Different Responses of Arbuscular Mycorrhizal Fungal Community Compositions in the Soil and Roots to Nitrogen Deposition in a Subtropical Cunninghamia lanceolata Plantation in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Description and Sample Collection
2.2. AM Fungal Colonization and Biomass Determination
2.3. DNA Extraction and Illumina Sequencing
2.4. Bioinformatics
2.5. Statistical Analysis
3. Results
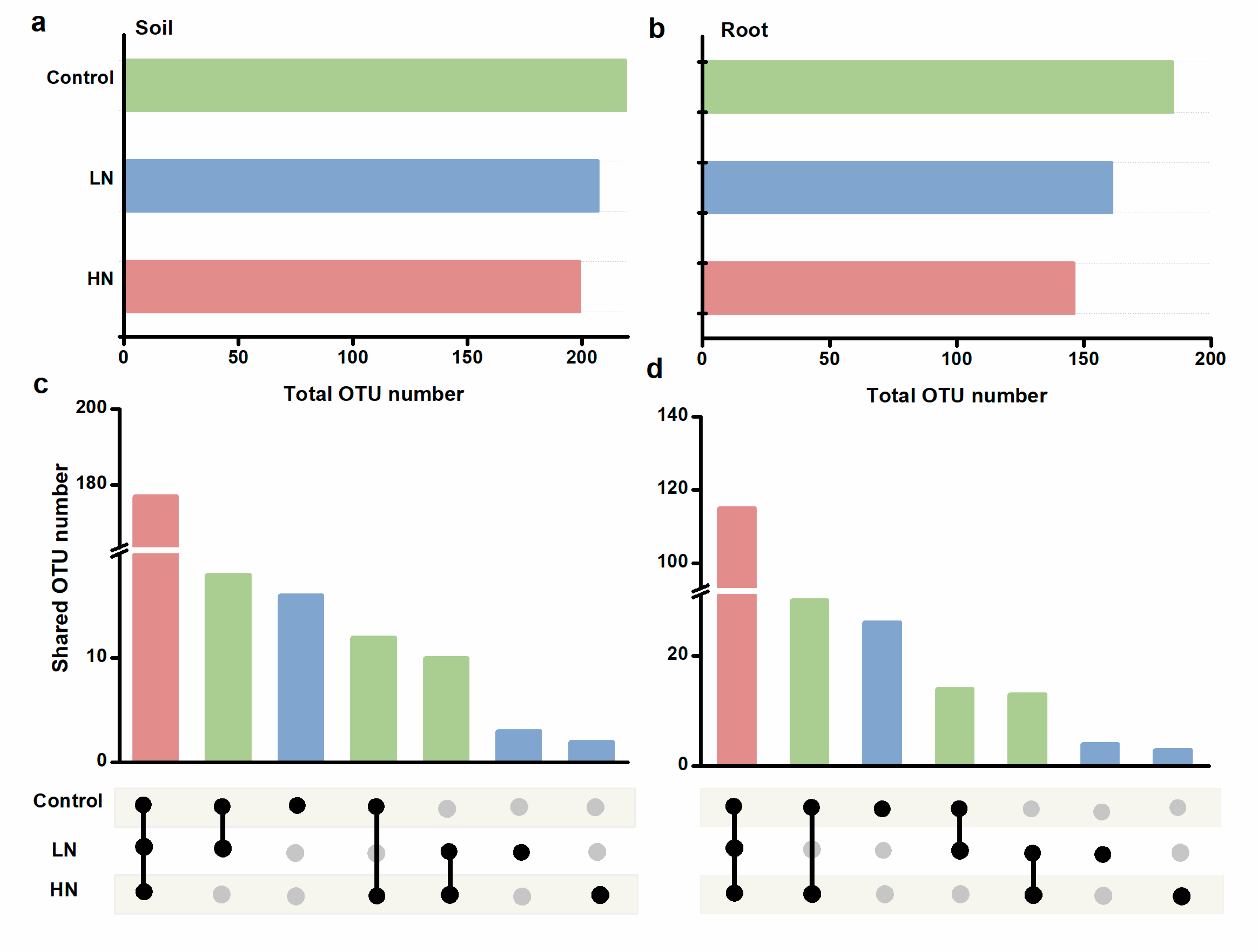
3.1. AM Fungal Abundance and Diversity
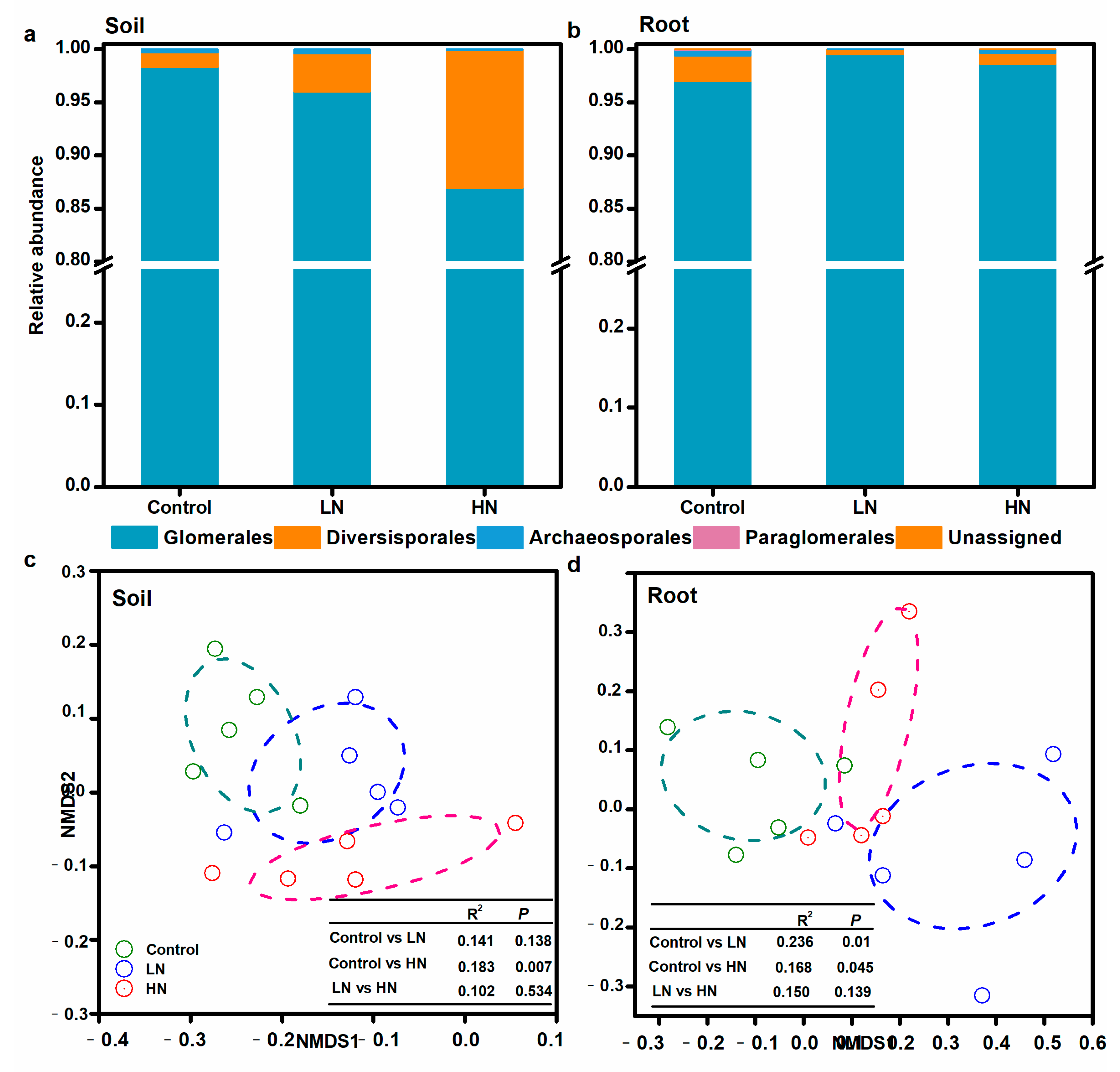
3.2. AM Fungal Community Structure
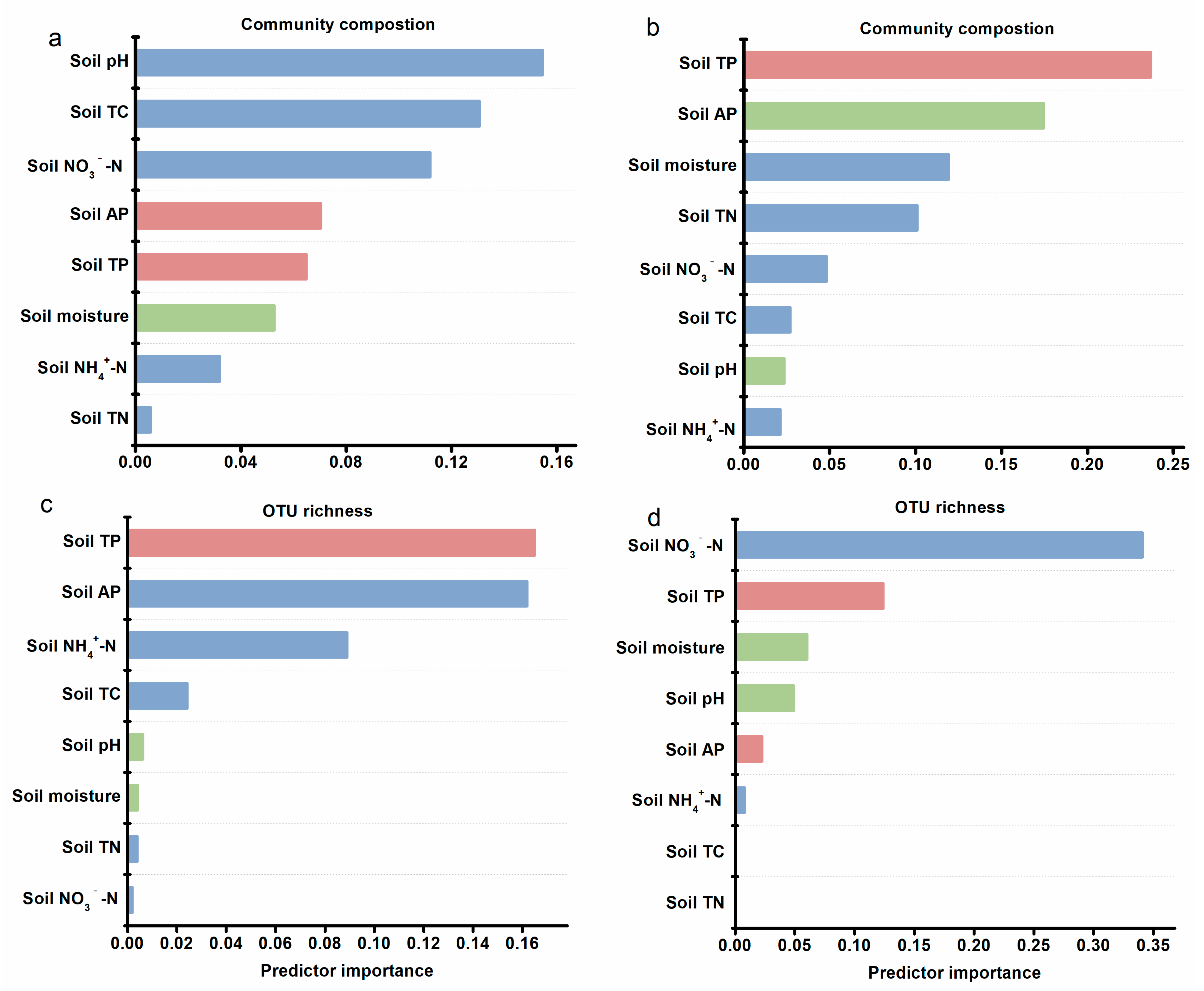
3.3. Diversity and Community Composition Linkages with Soil Properties
3.4. Community Assembly of AM Fungi in Soil and Roots
4. Discussion
4.1. Decreased AM Fungal Richness in Both Soil and Roots under N Addition Treatments
4.2. Distinct Responses of AM Fungal Taxa to N Addition between Soil and Roots
4.3. Differential Changing Patterns of Ecological Processes Driving AM Fungi between Soil and Roots under N Addition Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, J.J.; Dou, Y.X.; An, S.S. Plant community productivity is associated with multiple ecological stoichiometry in restoration grasslands. Ecol. Eng. 2023, 187, 106845. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Pẽnuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.C.; Chuyong, G.; Dobrowski, S.Z.; Grierson, P.; Harms, K.E.; Houlton, B.Z. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939. [Google Scholar] [CrossRef] [PubMed]
- Fortier, R.; Wright, S.J. Nutrient limitation of plant reproduction in a tropical moist forest. Ecology 2021, 102, e03469. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Simkin, S.M.; Allen, E.B.; Bowman, W.D.; Belnap, J.; Brooks, M.L.; Collins, S.L.; Geiser, L.H.; Gilliam, F.S.; Jovan, S.E.; et al. Potential vulnerability of 348 herbaceous species to atmospheric deposition of nitrogen and sulfur in the United States. Nat. Plants 2019, 5, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, X.; Chen, F. Shifts in plant community composition weaken the negative effect of nitrogen addition on community-level arbuscular mycorrhizal fungi colonization. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200483. [Google Scholar] [CrossRef]
