Influence of Irrigation on Biomass Partitioning in Above- and Belowground Organs of Trees Planted in Desert Sites of Mongolia
Abstract
:1. Introduction
2. Materials and Methods
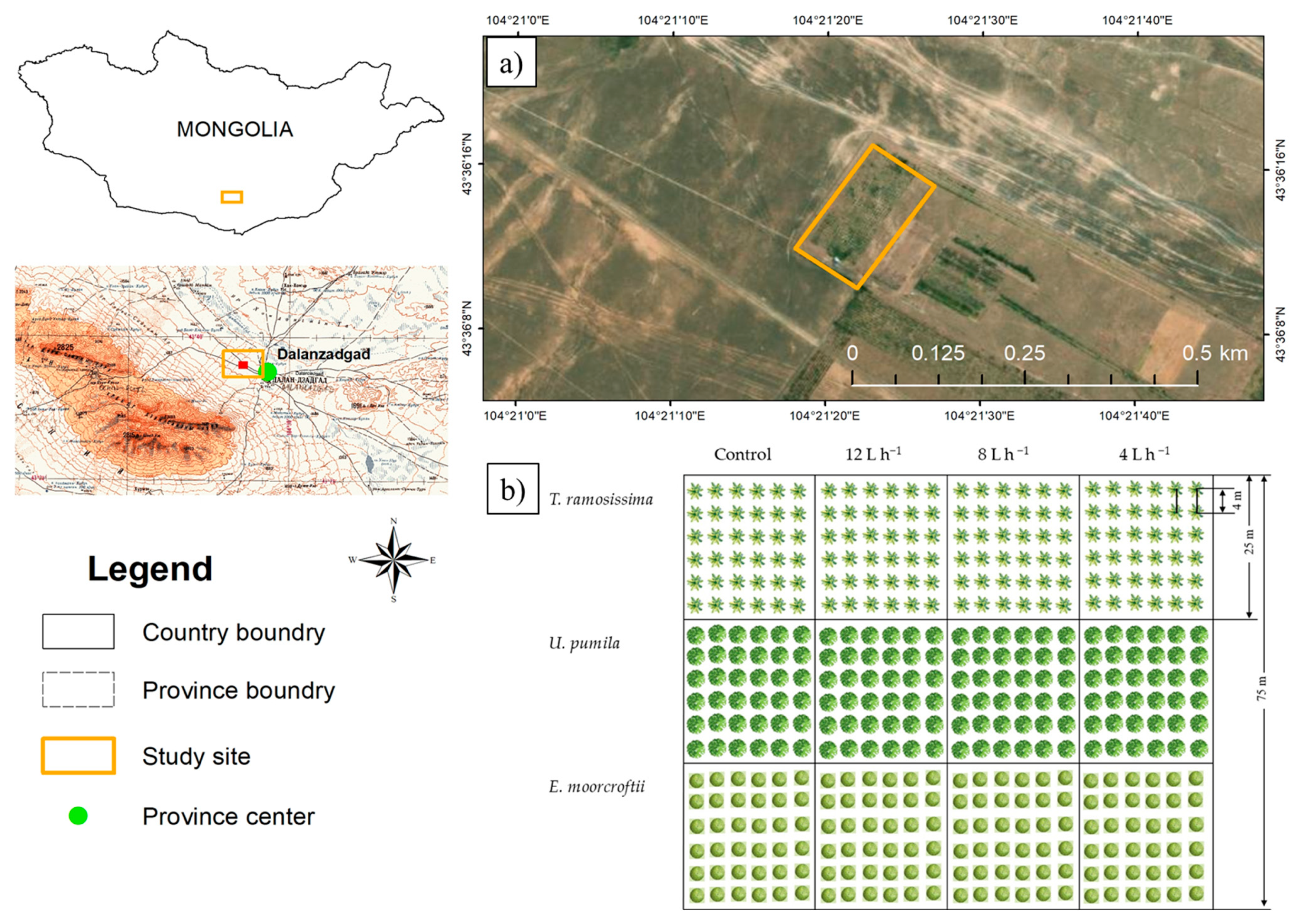
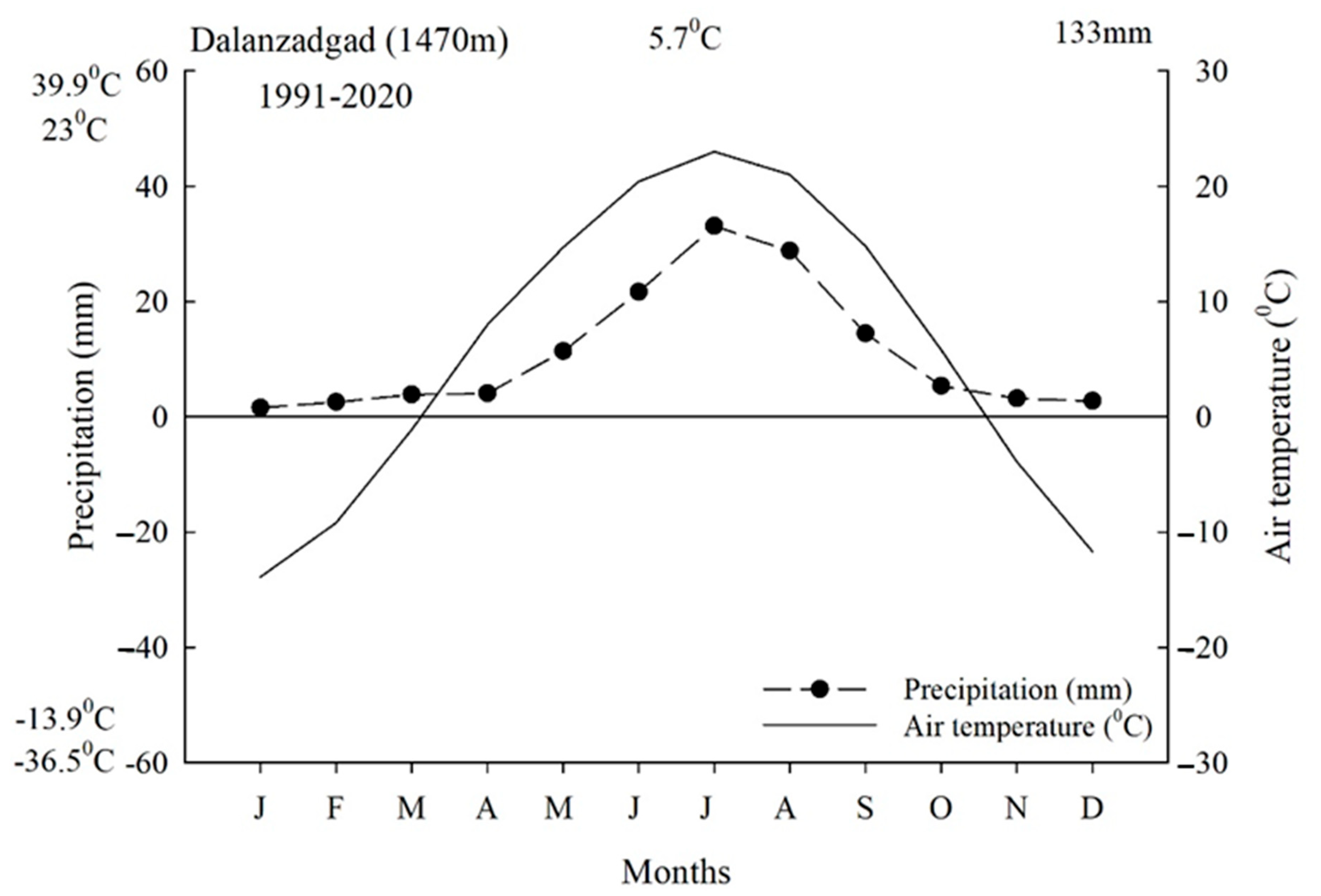
2.1. Study Site Description
2.2. Planting Scheme and Sampling Design
2.3. Measurement of Morphological Traits and Allocation of Biomass to Above and Below-Ground Organs
2.4. Soil Sampling and Chemical Analyses
2.5. Statistical Analysis
3. Results
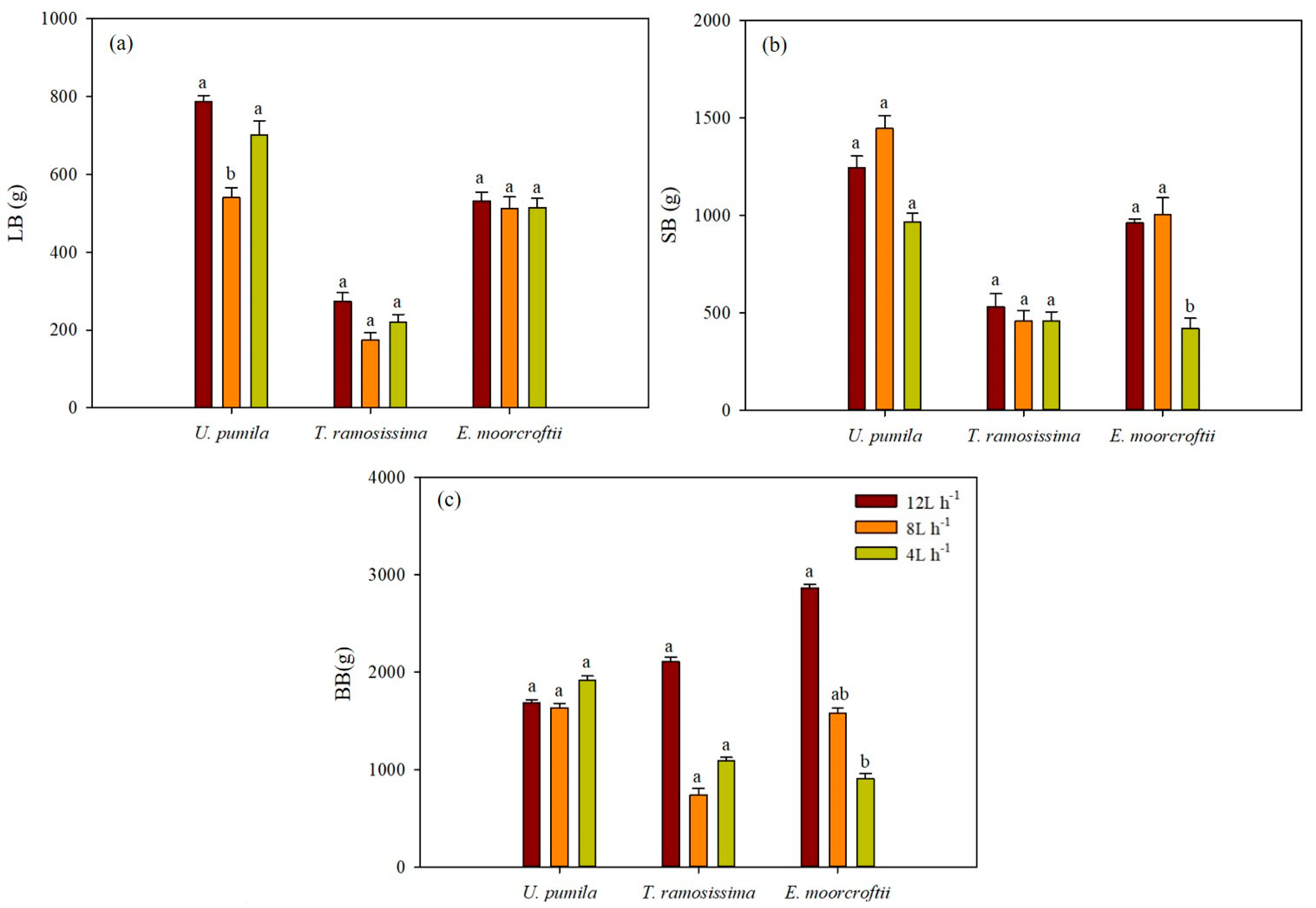
3.1. Effect of Irrigation Regimes on Morphological Traits of Above- and Below-Ground Organs of the Trees
3.2. Irrigation and Biomass Partitioning
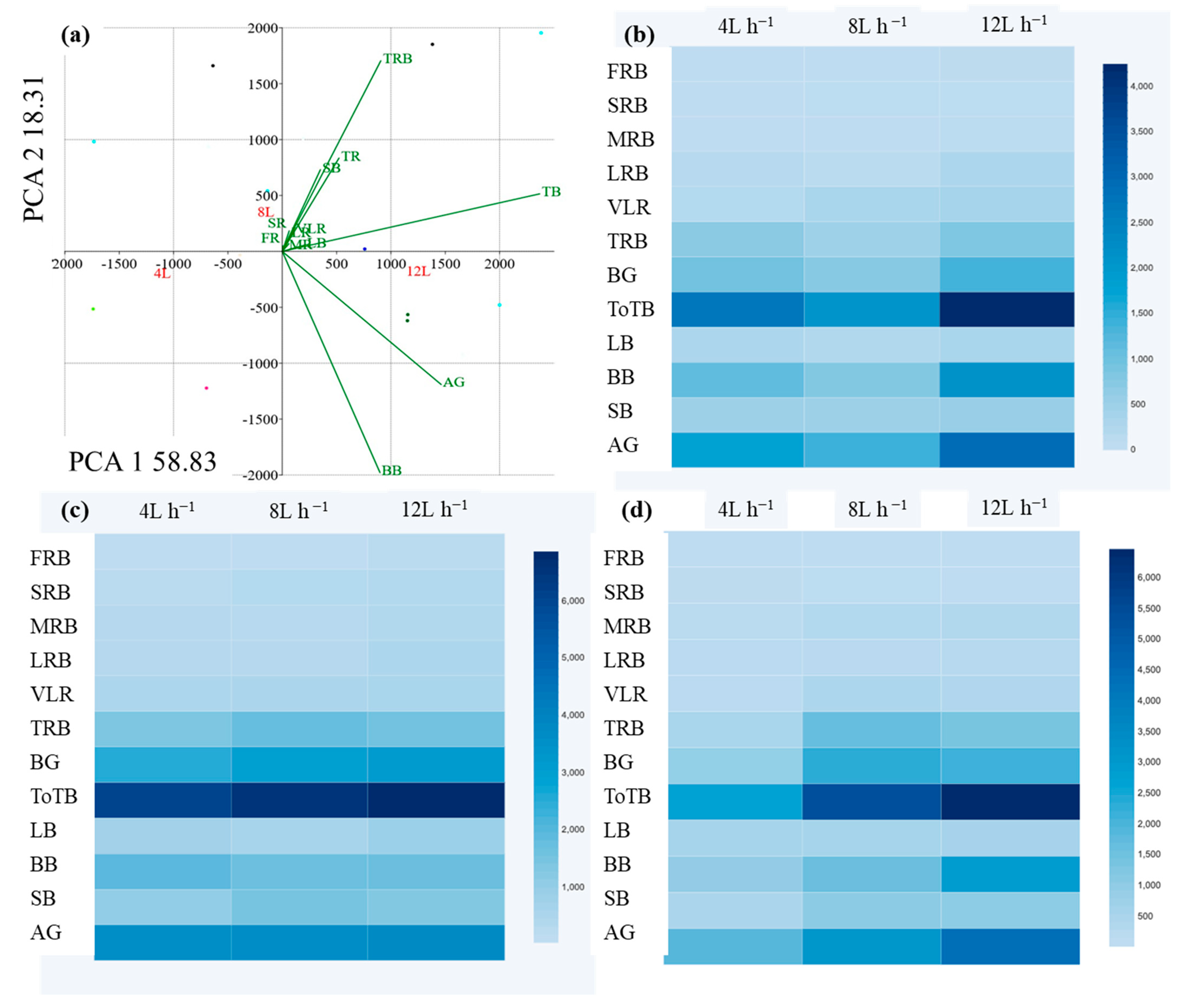
3.3. Statistical Analysis of the Variation of Biomass Partitioning between Above and Below-Ground Organs of Each Tree Species in Response to Different Irrigation Regimes
3.4. Carbon Content in the Tree
