Effects of Different Altitudes on Castanopsis hystrix, the Top Community-Building Species in Southern Subtropical China: Rhizospheric Soil Chemical Properties and Soil Microbiota
Abstract
:1. Introduction
2. Materials and Methods
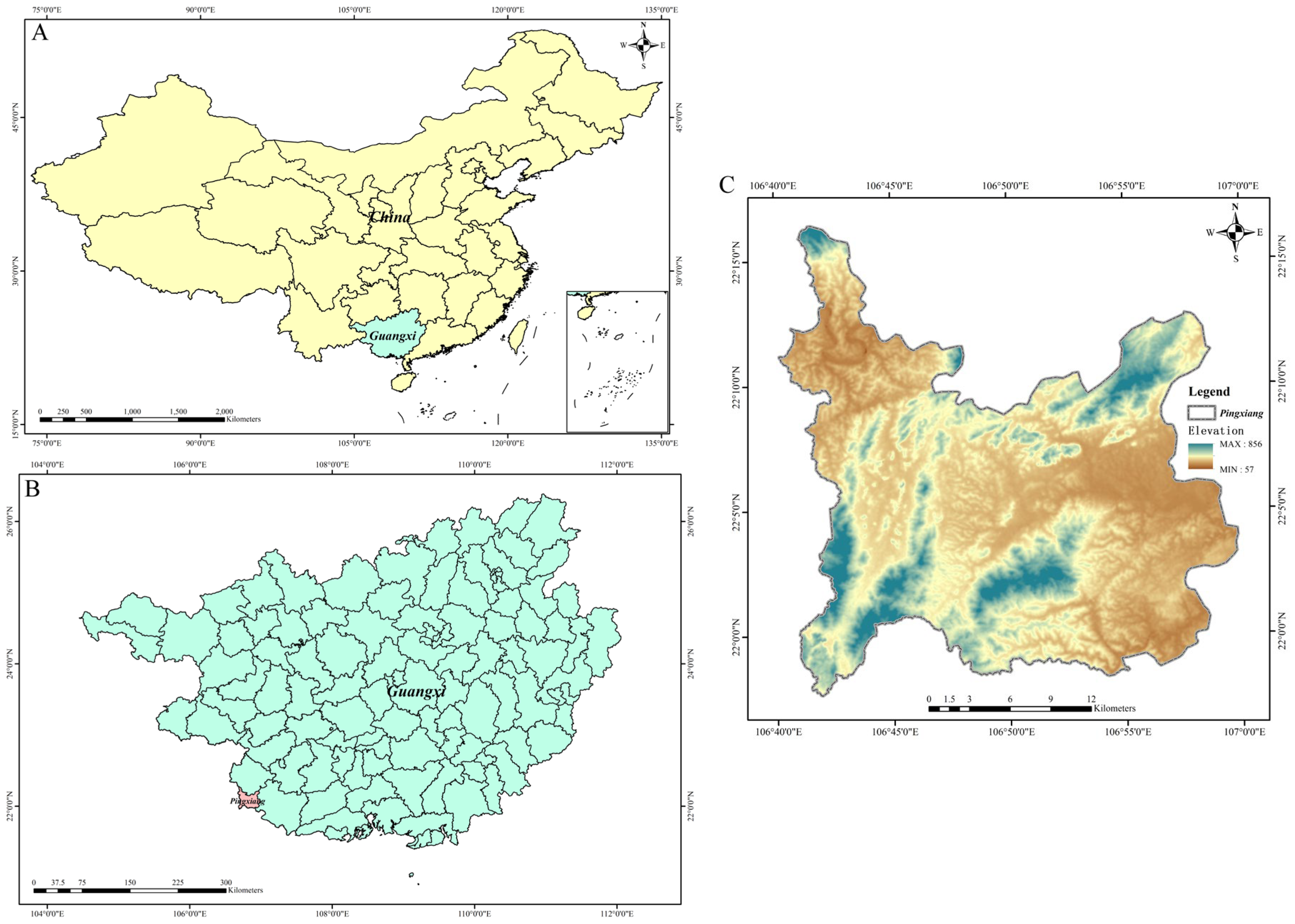
2.1. Overview of the Experimental Site and Experimental Materials
2.2. Sampling Method
2.3. Assessment of Soil Physical and Chemical Characteristics
2.4. Total DNA Extraction, PCR Amplification, and Sequencing of Soil Microorganisms
2.5. Data Analysis
3. Result and Analysis
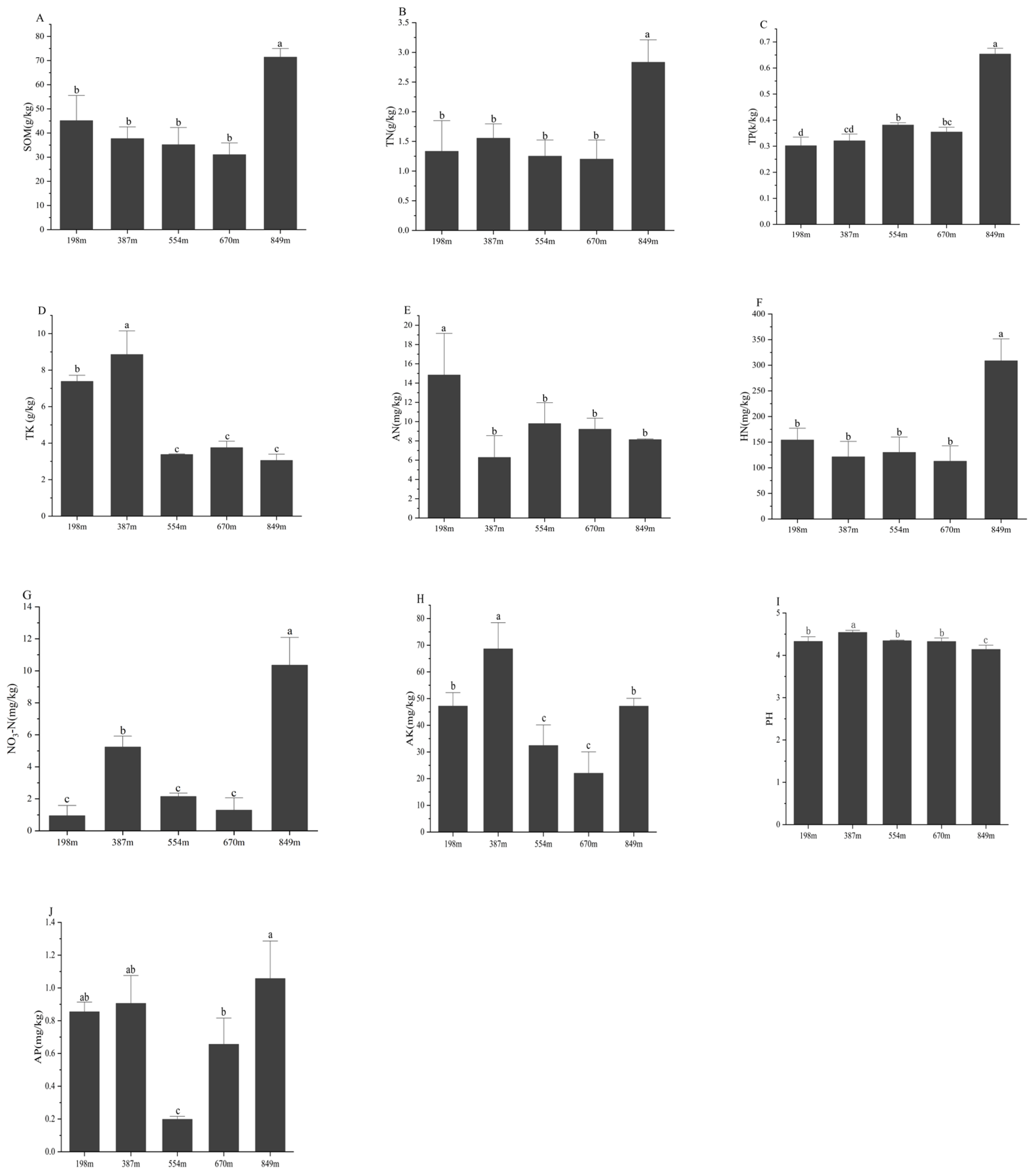
3.1. Analysis of Soil Physicochemical Properties
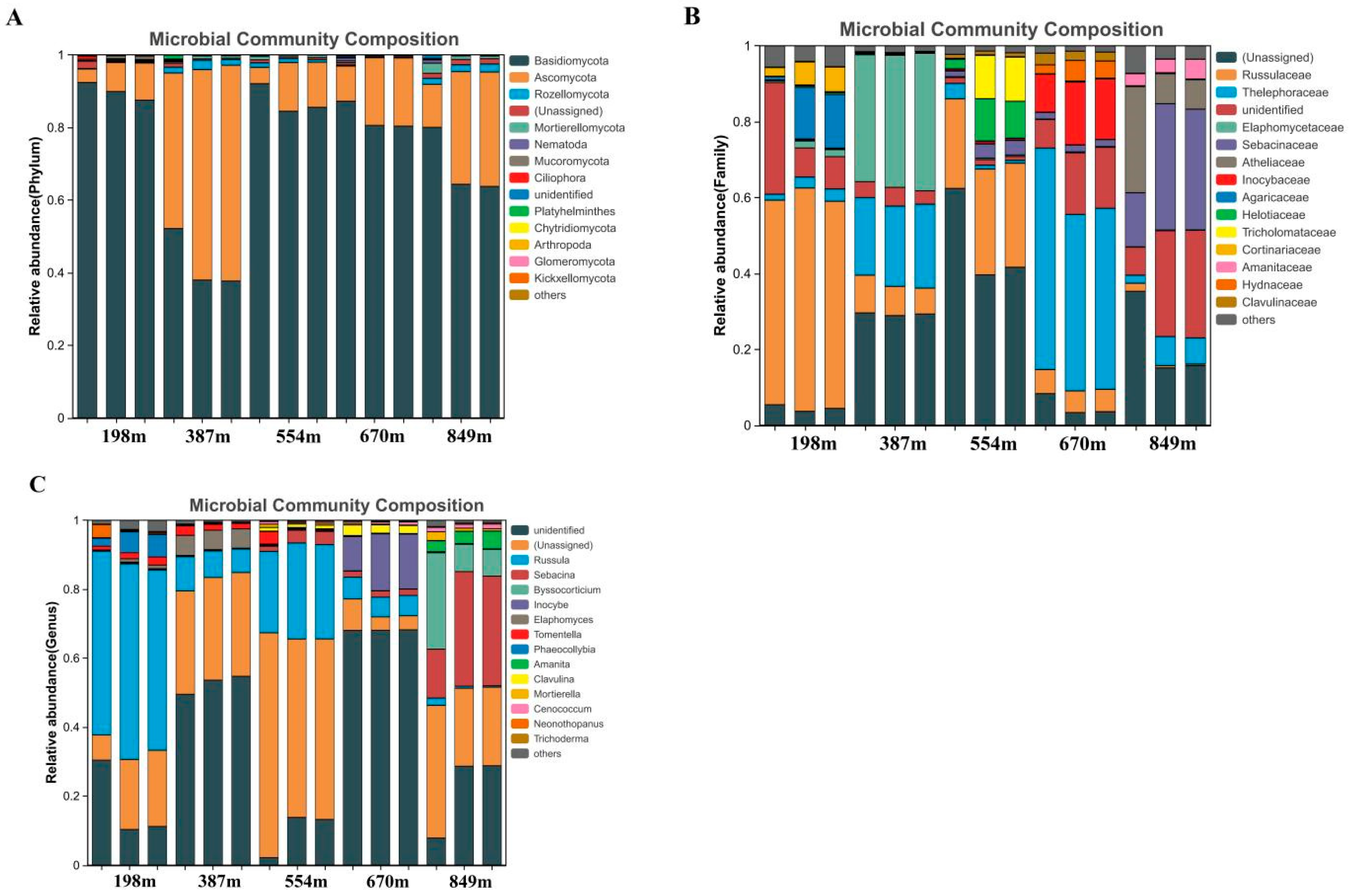
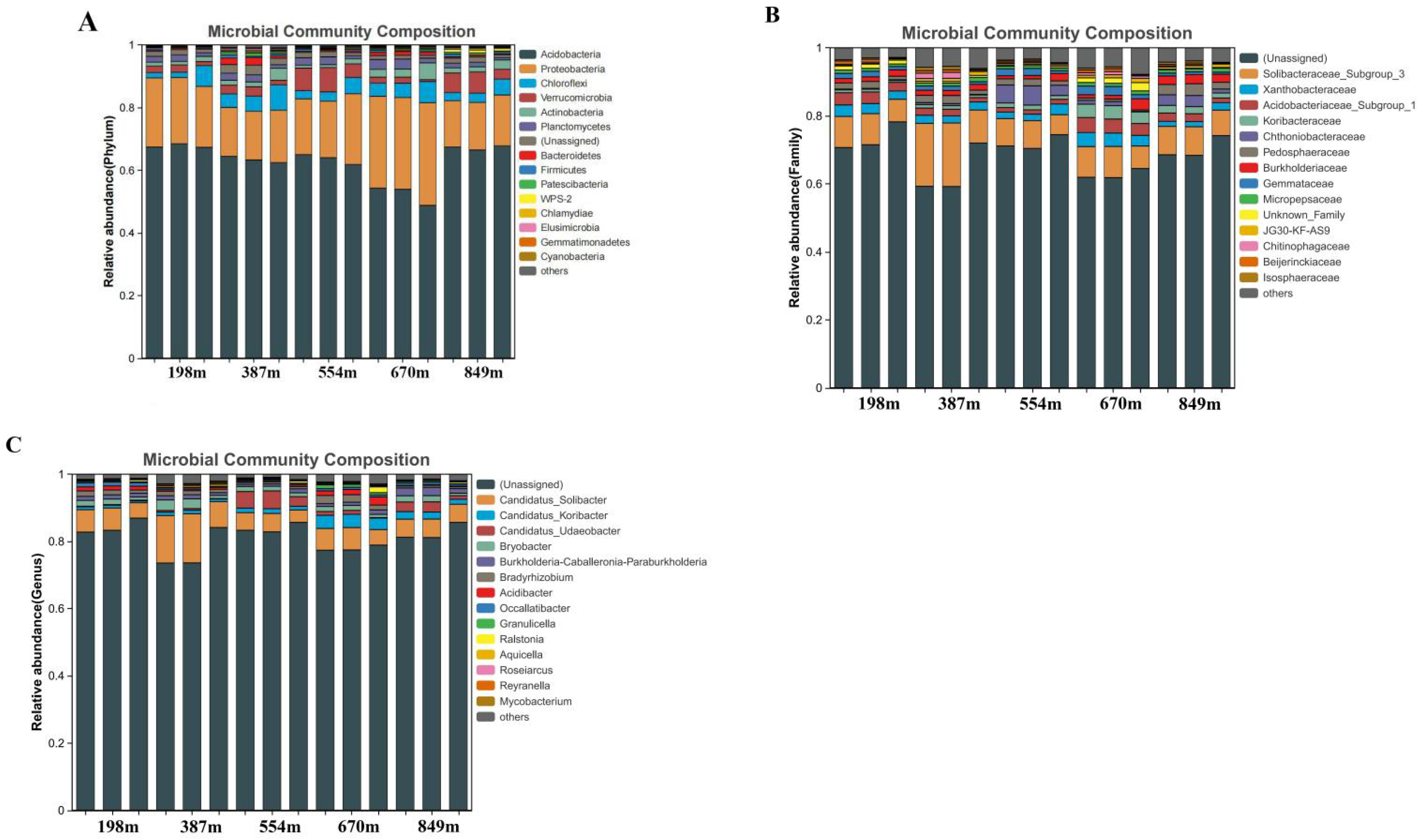
3.2. Microbial Community Characteristics
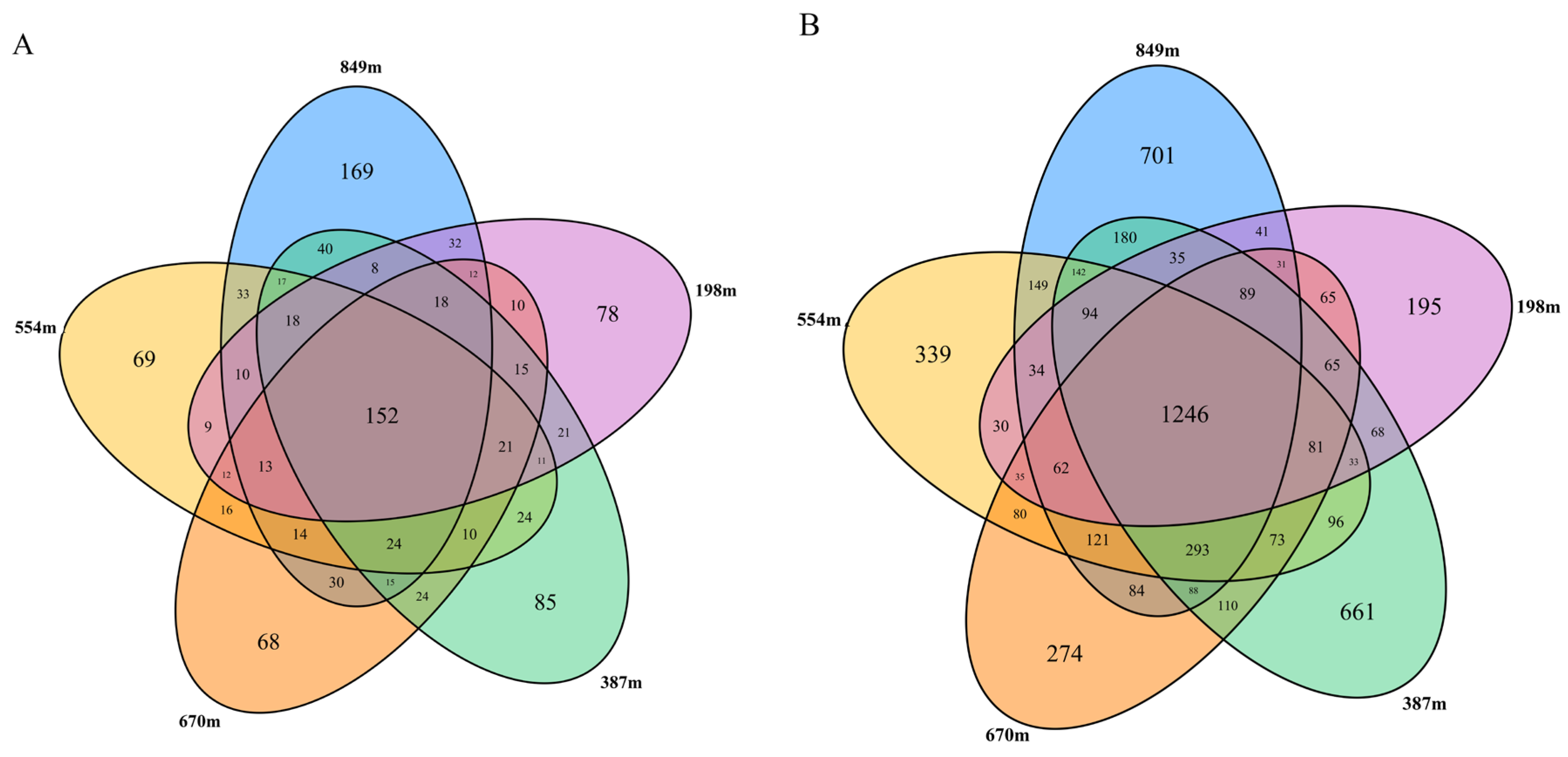
3.3. Microbial Biodiversity
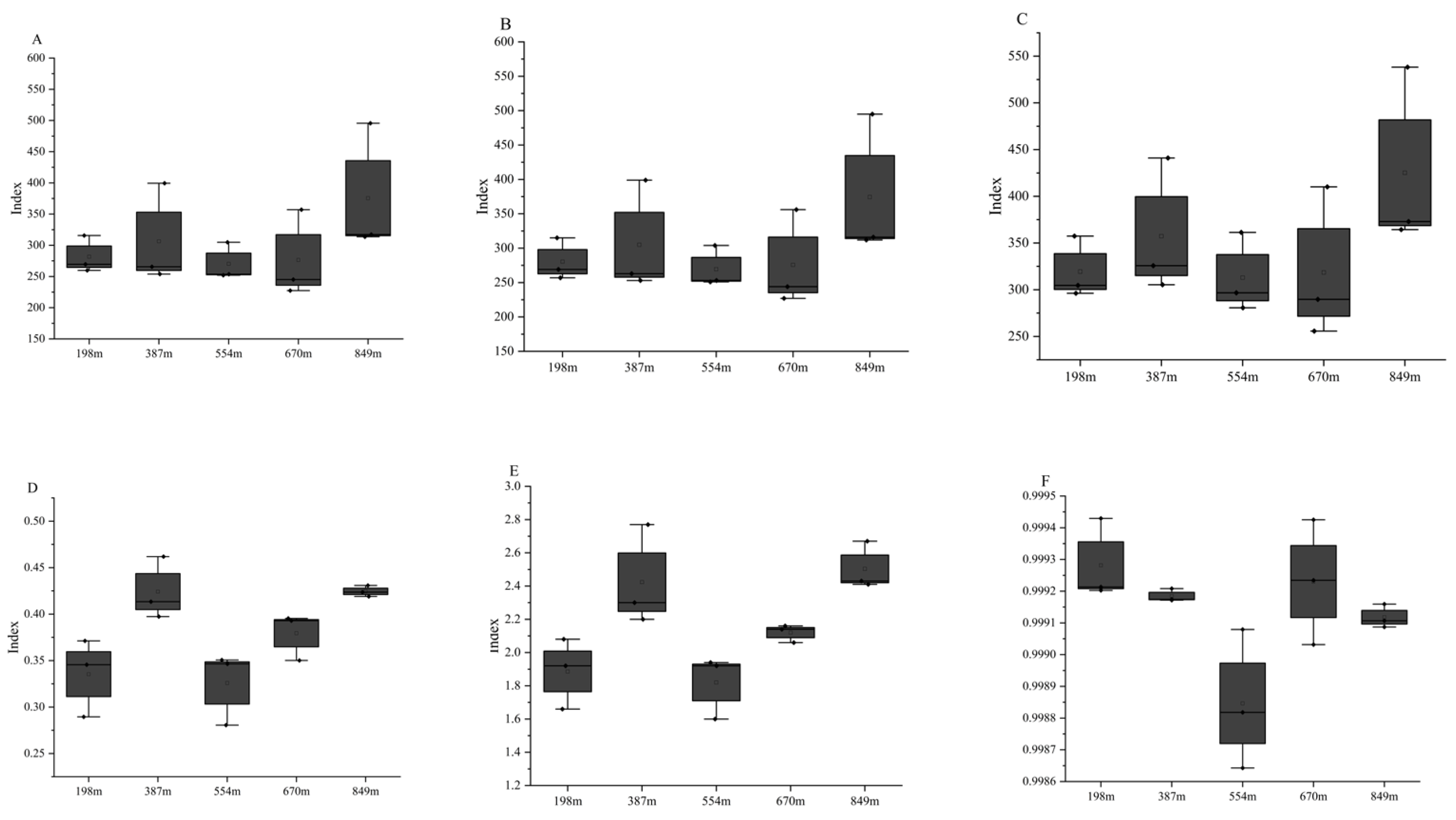
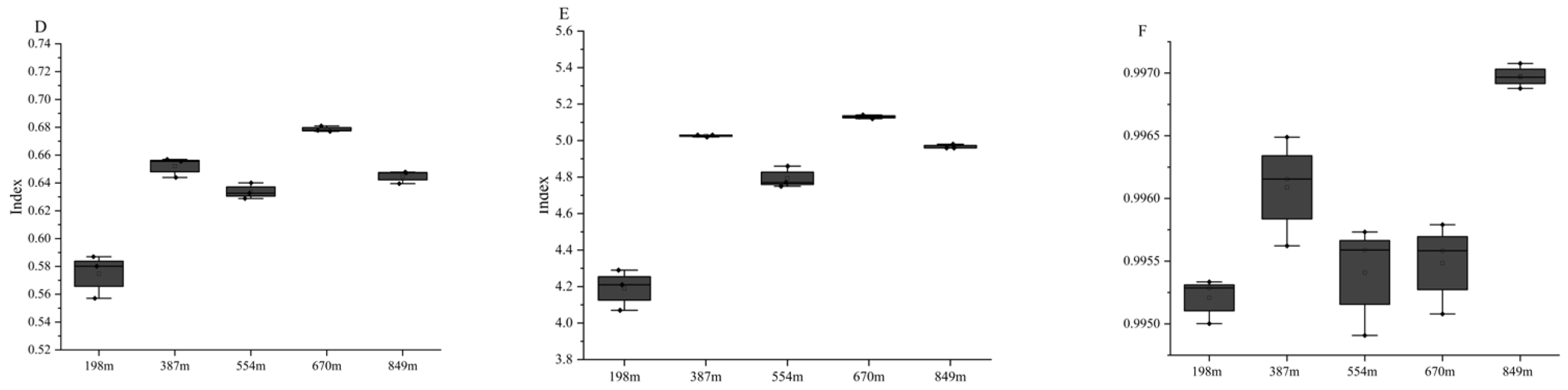
3.3.1. α Diversity Index Analysis
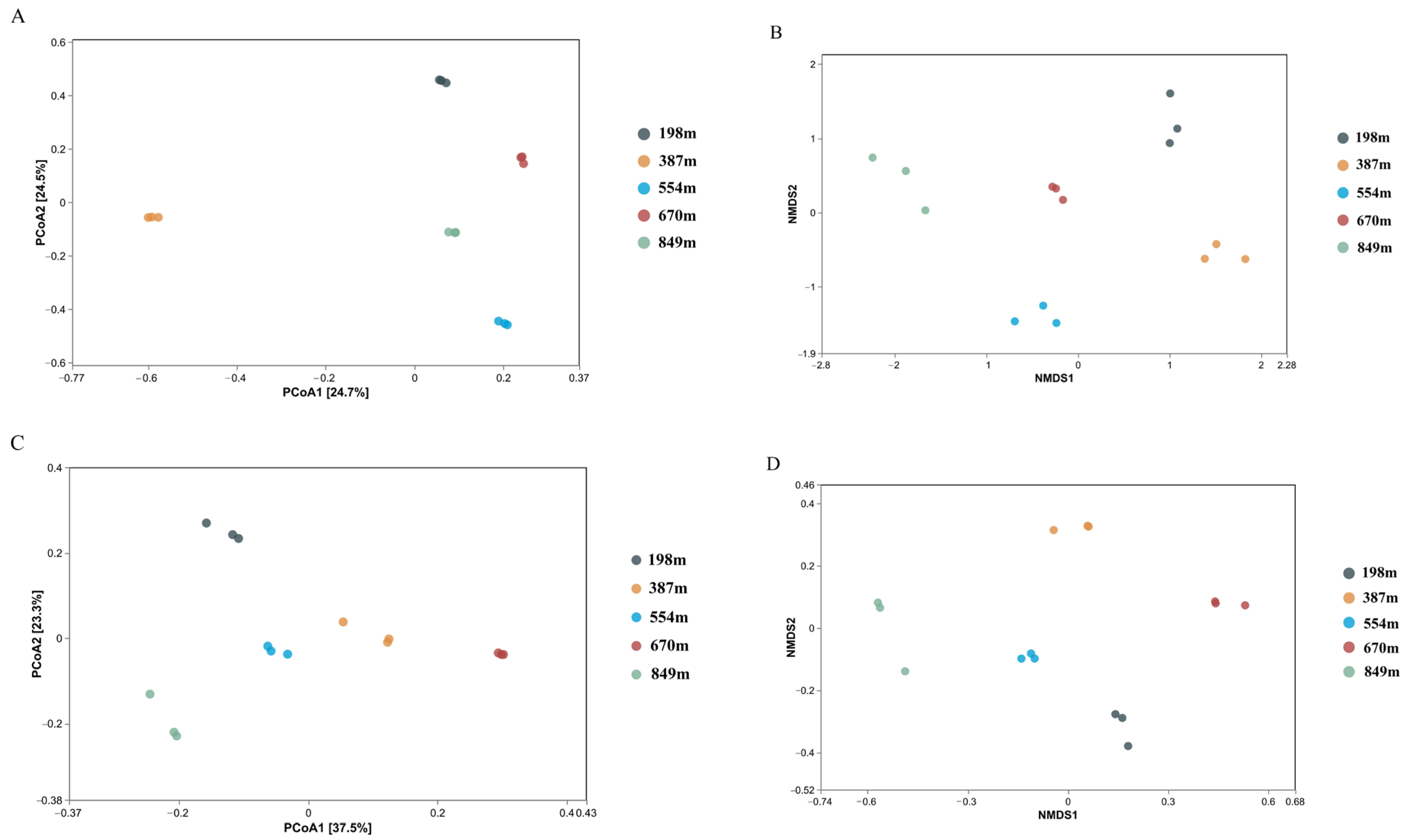
3.3.2. Beta Diversity Indices of the Microbial Communities
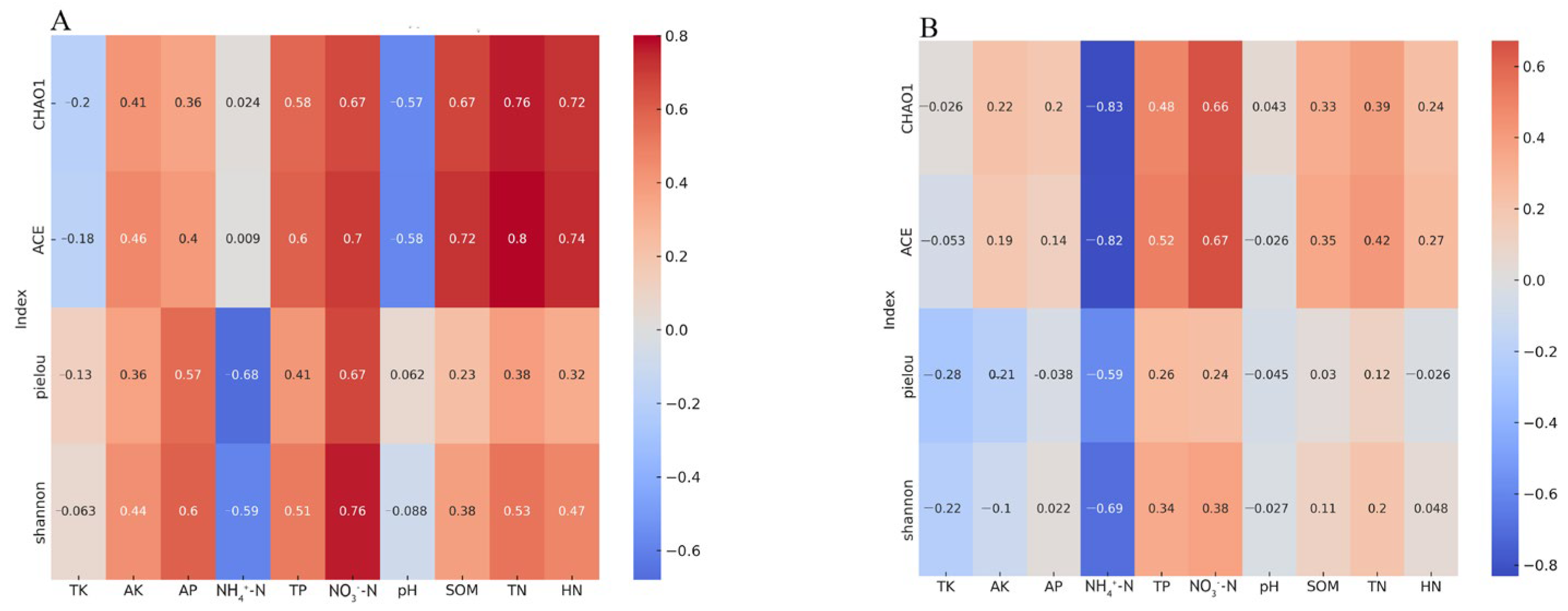
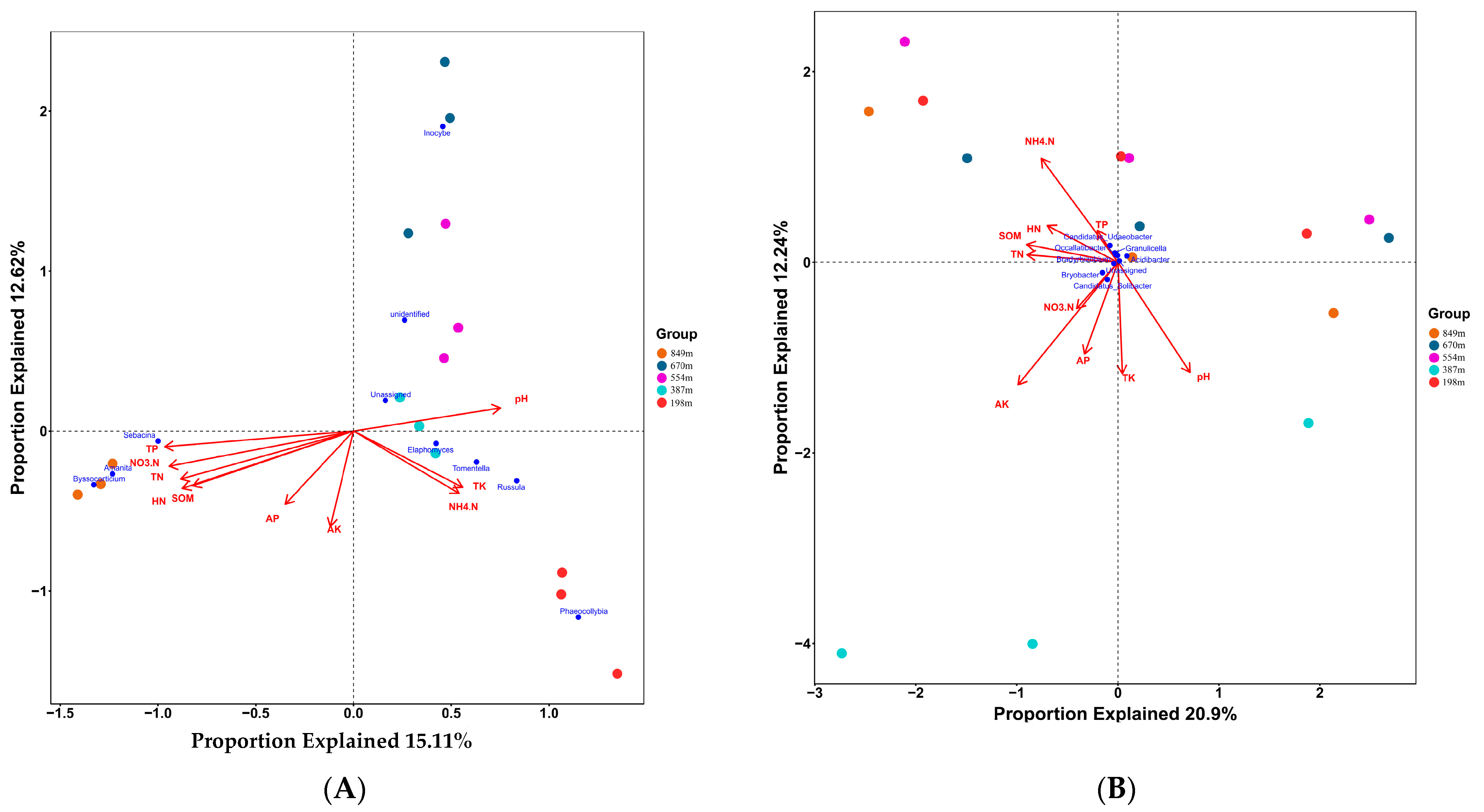
3.4. The Influence of Soil Physicochemical Properties on the Soil Microbial Community in the Rhizosphere of C. hystrix
4. Discussion
4.1. Physical and Chemical Characteristics and Microbial Community Structure of C. hystrix Rhizospheric Soil
4.2. The Impact of Altitude on the Microbial Community Structure in the Rhizosphere Soil of C. hystrix
4.3. Impact of Soil Physical and Chemical Characteristics on Bacterial and Fungal Community Configurations in the Rhizospheric Soil of C. hystrix
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Luo, Z.; Zhang, Y.; Liu, J.; Niu, Y.; Cai, L. Characteristics and ecological function prediction of alfalfa soil bacterial community in rain-fed areas of the Loess Plateau with different planting years. J. Grass Ind. 2021, 30, 54–67. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2023. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Franks, A.E.; Huang, X.; Yang, Q.; Cheng, Z.; Liu, Y.; Ma, B.; Xu, J.; He, Y. Loss of Microbial Diversity Weakens Specific Soil Functions, but Increases Soil Ecosystem Stability. Soil Biol. Biochem. 2023, 177, 108916. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, D.; Liu, J.; Qiao, P.; Zhou, X.; Lu, H.; Wu, X.; Liu, D.; Jin, X.; Wu, F. Changes in Rhizosphere Soil Microbial Communities in a Continuously Monocropped Cucumber (Cucumis Sativus L.) System. Eur. J. Soil Biol. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Solomon, W.; Janda, T.; Molnár, Z. Unveiling the Significance of Rhizosphere: Implications for Plant Growth, Stress Response, and Sustainable Agriculture. Plant Physiol. Biochem. 