- Wilcots, M.E.; Schroeder, K.M.; Lang, D.L.; Kjaer, S.J.; Hobbie, S.E.; Seabloom, E.W.; Borer, E.T. Realistic rates of nitrogen addition increase carbon flux rates but do not change soil carbon stocks in a temperate grassland. Glob. Chang. Biol. 2022, 16, 4819–4831. [Google Scholar] [CrossRef]
- Mellett, T.; Selvin, C.; Defforey, D.; Roberts, K.; Lecher, A.L.; Dennis, K.; Gutknecht, J.; Field, C.B.; Paytan, A. Assessing cumulative effects of climate change manipulations on phosphorus limitation in a californian grassland. Environ. Sci. Technol. 2018, 52, 98–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Lyu, M.; Xiong, D.; Wang, J.; Chen, Y.; Li, Y.; Wang, M.; Yang, Y. Short-term effects of soil warming and nitrogen addition on the N:P stoichiometry of Cunninghamia lanceolata in subtropical regions. Plant Soil. 2017, 411, 395–407. [Google Scholar] [CrossRef]
- Grant, T.; Sethuraman, A.; Escobar, M.A.; Vourlitis, G.L. Chronic dry nitrogen inputs alter soil microbial community composition in Southern California semi-arid shrublands. Appl. Soil Ecol. 2022, 176, 104496. [Google Scholar] [CrossRef]
- Gao, S.Q.; Song, Y.Y.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Gao, J.L.; Cheng, X.F.; Du, Y. Effects of temperature increase and nitrogen addition on the early litter decomposition in permafrost peatlands. Catena 2022, 209, 105801. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Pearson, J.N.; Jakobsen, I. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 1993, 124, 481–488. [Google Scholar] [CrossRef]
- Walder, F.; Niemann, H.; Natarajan, M.; Lehmann, M.F.; Boller, T.; Wiemken, A. Mycorrhizal networks: Common goods of plants shared under unequal terms of trade. Plant Physiol. 2012, 159, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.S.; Shew, H.D.; Rufty, T.W.; Hu, S.J. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.L.; He, X.H.; Liu, J.X. Glomalin-related soil protein responses to elevated CO2 and nitrogen addition in a subtropical forest: Potential consequences for soil carbon accumulation. Soil. Biol. Biochem. 2015, 83, 142–149. [Google Scholar] [CrossRef]
- Kang, F.; Yang, B.; Wujisiguleng; Yang, X.; Wang, L.; Guo, J.; Sun, W.; Zhang, Q.; Zhang, T. Arbuscular mycorrhizal fungi alleviate the negative effect of nitrogen deposition on ecosystem functions in meadow grassland. Land Degrad. Dev. 2020, 31, 748–759. [Google Scholar] [CrossRef]