3.5. Soil Chemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Entezari Zarch, A.; Ahmadi, H.; Moeini, A.; Pazira, E. Impacts of environmental and human factors on desertification-induced land degradation in arid areas. Arab. J. Geosci. 2021, 14, 2447. [Google Scholar] [CrossRef]
- Byambadorj, S.-O.; Chiatante, D.; Akhmadi, K.; Lunten, J.; Ochirbat, B.; Park, B.B.; Scippa, G.S.; Montagnoli, A.; Nyam-Osor, B. The effect of different watering regimes and fertilizer addition on the growth of tree species used to afforest the semi-arid steppe of Mongolia. Plant Biosyst. 2021, 155, 747–758. [Google Scholar] [CrossRef]
- Munkhzul, O.; Oyundelger, K.; Narantuya, N.; Tuvshintogtokh, I.; Oyuntsetseg, B.; Wesche, K.; Jäschke, Y. Grazing Effects on Mongolian Steppe Vegetation—A Systematic Review of Local Literature. Front. Ecol. Evol. 2021, 9, 703220. [Google Scholar] [CrossRef]
- Kosmas, C.; Kairis, O.; Karavitis, C.; Acikalin, S.; Alcalá, M.; Alfama, P.; Atlhopheng, J.; Barrera, J.; Belgacem, A.; Solé-Benet, A.; et al. An exploratory analysis of land abandonment drivers in areas prone to desertification. Catena 2015, 128, 252–261. [Google Scholar] [CrossRef]