2023, 206, 108290. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-Enhanced Plant Growth, Nutrient Acquisition, and Crop Protection: Advances in Soil, Plant, and Microbial Multifactorial Interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking Microbial Community Composition to Function in a Tropical Soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Li, X.; Qu, Z.; Zhang, Y.; Ge, Y.; Sun, H. Soil Fungal Community and Potential Function in Different Forest Ecosystems. Diversity 2022, 14, 520. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and Common Traits in Mycorrhizal Symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How Mycorrhizal Associations Drive Plant Population and Community Biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Cahanovitc, R.; Livne-Luzon, S.; Angel, R.; Klein, T. Ectomycorrhizal Fungi Mediate Belowground Carbon Transfer between Pines and Oaks. ISME J. 2022, 16, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Soudzilovskaia, N.A.; Van Bodegom, P.M.; Terrer, C.; Zelfde, M.V.; McCallum, I.; Luke McCormack, M.; Fisher, J.B.; Brundrett, M.C.; De Sá, N.C.; Tedersoo, L. Global Mycorrhizal Plant Distribution Linked to Terrestrial Carbon Stocks. Nat. Commun. 2019, 10, 5077. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Tian, L.; Nasir, F.; Chang, J.; Chang, C.; Zhang, J.; Li, X.; Tian, C. Impacts of Replanting American Ginseng on Fungal Assembly and Abundance in Response to Disease Outbreaks. Arch. Microbiol. 2021, 203, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Hättenschwiler, S.; Yang, X. The Plant Microbiome: A Missing Link for Understanding Community Dynamics and Multifunctionality in Forest Ecosystems. Appl. Soil Ecol. 2020, 145, 103345. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil Multifunctionality Is Affected by the Soil Environment and by Microbial Community Composition and Diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lucas-Borja, M.E.; Wang, Z.; Li, X.; Huang, Z. Microbial Diversity Regulates Ecosystem Multifunctionality during Natural Secondary Succession. J. Appl. Ecol. 2021, 58, 2833–2842. [Google Scholar] [CrossRef]
- Wu, B.; Luo, H.; Wang, X.; Liu, H.; Peng, H.; Sheng, M.; Xu, F.; Xu, H. Effects of Environmental Factors on Soil Bacterial Community Structure and Diversity in Different Contaminated Districts of Southwest China Mine Tailings. Sci. Total Environ. 2022, 802, 149899. [Google Scholar] [CrossRef]