- Storer, K.; Coggan, A.; Ineson, p.; Hodge, A. Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol. 2018, 220, 1285–1295. [Google Scholar] [CrossRef]
- Han, Y.; Feng, J.; Han, M.; Zhu, B. Responses of arbuscular mycorrhizal fungi to nitrogen addition: A meta-analysis. Glob. Chang. Biol. 2020, 26, 1539. [Google Scholar] [CrossRef]
- Weber, S.E.; Diez, J.M.; Andrews, L.V.; Goulden, M.L.; Aronson, E.L.; Allen, M.F. Responses of arbuscular mycorrhizal fungi to multiple coinciding global change drivers. Fungal Ecol. 2019, 40, 62–71. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Cui, M.M.; Wang, C.; Wang, J.; Wang, S.L.; Sun, Z.J.; Ren, F.R.; Wan, S.Q.; Han, S.J. Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Sci. Total Environ. 2022, 806, 150522. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, T.; Hempel, S.; Homeier, J.; Horn, S.; Velescu, A.; Wilcke, W.; Rillig, M.C. Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob. Chang. Biol. 2014, 20, 3646–3659. [Google Scholar] [CrossRef] [PubMed]
- Agnolucci, M.; Battini, F.; Cristani, C.; Giovannetti, M. Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates. Biol. Fertil. Soils 2015, 51, 379–389. [Google Scholar] [CrossRef]
- Mueller, R.C.; Bohannan, B.J.M. Shifts in the phylogenetic structure of arbuscular mycorrhizal fungi in response to experimental nitrogen and carbon dioxide additions. Oecologia 2015, 179, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Rowland, D.L.; Corkidi, L. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 2003, 84, 1895–1908. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Luo, J.; Qin, M.; Johnson, N.C.; Öpik, M.; Vasar, M.; Chai, Y.; Zhou, X.; Mao, L. Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem. New Phytol. 2018, 220, 1222–1235. [Google Scholar] [CrossRef]
- Thakur, M.P.; Del Real, I.M.; Cesarz, S.; Steinauer, K.; Reich, P.B.; Hobbie, S.; Ciobanu, M.; Rich, R.; Worm, K.; Eisenhauer, N. Soil microbial, nematode, and enzymatic responses to elevated CO2 N fertilization, warming, and reduced precipitation. Soil Biol. Biochem. 2019, 135, 184–193. [Google Scholar] [CrossRef]