- Goffner, D.; Sinare, H.; Gordon, L.J. The great Green Wall for the Sahara and the Sahel initiative as an opportunity to enhance resilience in Sahelian landscapes and livelihoods. Reg. Environ. Chang. 2019, 19, 2139–2140. [Google Scholar] [CrossRef]
- Saley, I.A.; Salack, S.; Sanda, I.S.; Moussa, M.S.; Bonkaney, A.L.; Ly, M.; Fodé, M. The possible role of the Sahel Greenbelt on the occurrence of climate extremes over the West African Sahel. Atmos. Sci. Lett. 2019, 20, e927. [Google Scholar] [CrossRef]
- Liu, X. Comment on “why large-scale afforestation efforts in China have failed to solve the desertification problem”. Environ. Sci. Technol. 2008, 42, 8165. [Google Scholar] [CrossRef] [PubMed]
- Idris Medugu, N.; Rafee Majid, M.; Johar, F.; Choji, I.D. The role of afforestation programme in combating desertification in Nigeria. Int. J. Clim. Chang. Strateg. Manag. 2010, 2, 35–47. [Google Scholar] [CrossRef]
- Beiser, V. China’s Green Great Wall is on the Front Line of Its Fight against Desertification, but Is It Sustainable? Available online: https://www.scmp.com/magazines/post-magazine/long-reads/article/2177586/chinas-green-great-wall-front-line-itsfight (accessed on 12 October 2023).
- Sarlak, M.; Ferretti, L.V.; Biasi, R. The productive landscape in the desert margin for the sustainable development of rural settlements: An innovative greenbelt for maranjab desert in Iran. Sustainability 2021, 13, 2077. [Google Scholar] [CrossRef]
- MNE Report on the State of the Environment of Mongolia 2019–2020; Ministry of Environment and Tourism: Ulaanbaatar, Mongolia, 2020.
- National Committee on Combatting Desertification of Mongolia National Report on Voluntary Target Setting to Achieve Land Degradation Neutrality in Mongolia. 2018. Available online: https://www.unccd.int/our-work-impact/country-profiles/mongolia (accessed on 2 September 2023).