- Zuo, X.; Sun, S.; Wang, S.; Yue, P.; Hu, Y.; Zhao, S.; Guo, X.; Li, X.; Chen, M.; Ma, X.; et al. Contrasting Relationships between Plant-Soil Microbial Diversity Are Driven by Geographic and Experimental Precipitation Changes. Sci. Total Environ. 2023, 861, 160654. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.; Zhou, J.; Bing, H.; Zhu, H. Climate Influences the Alpine Soil Bacterial Communities by Regulating the Vegetation and the Soil Properties along an Altitudinal Gradient in SW China. Catena 2020, 195, 104727. [Google Scholar] [CrossRef]
- Ji, L.; Shen, F.; Liu, Y.; Yang, Y.; Wang, J.; Purahong, W.; Yang, L. Contrasting Altitudinal Patterns and Co-Occurrence Networks of Soil Bacterial and Fungal Communities along Soil Depths in the Cold-Temperate Montane Forests of China. Catena 2022, 209, 105844. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, F.; Xie, C.; Qu, P.; Jiang, K.; Du, H.; Zhao, M.; Lu, Y.; Wang, B.; Shi, X.; et al. Effects of Different Altitudes on Coffea Arabica Rhizospheric Soil Chemical Properties and Soil Microbiota. Agronomy 2023, 13, 471. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Li, M.; Yang, X.J.; Su, Y.; Wang, X.Q. Composition and Altitudinal Response of Soil Fungal Communities around Picea crassifolia in Qinghai. Mycology 2023, 42, 1635–1650. (In Chinese) [Google Scholar] [CrossRef]
- Shen, C.; Gunina, A.; Luo, Y.; Wang, J.; He, J.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting Patterns and Drivers of Soil Bacterial and Fungal Diversity across a Mountain Gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, W.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential Responses of Soil Microbial Biomass, Diversity, and Compositions to Altitudinal Gradients Depend on Plant and Soil Characteristics. Sci. Total Environ. 2018, 610–611, 750–758. [Google Scholar] [CrossRef]
- Xue, Guangyu; Deng, Zhiwen; Zhu, Xueping; Wu, Junduo; Dong, Shitao; Xie, Xianjin; Zeng, Ji Genomic characterisation and phylogenetic analysis of Castanopsis hystrix chloroplasts. J. Biol. Eng. 2023, 39, 670–684. (In Chinese) [CrossRef]
- Zhao, Z.; Liu, Y.; Jia, H.; Sun, W.; Ming, A.; Pang, S.; An, N.; Zhang, J.; Tang, C.; Dong, S. Influence of Slope Direction on the Soil Seed Bank and Seedling Regeneration of Castanopsis hystrix Seed Rain. Forests 2021, 12, 500. [Google Scholar] [CrossRef]
- Xue, G.; Wu, J.; Zhou, B.; Zhu, X.; Zeng, J.; Ma, Y.; Wang, Y.; Jia, H. Effects of Shading on the Growth and Photosynthetic Fluorescence Characteristics of Castanopsis hystrix Seedlings of Top Community-Building Species in Southern Subtropical China. Forests 2023, 14, 1659. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Yang, F.; Chen, B.; Liang, J.; Lu, J. Structure of mycorrhizal microbial communities in a mixed Castanopsis hystrix-Pinus massoniana forest in Pingxiang, Guangxi. J. Mycol. 