- Stevens, B.M.; Propster, J.R.; Öpik, M.; Wilson, G.W.T.; Alloway, S.L.; Mayemba, E.; Johnson, N.C. Arbuscular mycorrhizal fungi in roots and soil respond differently to biotic and abiotic factors in the Serengeti. Mycorrhiza 2020, 30, 79–95. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Gao, C.; He, X.; Ding, Q.; Kim, Y.; Rui, Y.; Wang, S.; Guo, L.D. The arbuscular mycorrhizal fungal community response to warming and grazing differs between soil and roots on the Qinghai-Tibetan plateau. PLoS ONE 2013, 8, e76447. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Feng, X.X.; Guo, Y.X.; Ren, C.J.; Wang, J.; Doughty, R. Elevation gradients affect the differences of arbuscular mycorrhizal fungi diversity between root and rhizosphere soil. Agric. For. Meteorol. 2020, 284, 107894. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Piao, H.C.; Liu, C.Q. Variations in nitrogen, zinc, and sugar concentrations in Chinese fir seedlings grown on shrubland and plowed soils in response to arbuscular mycorrhizae-mediated process. Biol. Fert. Soils 2011, 47, 721–727. [Google Scholar] [CrossRef]
- Miller, A.J.; Schuur, E.A.G.; Chadwick, O.A. Redox control of phosphorus pools in Hawaiian montane forest soils. Geoderma 2001, 102, 219–237. [Google Scholar] [CrossRef]
- Zhang, W. Observation of N/S Deposition Fluxes and Investigation of Simulated S Deposition Effect on soil N2O Production of Castanopsis Carlessii Forests; Fujian Normal University: Fuzhou, China, 2013. (In Chinese) [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Olsson, P.A.; Bååth, E.; Jakobsen, I.; Söderström, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Van, G.M.; Busschaert, P.; Honnay, O.; Lievens, B. Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. J. Microbiol. Methods 2014, 106, 93–100. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of highthroughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Opik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, U.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. 2013. Available online: http://purl.oclc.org/estimates (accessed on 1 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Luo, Y.Q.; Hui, D.F.; Zhang, D.Q. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef]
- Hauke, J.; Kossowski, T. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaest. Geogr. 2011, 30, 87–93. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R.N.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E Ltd: Plymouth, UK, 2014. [Google Scholar]
- Stegen, J.C.; Lin, X.J.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Xu, Z.W.; Xu, T.L.; Veresoglou, S.D.; Yang, G.W.; Chen, B.D. Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2017, 115, 233–242. [Google Scholar] [CrossRef]