- Domke, G.M.; Oswalt, S.N.; Walters, B.F.; Morin, R.S. Tree planting has the potential to increase carbon sequestration capacity of forests in the United States. Proc. Natl. Acad. Sci. USA 2020, 117, 24649–24651. [Google Scholar] [CrossRef]
- Kassa, G.; Bekele, T.; Demissew, S.; Abebe, T. Above- and belowground biomass and biomass carbon stocks in homegarden agroforestry systems of different age groups at three sites of southern and southwestern Ethiopia. Carbon Manag. 2022, 13, 531–549. [Google Scholar] [CrossRef]
- Batkhuu, N.-O.; Khaulenbek, A.; Batkhishig, O.; Janchivdorj, L.; Bayasgalan, D.; Tsagaantsooj, N.; Ser-Oddamba, B.; Enkhchimeg, T.; Oyuntugs, A.; Zoljargal, S.; et al. Afforestation Practice in Arid and Semi-Arid Regions of Mongolia; Chultem, D., Ed.; Hunnu Press LLC: Ulaanbaatar, Mongolia, 2018; ISBN 978-99978-4-889-5. [Google Scholar]
- Avirmed, T.; Byambadorj, S.-O.; Chiatante, D.; Sharavdorj, K.; Ganbat, B.; Sukhbaatar, G.; Nyam-Osor, B. Afforestation of semi-arid regions of Mongolia: Carbon sequestration in trees and increase of soil organic carbon. Plant Biosyst.—An Int. J. Deal. All Asp. Plant Biol. 2023, 157, 1. [Google Scholar] [CrossRef]
- Byambadorj, S.O.; Hernandez, J.O.; Lkhagvasuren, S.; Erma, G.; Sharavdorj, K.; Park, B.B.; Nyam-Osor, B. Leaf morpho-physiological traits of Populus sibirica and Ulmus pumila in different irrigation regimes and fertilizer types. PeerJ 2023, 11, e16107. [Google Scholar] [CrossRef] [PubMed]
- Ser-Oddamba, B.; Khaulenbek, A.; Janchivdorj, L.; Batkhishig, O.; Park, B.B.; Batkhuu, N.-O. The Effect of Different Watering Regimes on the Growth of Trees and Shrubs in Desert Regions of Mongolia. Mong. J. Biol. Sci. 2020, 18, 55–64. [Google Scholar] [CrossRef]
- Nyam-Osor, B.; Byambadorj, S.; Park, B.B.; Terzaghi, M.; Gabriella, S.S.; Stanturf, J.A.; Chiatante, D.; Montagnoli, A. Root Biomass Distribution of Populus sibirica and Ulmus pumila Afforestation Stands Is Affected by Watering Regimes and Fertilization in the Mongolian Semi-arid Steppe. Front. Plant Sci. 2021, 12, 638828. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, A.; Lasserre, B.; Terzaghi, M.; Byambadorj, S.O.; Nyam-Osor, B.; Scippa, G.S.; Chiatante, D. Fertilization reduces root architecture plasticity in Ulmus pumila used for afforesting Mongolian semi-arid steppe. Front. Plant Sci. 2022, 13, 878299. [Google Scholar] [CrossRef] [PubMed]
- Byambadorj, S.-O.; Nyam-Osor, B.; Park, B.B.; Avirmed, T.; Scippa, G.S.; Chiatante, D.; Montagnoli, A.; Dimitrova, A. Afforestation of Mongolian steppe: Patterns of biomass partitioning in Populus sibirica and Ulmus pumila trees in response to management supporting measures. Plant Biosyst.—An Int. J. Deal. All Asp. Plant Biol. 2021, 156, 969–981. [Google Scholar] [CrossRef]
- Cho, S.; Ser-Oddamba, B.; Batkhuu, N.-O.; Seok Kim, H. Comparison of water use efficiency and biomass production in 10-year-old Populus sibirica and Ulmus pumila plantations in Lun soum, Mongolia. Forest Sci. Technol. 2019, 15, 147–158. [Google Scholar] [CrossRef]
- Chen, S.; Wang, D.; Sheng, X.; Zhang, C.; Li, W.; Xiao, N.; Wang, L.; Lu, Z. Comparison of Stomatal Structure and Distribution between Ovules and Leaves in Ginkgo biloba. Forests 2022, 13, 1801. [Google Scholar] [CrossRef]
- Yan, X.L.; Dai, T.F.; Jia, L.M. Evaluation of the cumulative effect of drip irrigation and fertigation on productivity in a poplar plantation. Ann. For. Sci. 2018, 75, 5. [Google Scholar] [CrossRef]
- Byambadorj, S.-O.; Park, B.B.; Hernandez, J.O.; Dulamsuren, N.; Sainbuyan, Z.; Altantugs, O.; Sharavdorj, K.; Seong, I.K. Optimal Irrigation Regime for Woody Species Potentially Suitable for Effective and Sustainable Afforestation in the Desert Region of Mongolia. Land 2021, 10, 212. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world—Challenges and progress: Introduction to a Virtual Issue on carbon allocation: Introduction to a virtual issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, Y.; Tissue, D.T.; Bruna, S.; Maier, C.; Dijkstra, F.A. Drought Impacts on Tree Root Traits Are Linked to Their Decomposability and Net Carbon Release. Front. For. Glob. Change 2022, 5, 836062. [Google Scholar] [CrossRef]
- Ma, X.; Ma, F.; Li, C.; Mi, Y.; Bai, T.; Shu, H. Biomass accumulation, allocation, and water-use efficiency in 10 Malus rootstocks under two watering regimes. Agrofor. Syst. 2010, 80, 283–294. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Fang, C.; Chen, J.; Li, B. The effects of plantation practice on soil properties based on the comparison between natural and planted forests: A meta-analysis. Glob. Ecol. Biogeogr. 2012, 21, 318–327. [Google Scholar] [CrossRef]
- Ulziykhutag, N. Overview of the Flora of Mongolia; State Publishing: Ulaanbaatar, Mongolia, 1989. [Google Scholar]
- Wesche, K.; Miehe, S.; Miehe, G. Plant communities of the Gobi Gurvan Sayhan National Park (South Gobi Aymak, Mongolia). Candollea 2005, 60, 149–205. [Google Scholar]
- Batkhuu, N.-O.; Khaulenbek, A.; Bayasgalan, D.; Batkhishig, O.; Janchivdorj, L.; Tsagaantsooj, N.; Ser-Oddamba, B. Mongolia-Korea Joint “Green Belt” Project Research Report; Art Soft LLC: Ulaanbaatar, Mongolia, 2012. [Google Scholar]
- NAMEM The National Agency for Meteorology and Environmental Monitoring of Mongolia. Weather Data. Available online: http://namem.gov.mn/eng/?p=56 (accessed on 2 October 2020).