2021, 40, 1343–1356. (In Chinese) [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, L.; Liang, T.; Man, J.; Wang, Y.; Li, Y.; Chen, H.; Zhang, T. Deciphering Rhizosphere Microbiome Assembly of Castanea Henryi in Plantation and Natural Forest. Microorganisms 2021, 10, 42. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, H.; Ullah, N.; Tung, S.A.; Ali, B.; Li, X.; Chen, S.; Xu, L. Insights into Accumulation of Active Ingredients and Rhizosphere Microorganisms between Salvia miltiorrhiza and S. castanea. FEMS Microbiol. Lett. 2023, 370, fnad102. [Google Scholar] [CrossRef]
- Yao, X.; Hui, D.; Hou, E.; Xiong, J.; Xing, S.; Deng, Q. Differential Responses and Mechanistic Controls of Soil Phosphorus Transformation in Eucalyptus Plantations with N Fertilization and Introduced N2-fixing Tree Species. New Phytol. 2023, 237, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Li, Y.; Zhou, X.; Wen, Y.; Zhu, H.; Qin, Z.; Cai, D.; Jia, H.; Li, X.; You, Y. Natural regeneration and environmental interpretations of Castanopsis hystrixs in a southern subtropical Pinus massoniana × Castanopsis hystrix mixed forest. Guangxi Sci. 2019, 26, 207–214. (In Chinese) [Google Scholar] [CrossRef]
- Lu, L.H.; Wang, B.G.; He, Z.M. Effects of stand and cultivation pattern on the growth of Castanopsis hystrix. For. Sci. Res. 1999, 519–523. (In Chinese) [Google Scholar]
- Schmidt, S.K.; King, A.J.; Meier, C.L.; Bowman, W.D.; Farrer, E.C.; Suding, K.N.; Nemergut, D.R. Plant–Microbe Interactions at Multiple Scales across a High-Elevation Landscape. Plant Ecol. Divers. 2015, 8, 703–712. [Google Scholar] [CrossRef]
- Huo, C.; Lu, J.; Yin, L.; Wang, P.; Cheng, W. Rhizosphere Effects along an Altitudinal Gradient of the Changbai Mountain, China. Forests 2022, 13, 1104. [Google Scholar] [CrossRef]
- Xie, L.; Li, W.; Pang, X.; Liu, Q.; Yin, C. Soil Properties and Root Traits Are Important Factors Driving Rhizosphere Soil Bacterial and Fungal Community Variations in Alpine Rhododendron Nitidulum Shrub Ecosystems along an Altitudinal Gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Sun, Z.; Liu, S.; Wang, Q.; Zeng, Z. Rhizosphere Effects on Soil Organic Carbon Processes in Terrestrial Ecosystems: A Meta-Analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Huo, C.; Lu, J.; Yin, L.; Wang, P.; Cheng, W. Strong Linkage of Carbon and Nitrogen Mineralization in Rhizosphere Soils along an Altitudinal Forest Gradient of Changbai Mountain. 2022. Available online: https://www.researchsquare.com/article/rs-1588844/v1 (accessed on 10 May 2023).