- Liu, Y.J.; Johnson, N.C.; Mao, L.; Shi, G.X.; Jiang, S.J.; Ma, X.J.; Du, G.Z.; An, L.Z.; Feng, H.Y. Phylogenetic structure of arbuscular mycorrhizal community shifts in response to increasing soil fertility. Soil Biol. Biochem. 2015, 89, 196–205. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, L.T.A.; Lilleskov, E.A.; Pregitzer, K.S.; Miller, R.M. Decline of arbuscular mycorrhizal fungi in northern hardwood forests exposed to chronic nitrogen additions. New Phytol. 2007, 176, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, A.P.; Dalpe, Y.; Lapointe, L.; Piche, Y. Soil pH-induced changes in root colonization, diversity, and reproduction of symbiotic arbuscular mycorrhizal fungi from healthy and declining maple forests. Can. J. For. Res. 2000, 30, 1543–1554. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Menge, D.N.L.; Field, C.B. Simulated global changes alter phosphorus demand in annual grassland. Glob. Change Biol. 2007, 13, 2582–2591. [Google Scholar] [CrossRef]
- Cao, J.L.; Lin, T.-C.; Yang, Z.J.; Zheng, Y.; Xie, L.; Xiong, D.C.; Yang, Y.S. Warming exerts a stronger effect than nitrogen addition on the soil arbuscular mycorrhizal fungal community in a young subtropical Cunninghamia lanceolata plantation. Geoderma 2020, 367, 114273. [Google Scholar] [CrossRef]
- Berendse, F.; Nvan, B.; Rydin, H.; Buttler, A.; Heijmans, M.; Hoosbeek, M.R.; Lee, J.A.; Mitchell, E.; Saarinen, T.; Vasander, H. Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Glob. Chang. Biol. 2001, 7, 591–598. [Google Scholar] [CrossRef]
- Emery, S.M.; Bell-Dereske, L.; Stahlheber, K.A.; Gross, K.L. Arbuscular mycorrhizal fungal community responses to drought and nitrogen fertilization in switchgrass stands. Appl. Soil Ecol. 2022, 2022, 104218. [Google Scholar] [CrossRef]
- Drigo, B.; Pijl, A.S.; Duyts, H.; Kielak, A.M.; Gamper, H.A.; Houtekamer, M.J.; Boschker, H.T.S.; Bodelier, P.L.E.; Whiteley, A.S.; Veen, J.A.V. Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc. Natl. Acad. Sci. USA 2010, 107, 10938. [Google Scholar] [CrossRef] [PubMed]
- Egerton-Warburton, L.M.; Allen, E.B. Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol. Appl. 2000, 10, 484–496. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Treseder, K.K.; Vitousek, P.M. Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 2001, 82, 946–954. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography Princeton University Press: Princeton, NJ, USA, 2001; Volume 32.