- Batkhishig, O. Soil classification of Mongolia. J. Mong. Soil Sci. 2016, 1, 18–31. [Google Scholar]
- Thompson, J.R.; Schultz, R.C. Root system morphology of Quercus rubra L. planting stock and 3-year field performance in Iowa. New For. 1995, 9, 225–236. [Google Scholar] [CrossRef]
- Böhm, W. Methods of Studying Root Systems; Springer-Verlag: Berlin, Germany, 1979; ISBN 978-3-642-67282-8. [Google Scholar]
- Zobel, R.W.; Waisel, Y. A plant root system architectural taxonomy: A framework for root nomenclature. Plant Biosyst. 2010, 144, 507–512. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Brown, S.L.; Birdsey, R.A. Measurement Guidelines for the Sequestration of Forest Carbon; US Department of Agriculture, Forest Service, Northern Research Station: Washington, DC, USA, 2007; Volume 18, 42p.
- Chow, P.; Rolfe, G. Carbon and hydrogen contents of short-rotation biomass of five hardwood species. Wood fiber Sci. 1989, 21, 30–36. [Google Scholar]
- MacDicken, K.G. A Guide to Monitoring Carbon Storage in Forestry and Agroforestry Projects; Winrock International Institute for Agricultural Development: Arlington, VA, USA, 1997. [Google Scholar]
- Eggleston, H.S.; Miwa, K.; Srivastava, N.; Tanabe, K. IPCC 2006 IPCC Guidelines for National Greenhouse Inventories—A Primer, Prepared by the National Greenhouse Gas Inventories Programme. Available online: https://www.osti.gov/etdeweb/biblio/20880391 (accessed on 10 October 2023).
- Thurner, M.; Beer, C.; Santoro, M.; Carvalhais, N.; Wutzler, T.; Schepaschenko, D.; Shvidenko, A.; Kompter, E.; Ahrens, B.; Levick, S.R.; et al. Carbon stock and density of northern boreal and temperate forests. Glob. Ecol. Biogeogr. 2014, 23, 297–310. [Google Scholar] [CrossRef]
- Meragiaw, M.; Singh, B.R.; Woldu, Z.; Martinsen, V. Carbon stocks of above- And belowground tree biomass in Kibate Forest around Wonchi Crater Lake, Central Highland of Ethiopia. PLoS ONE 2021, 16, e0254231. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D., Ed.; SSSA, ASA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- ASTM D4373-96; Standard Test Method for Calcium Carbonate Content of Soils. ASTM International: West Conshohocken, PA, USA, 1996.
- ISO 10390:2001; Soil Quality. Determination of pH. International Standard: Vernier, Geneva, 2001.
- MNS 3310:1991; The Soil. Methods of Determination of the Agrochemical Characteristics of Soil. International Standard: Vernier, Geneva, 1991.
- SSIR Soil survey laboratory methods manual. In Soil Survey Investigations Report No.42; Burt, R. (Ed.) USDA-NRCS: Lincoln, NE, USA, 2004; pp. 610–613. [Google Scholar]
- SAS Institute Inc. SAS Software 9.4; SAS Inst. Inc.: Cary, NC, USA, 2014; p. 25. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis, Version 4.03; Palaeontologia Electronica: Seattle, DC, USA, 2001. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing 2021. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 15 April 2023).
- Systat Software, I. Sigma Plot Version 12.5; Systat Softw. Inc.: San Jose, CA, USA, 2013. [Google Scholar]
- FAO Helping Forests Take Cover: On forest Protection, Increasing Forest Cover and Future Approaches to Reforesting Degraded Tropical Landscapes in Asia and the Pacific; FOA: Bangkok, Thailand, 2005.