- Cairney, J.W.G. Basidiomycete Mycelia in Forest Soils: Dimensions, Dynamics and Roles in Nutrient Distribution. Mycol. Res. 2005, 109, 7–20. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying Enzymes in Filamentous Basidiomycetes—Ecological, Functional and Phylogenetic Review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Dörfelt, H.; Gube, M.; Jackson, D.J.; Reitner, J.; Seyfullah, L.J.; et al. Estimating the Phanerozoic History of the Ascomycota Lineages: Combining Fossil and Molecular Data. Mol. Phylogenet. Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef]
- Bahram, M.; Põlme, S.; Kõljalg, U.; Zarre, S.; Tedersoo, L. Regional and Local Patterns of Ectomycorrhizal Fungal Diversity and Community Structure along an Altitudinal Gradient in the Hyrcanian Forests of Northern Iran. New Phytol. 2012, 193, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Claridge, A.W.; May, T.W. Mycophagy among Australian Mammals. Aust. J. Ecol. 1994, 19, 251–275. [Google Scholar] [CrossRef]
- Beenken, L. Die Gattung Russula: Untersuchungen zu ihrer Systematik anhand von Ektomykorrhizen. Ph.D. Thesis, Universität München, München, Germany, 2004. [Google Scholar]
- Zu, M.; Yuan, Y.; Zuo, J.; Sun, L.; Tao, J. Microbiota Associated with the Rhizosphere of Paeonia lactiflora Pall. (Ornamental Cultivar). Appl. Soil Ecol. 2022, 169, 104214. [Google Scholar] [CrossRef]
- Taylor, D.L.; Bruns, T.D. Community Structure of Ectomycorrhizal Fungi in a Pinus Muricata Forest: Minimal Overlap between the Mature Forest and Resistant Propagule Communities. Mol. Ecol. 1999, 8, 1837–1850. [Google Scholar] [CrossRef]
- Han, Q.; Huang, J.; Long, D.; Wang, X.; Liu, J. Diversity and Community Structure of Ectomycorrhizal Fungi Associated with Larix Chinensis across the Alpine Treeline Ecotone of Taibai Mountain. Mycorrhiza 2017, 27, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.A.; Wilson, J.; Last, F.T.; Walker, C. The Concept of Succession in Relation to the Spread of Sheathing Mycorrhizal Fungi on Inoculated Tree Seedlings Growing in Unsterile Soils. Plant Soil 1983, 71, 247–256. [Google Scholar] [CrossRef]
- Lu, X.; Cao, T.; Nguyễn, T.T.T.; Yuan, H.-S. Six New Species of Tomentella (Thelephorales, Basidiomycota) from Tropical Pine Forests in Central Vietnam. Front. Microbiol. 2022, 13, 864198. [Google Scholar] [CrossRef]
- Redhead, S.A.; Malloch, D.W. The Genus Phaeocollybia (Agaricales) in Eastern Canada and Its Biological Status. Can. J. Bot. 1986, 64, 1249–1254. [Google Scholar] [CrossRef]
- Argüelles-Moyao, A.; Garibay-Orijel, R.; Márquez-Valdelamar, L.M.; Arellano-Torres, E. Clavulina-Membranomyces Is the Most Important Lineage within the Highly Diverse Ectomycorrhizal Fungal Community of Abies religiosa. Mycorrhiza 2017, 27, 53–65. [Google Scholar] [CrossRef]
