menziesii var. menziesii | GAM | 0.85 | 0.80 | 0.63 | 0.89 |
| P. menziesii var. menziesii | GLM | 0.89 | 0.79 | 0.67 | 0.91 |
| P. menziesii var. menziesii | MAXENT | 0.84 | 0.79 | 0.63 | 0.90 |
| P. menziesii var. menziesii | RF | 0.85 | 0.91 | 0.98 | 1 |
| Q. rubra | BRT | 0.81 | 0.85 | 0.69 | 0.92 |
| Q. rubra | GAM | 0.85 | 0.83 | 0.68 | 0.92 |
| Q. rubra | GLM | 0.85 | 0.73 | 0.57 | 0.85 |
| Q. rubra | MAXENT | 0.79 | 0.83 | 0.64 | 0.91 |
| Q. rubra | RF | 0.85 | 0.87 | 0.98 | 1 |
| R. pseudoacacia | BRT | 0.94 | 0.91 | 0.86 | 0.97 |
| R. pseudoacacia | GAM | 0.99 | 0.79 | 0.79 | 0.89 |
| R. pseudoacacia | GLM | 0.87 | 0.86 | 0.75 | 0.94 |
| R. pseudoacacia | MAXENT | 0.91 | 0.92 | 0.83 | 0.96 |
| R. pseudoacacia | RF | 0.96 | 0.96 | 0.99 | 1 |
| Species | SE 1 | SP 1 | TSS | AUC |
|---|---|---|---|---|
| A. grandis | 0.98 | 0.96 | 0.96 | 1 |
| L. tulipifera | 0.99 | 0.98 | 0.98 | 1 |
| P. menziesii var. menziesii | 0.95 | 0.92 | 0.89 | 0.99 |
| P. ponderosa | 0.93 | 0.90 | 0.86 | 0.98 |
| Q. rubra | 0.88 | 0.83 | 0.79 | 0.97 |
| R. pseudoacacia | 0.97 | 0.93 | 0.93 | 1 |
| Mean | 0.95 | 0.92 | 0.90 | 0.99 |
| Bioclim 5 | Bioclim 6 | Bioclim 18 | Bioclim 19 | CCI | GDD | AWC | pH | |
|---|---|---|---|---|---|---|---|---|
| Mean | 0.25 | 0.32 | 0.29 | 0.17 | 0.12 | 0.13 * | 0.04 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, A.T.; Heinen, H.; Koch, O.; de Avila, A.L.; Hinze, J. The Range Potential of North American Tree Species in Europe. Forests 2024, 15, 130. https://doi.org/10.3390/f15010130
Albrecht AT, Heinen H, Koch O, de Avila AL, Hinze J. The Range Potential of North American Tree Species in Europe. Forests. 2024; 15(1):130. https://doi.org/10.3390/f15010130
Chicago/Turabian StyleAlbrecht, Axel Tim, Henry Heinen, Olef Koch, Angela Luciana de Avila, and Jonas Hinze. 2024. "The Range Potential of North American Tree Species in Europe" Forests 15, no. 1: 130. https://doi.org/10.3390/f15010130