- Del Campo, A.D.; González-Sanchis, M.; García-Prats, A.; Ceacero, C.J.; Lull, C. The impact of adaptive forest management on water fluxes and growth dynamics in a water-limited low-biomass oak coppice. Agric. For. Meteorol. 2019, 264, 266–282. [Google Scholar] [CrossRef]
- Kambo, D.; Danby, R. Factors influencing the establishment and growth of tree seedlings at Subarctic alpine treelines.pdf. Ecosphere 2018, 9, e02176. [Google Scholar] [CrossRef]
- García-Morote, F.A.; López-Serrano, F.R.; Martínez-García, E.; Andrés-Abellán, M.; Dadi, T.; Candel, D.; Rubio, E.; Lucas-Borja, M.E. Stem biomass production of Paulownia elongata × P. fortunei under low irrigation in a semi-arid environment. Forests 2014, 5, 2505–2520. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, X.; Yi, H.; Li, Y.; Xu, X.; He, J.; He, L. Plant diversity drives soil carbon sequestration: Evidence from 150 years of vegetation restoration in the temperate zone. Front. Plant Sci. 2023, 14, 1191704. [Google Scholar] [CrossRef] [PubMed]
- Aponte, C.; Kasel, S.; Nitschke, C.R.; Tanase, M.A.; Vickers, H.; Parker, L.; Fedrigo, M.; Kohout, M.; Ruiz-Benito, P.; Zavala, M.A.; et al. Structural diversity underpins carbon storage in Australian temperate forests. Glob. Ecol. Biogeogr. 2020, 29, 789–802. [Google Scholar] [CrossRef]
- Besnard, S.; Santoro, M.; Cartus, O.; Fan, N.; Linscheid, N.; Nair, R.; Weber, U.; Koirala, S.; Carvalhais, N. Global sensitivities of forest carbon changes to environmental conditions. Glob. Change Biol. 2021, 27, 6467–6483. [Google Scholar] [CrossRef]
- Poudel, K.P.; Temesgen, H. Methods for estimating aboveground biomass and its components for Douglas-fir and lodgepole pine trees. Can. J. For. Res. 2015, 46, 77–87. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants—An economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Herrera-Ramírez, D.; Muhr, J.; Hartmann, H.; Römermann, C.; Trumbore, S.; Sierra, C.A. Probability distributions of nonstructural carbon ages and transit times provide insights into carbon allocation dynamics of mature trees. New Phytol. 2020, 226, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xi, B.; Li, G.; Wang, Y.; Jia, L.; Zhao, D. Influence of drip irrigation, nitrogen fertigation, and precipitation on soil water and nitrogen distribution, tree seasonal growth and nitrogen uptake in young triploid poplar (Populus tomentosa) plantations. Agric. Water Manag. 2021, 243, 106460. [Google Scholar] [CrossRef]
- Abdoun, K.A.; Altahir, O.A.; Alsagan, A.A.; Alsaiady, M.Y.; Alshaikhi, A.M.; Alshamiry, F.A.; Al-Haidary, A.A. Effect of irrigation frequency and selenium fertilization on the vegetative growth and biomass yield of Moringa oleifera and Moringa peregrina. Sci. Rep. 2022, 12, 22379. [Google Scholar] [CrossRef] [PubMed]
- Noulèkoun, F.; Khamzina, A.; Naab, J.B.; Lamers, J.P.A. Biomass allocation in five semi-arid afforestation species is driven mainly by ontogeny rather than resource availability. Ann. For. Sci. 2017, 74, 78. [Google Scholar] [CrossRef]