- Yang, A.; Lu, Y.; Zhang, J.; Wu, J.; Xu, J.; Tong, Z. Changes in soil nutrients and Acidobacterium community structure in a Cunninghamia lanceolata plantation forest. For. Sci. 2019, 55, 119–127. (In Chinese) [Google Scholar]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial Populations and Environmental Factors Controlling Cellulose Degradation in an Acidic Sphagnum Peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Rentz, J.A.; Lilburn, T.G.; Davis, R.E.; Aldrich, H.; Chan, C.; Moyer, C.L. A Novel Lineage of Proteobacteria Involved in Formation of Marine Fe-Oxidizing Microbial Mat Communities. PLoS ONE 2007, 2, e667. [Google Scholar] [CrossRef]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Schleifer, K.-H.; Stackebrandt, E. (Eds.) The Prokaryotes; Springer: New York, NY, USA, 2006; ISBN 978-0-387-25495-1. [Google Scholar]
- Yu, Z.; Li, Y.; Wang, G.; Liu, J.; Liu, J.; Liu, X.; Herbert, S.J.; Jin, J. Effectiveness of Elevated CO2 Mediating Bacterial Communities in the Soybean Rhizosphere Depends on Genotypes. Agric. Ecosyst. Environ. 2016, 231, 229–232. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Brunner, I.; Widmer, F.; Zeyer, J.; Frey, B. Vertical Distribution of the Soil Microbiota along a Successional Gradient in a Glacier Forefield. Mol. Ecol. 2015, 24, 1091–1108. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Rodríguez-Echeverría, S.; González, L.; Freitas, H. Effect of Invasive Acacia Dealbata Link on Soil Microorganisms as Determined by PCR-DGGE. Appl. Soil Ecol. 2010, 44, 245–251. [Google Scholar] [CrossRef]
- Liao, M.; Xie, X.; Peng, Y.; Chai, J.; Chen, N. Characteristics of Soil Microbial Community Functional and Structure Diversity with Coverage of Solidago Canadensis L. J. Cent. South Univ. 2013, 20, 749–756. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.T.; Mitkowski, N.A.; Aldrich-Wolfe, L.; Emele, L.R.; Jurkonie, D.D.; Ficke, A.; Maldonado-Ramirez, S.; Lynch, S.T.; Nelson, E.B. Methods for Assessing the Composition and Diversity of Soil Microbial Communities. Appl. Soil Ecol. 2000, 15, 25–36. [Google Scholar] [CrossRef]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal Networks: Mechanisms, Ecology and Modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Drenkhan, R.; Pritsch, K.; Buegger, F.; Padari, A.; Hagh-Doust, N.; Mikryukov, V.; Gohar, D.; et al. Regional-Scale In-Depth Analysis of Soil Fungal Diversity Reveals Strong pH and Plant Species Effects in Northern Europe. Front. Microbiol. 2020, 11, 1953. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and Nutrients Shape the Vertical Distribution of Microbial Communities in an Alpine Wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Nacke, H.; Thürmer, A.; Wollherr, A.; Will, C.; Hodac, L.; Herold, N.; Schöning, I.; Schrumpf, M.; Daniel, R. Pyrosequencing-Based Assessment of Bacterial Community Structure Along Different Management Types in German Forest and Grassland Soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef]
- Gao, X.-H.; Li, M.; Lu, P.; Lv, G.-F.; Niu, Y.-F. Research on the structure of soil bacterial community in the inter-root of Betula platyphylla in Dazhoushan, Hohhot. J. Ecol. 2019, 39, 3586–3596. (In Chinese) [Google Scholar]
- Juhnke, M.E.; Mathre, D.E.; Sands, D.C. Identification and Characterization of Rhizosphere-Competent Bacteria of Wheat. Appl. Environ. Microbiol. 1987, 53, 2793–2799. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH Drives the Spatial Distribution of Bacterial Communities along Elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a Fungal World: Impact of Fungi on Soil Bacterial Niche Development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering Fungal: Bacterial Dominance in Soils—Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Romanowicz, K.J.; Freedman, Z.B.; Upchurch, R.A.; Argiroff, W.A.; Zak, D.R. Active Microorganisms in Forest Soils Differ from the Total Community yet Are Shaped by the Same Environmental Factors: The Influence of pH and Soil Moisture. FEMS Microbiol. Ecol. 2016, 92, fiw149. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.; Chen, L.; Wang, J.; Zhang, L. Soil pH Determines Fungal Diversity along an Elevation Gradient in Southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Li, J.H.; Zhang, G.Y.; Wang, N.; Tan, W.L.; Wang, Y.P.; Wang, C.Y.; Wang, H.F. Analysis of the diversity and community composition of soil fungi in Hevea brasiliensis forests based on high-throughput sequencing. South. J. Agric. 2018, 49, 1729–1735. (In Chinese) [Google Scholar]
- Zhang, J.; Zheng, M.; Zhang, Y.; Wang, J.; Shen, H.; Lin, Y.; Tang, X.; Hui, D.; Lambers, H.; Sardans, J.; et al. Soil Phosphorus Availability Affects Diazotroph Communities during Vegetation Succession in Lowland Subtropical Forests. Appl. Soil Ecol. 2021, 166, 104009. [Google Scholar] [CrossRef]