- Pereira, C.M.R.; López-García, Á.; Silva, D.K.A.D.; Maia, L.C.; Frøslev, T.C.; Kjøller, R.; Rosendahl, S. Tropical forest type influences community assembly processes in arbuscular mycorrhizal fungi. J. Biogeogr. 2020, 47, 434–444. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Teixeira, H.; Correia, M.; Timóteo, S.; Heleno, R.; Öpik, M.; Moora, M. Arbuscular mycorrhizal fungi communities from tropical Africa reveal strong ecological structure. New Phytol. 2017, 213, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Bouffaud, M.L.; Bragalini, C.; Berruti, A.; Peyret-Guzzon, M.; Voyron, S.; Stockinger, H.; Girlanda, M. Arbuscular mycorrhizal fungal community differences among European long-term observatories. Mycorrhiza 2017, 27, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.; Heuck, M.K.; Aguilar-Trigueros, C.A. Host filtering, not competitive exclusion, may be the main driver of arbuscular mycorrhizal fungal community assembly under high phosphorus. Funct. Ecol. 2023, 37, 1856–1869. [Google Scholar] [CrossRef]
- Vályi, K.; Mardhiah, U.; Rillig, M.C.; Hempel, S. Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 2016, 10, 2341. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, Q.K.; Shen, C.C.; Yang, F.T.; Wang, J.C.; Ge, Y. Significant dose effects of fertilizers on soil diazotrophic diversity, community composition, and assembly processes in a long-term paddy field fertilization experiment. Land Degrad. Dev. 2021, 32, 420–429. [Google Scholar] [CrossRef]
- Xiong, D.C.; Yang, Z.J.; Chen, G.S.; Liu, X.F.; Lin, W.S.; Huang, J.X.; Bowles, F.P.; Lin, C.F.; Xie, J.S.; Li, Y.Q.; et al. Interactive effects of warming and nitrogen addition on fine root dynamics of a young subtropical plantation. Soil Biol. Biochem. 2018, 123, 180–189. [Google Scholar] [CrossRef]



| Observed OTUs | Chao1 | Shannon | ACE | ||
|---|---|---|---|---|---|
| Root | Control | 79.8 ± 7.79 a | 102.0 ± 15.9 a | 1.51 ± 0.16 a | 103.2 ± 19.3 a |
| LN | 62.6 ± 8.44 ab | 79.9 ± 8.14 ab | 0.95 ± 0.17 b | 79.8 ± 8.22 ab | |
| HN | 59.8 ± 15.4 b | 70.6 ± 15.3 b | 0.93 ± 0.16 b | 73.6 ± 13.3 b | |
| Soil | Control | 111.2 ± 7.69 a | 158.9 ± 22.5 a | 2.34 ± 0.04 a | 154.1 ± 15.9 a |
| LN | 111.8 ± 6.38 a | 133.1 ± 17.0 ab | 2.24 ± 0.11 ab | 138.5 ± 18.9 ab | |