- Jian, Z.; Ni, Y.; Zeng, L.; Lei, L.; Xu, J.; Xiao, W.; Li, M.-H. Latitudinal Patterns of Soil Extracellular Enzyme Activities and Their Controlling Factors in Pinus massoniana Plantations in Subtropical China. For. Ecol. Manag. 2021, 495, 119358. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between Plant Roots, Rhizosphere Microorganisms, and Nitrogen and Its Special Focus on Rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Chen, L.; Xu, H.; Wu, S.; Baoyin, T. Plant and Soil Properties Mediate the Response of Soil Microbial Communities to Moderate Grazing in a Semiarid Grassland of Northern China. J. Environ. Manag. 2021, 284, 112005. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Koziol, L.; Foster, B.L.; Bever, J.D. Microbial Mediators of Plant Community Response to Long-term N and P Fertilization: Evidence of a Role of Plant Responsiveness to Mycorrhizal Fungi. Glob. Chang. Biol. 2022, 28, 2721–2735. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic Colonization of Plant Roots by Nitrogen-Fixing Bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Haro, R.; Benito, B. The Role of Soil Fungi in K+ Plant Nutrition. Int. J. Mol. Sci. 2019, 20, 3169. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Exploring the Potential: Can Mycorrhizal Fungi and Hyphosphere Silicate-Solubilizing Bacteria Synergistically Alleviate Cadmium Stress in Plants? Curr. Res. Biotechnol. 2023, 6, 100158. [Google Scholar] [CrossRef]
- Basak, B.B.; Maity, A.; Ray, P.; Biswas, D.R.; Roy, S. Potassium Supply in Agriculture through Biological Potassium Fertilizer: A Promising and Sustainable Option for Developing Countries. Arch. Agron. Soil Sci. 2022, 68, 101–114. [Google Scholar] [CrossRef]
- Huang, M.; Xin, W.; Lei, X.; Zhang, Z.; Ye, X.; Sun, Y. Effects of different ratios of nitrogen, phosphorus and potassium on the structure of soil bacterial communities in the inter-root of young Camellia oleifera forests. South. For. Sci. 2021, 49, 32–36. (In Chinese) [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, G.; Xiong, J.; Tang, L.; Zhang, Q.; Zeng, J.; Zhao, C.; Wu, J.; Dong, S.; Zhu, X. Effects of Different Altitudes on Castanopsis hystrix, the Top Community-Building Species in Southern Subtropical China: Rhizospheric Soil Chemical Properties and Soil Microbiota. Forests 2024, 15, 187. https://doi.org/10.3390/f15010187
Xue G, Xiong J, Tang L, Zhang Q, Zeng J, Zhao C, Wu J, Dong S, Zhu X. Effects of Different Altitudes on Castanopsis hystrix, the Top Community-Building Species in Southern Subtropical China: Rhizospheric Soil Chemical Properties and Soil Microbiota. Forests. 2024; 15(1):187. https://doi.org/10.3390/f15010187
Chicago/Turabian StyleXue, Guangyu, Junfei Xiong, Li Tang, Quanxin Zhang, Ji Zeng, Chenchi Zhao, Junduo Wu, Shitao Dong, and Xueping Zhu. 2024. "Effects of Different Altitudes on Castanopsis hystrix, the Top Community-Building Species in Southern Subtropical China: Rhizospheric Soil Chemical Properties and Soil Microbiota" Forests 15, no. 1: 187. https://doi.org/10.3390/f15010187