| HN | 97.4 ± 8.85 b | 121.5 ± 12.6 b | 2.14 ± 0.15 b | 122.7 ± 13.2 b |
| Soil | Root | |||||
|---|---|---|---|---|---|---|
| Control | LN | HN | Control | LN | HN | |
| Glomeraceae | 93.9 ± 4.1 a | 90.8 ± 6.7 ab | 83.5 ± 9.7 b | 96.9 ± 2.1 b | 99.5 ± 0.5 a | 98.6 ± 2.3 a |
| Gigasporaceae | 1.58 ± 0.48 b | 3.27 ± 0.28 a | 4.12 ± 0.48 a | 1.67 ± 0.17 a | 0.37 ± 0.04 b | 0.33 ± 0.03 b |
| Acaulosporaceae | 0.66 ± 0.08 a | 0.35 ± 0.03 b | 0.17 ± 0.02 c | 1.26 ± 0.16 a | 0.26 ± 0.03 b | 0.76 ± 0.02 b |
| Ambisporaceae | 1.38 ± 0.12 c | 3.61 ± 0.48 b | 11.0 ± 0.78 a | 0.56 ± 0.07 a | 0.03 ± 0.005 b | 0.38 ± 0.07 a |
| Claroideoglomeraece | 2.17 ± 0.29 a | 1.50 ± 0.08 b | 1.11 ± 0.17 b | 0.002 | ND | ND |
| Paraglomeraceae | 0.31 ± 0.06 a | 0.43 ± 0.08 a | 0.08 ± 0.001 a | 0.060 | ND | ND |
| Diversisporaceae | 0.02 | ND a | ND | 0.002 | ND | ND |
| unassigned | ND | ND | ND | 0.002 | ND | ND |
| Matrix | Soil | Root | ||
|---|---|---|---|---|
| r | p | r | p | |
| Soil pH | 0.422 | 0.005 | 0.087 | 0.221 |
| Soil moisture | −0.109 | 0.731 | −0.140 | 0.786 |
| Soil TC | −0.075 | 0.697 | −0.068 | 0.682 |
| Soil TN | −0.051 | 0.639 | 0.062 | 0.325 |
| Soil TP | −0.246 | 0.952 | 0.146 | 0.181 |
| Soil AP | −0.068 | 0.290 | 0.460 | 0.006 |
| Soil NH4+−N | −0.066 | 0.653 | −0.101 | 0.734 |
| Soil NO3−−N | 0.160 | 0.177 | −0.058 | 0.569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Liu, Z.; Li, S.; Lai, F.; Chi, C.; Yang, Y.; Cao, J. Different Responses of Arbuscular Mycorrhizal Fungal Community Compositions in the Soil and Roots to Nitrogen Deposition in a Subtropical Cunninghamia lanceolata Plantation in China. Forests 2024, 15, 27. https://doi.org/10.3390/f15010027
Han Y, Liu Z, Li S, Lai F, Chi C, Yang Y, Cao J. Different Responses of Arbuscular Mycorrhizal Fungal Community Compositions in the Soil and Roots to Nitrogen Deposition in a Subtropical Cunninghamia lanceolata Plantation in China. Forests. 2024; 15(1):27. https://doi.org/10.3390/f15010027
Chicago/Turabian StyleHan, Yu, Zhiyuan Liu, Siyao Li, Faying Lai, Chunghao Chi, Yusheng Yang, and Jiling Cao. 2024. "Different Responses of Arbuscular Mycorrhizal Fungal Community Compositions in the Soil and Roots to Nitrogen Deposition in a Subtropical Cunninghamia lanceolata Plantation in China" Forests 15, no. 1: 27. https://doi.org/10.3390/f15010027