- He, Y.; Xi, B.; Bloomberg, M.; Jia, L.; Zhao, D. Effects of drip irrigation and nitrogen fertigation on stand growth and biomass allocation in young triploid Populus tomentosa plantations. For. Ecol. Manag. 2020, 461, 117937. [Google Scholar] [CrossRef]
- Imada, S.; Matsuo, N.; Acharya, K.; Yamanaka, N. Effects of salinity on fine root distribution and whole plant biomass of Tamarix ramosissima cuttings. J. Arid Environ. 2015, 114, 84–90. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, W.; Li, R. Visual preference of trees: The effects of tree attributes and seasons. Urban For. Urban Green. 2017, 25, 19–25. [Google Scholar] [CrossRef]
- David, M.; Eissenstat, R.D.Y. Root Life Span, Efficiency, and Turnover. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; p. 28. ISBN 9780429221859. [Google Scholar]
- Gu, J.; Wang, Y.; Fahey, T.J.; Wang, Z. Effects of root diameter, branch order, soil depth and season of birth on fine root life span in five temperate tree species. Eur. J. For. Res. 2017, 136, 727–738. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Galiano, L.; Gessler, A. Plasticity of fine-root traits under long-term irrigation of a water-limited scots pine forest. Front. Plant Sci. 2019, 10, 701. [Google Scholar] [CrossRef]
- Liu, F.; You, Q.; Xue, X.; Peng, F.; Huang, C.; Ma, S.; Pan, J.; Shi, Y.; Chen, X. The Stem Sap Flow and Water Sources for Tamarix ramosissima in an Artificial Shelterbelt With a Deep Groundwater Table in Northwest China. Front. Plant Sci. 2022, 13, 794084. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Norby, R.J.; Andersen, K.M.; Valverde-Barrantes, O.; Fuchslueger, L.; Oblitas, E.; Hartley, I.P.; Iversen, C.M.; Gonçalves, N.B.; Takeshi, B.; et al. Fine-root dynamics vary with soil depth and precipitation in a low-nutrient tropical forest in the Central Amazonia. Plant-Environ. Interact. 2020, 1, 3–16. [Google Scholar] [CrossRef]
- Vieira, D.A.; Martins, T.O.; Matias, R.A.M.; Silva, P.H.F.; Valadão, M.B.X.; Barbosa, P.V.G.; Gatto, A.; Pinto, J.R.R.; Bussinguer, A.P.; Ribeiro, F.P. Relationships between soil properties and species establishment in the restoration of mined soils in the Cerrado biome. PLoS ONE 2022, 17, e0277193. [Google Scholar] [CrossRef]
- He, Z.; Hu, Q.; Zhang, Y.; Cao, H.; Nan, X. Effects of irrigation and nitrogen management strategies on soil nitrogen and apple yields in loess plateau of China. Agric. Water Manag. 2023, 280, 108220. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, W.; Ma, Y.; Wang, X.; Li, Z.; Zhai, B.; Wang, Z. Responses of soil water, nitrate and yield of apple orchard to integrated soil management in Loess Plateau, China. Agric. Water Manag. 2020, 240, 106325. [Google Scholar] [CrossRef]



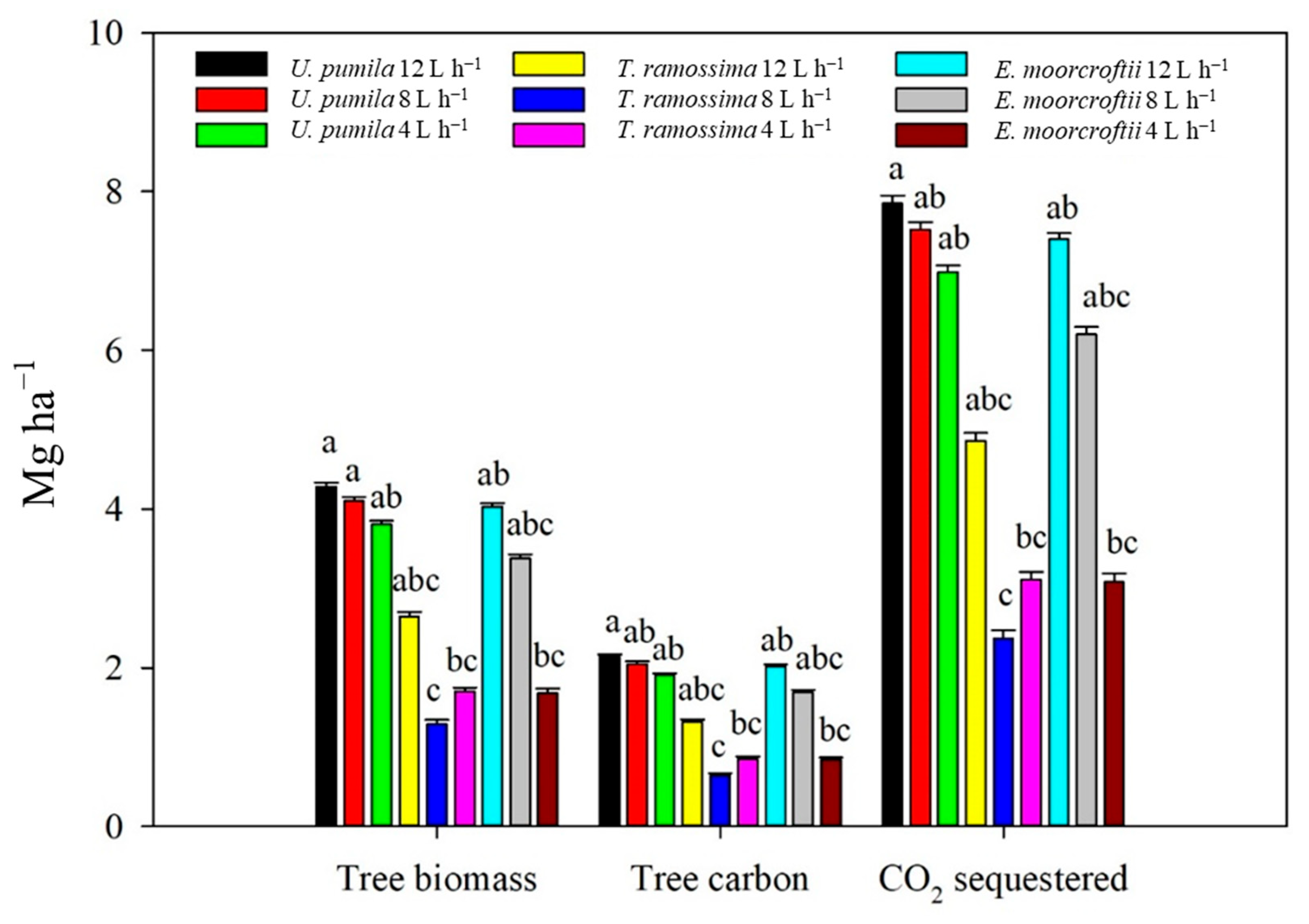
| Plant Species | Treatments | %, LB/ToTB | %, BB/ToTB | %, SB/ToTB | %, AG/ToTB | %, BG/ToTB |
|---|---|---|---|---|---|---|
| U. pumila | 12 L h−1 | 11.49 ± 1.29 a | 24.62 ± 1.58 b | 18.2 ± 1.43 a,b | 54.32 ± 5.05 a | 45.67 ± 3.45 a |
| 8 L h−1 | 8.23 ± 1.13 b | 24.94 ± 1.65 b | 22.05 ± 1.44 a | 55.24 ± 5.12 a | 44.75 ± 3.48 a | |
| 4 L h−1 | 11.5 ± 1.25 a | 31.5 ± 1.72 a | 15.86 ± 1.38 b | 58.87 ± 5.35 a | 41.12 ± 3.21 a | |
| T. ramosissima | 12 L h−1 | 6.47 ± 0.98 b | 49.74 ± 2.58 a | 12.5 ± 1.12 b | 68.72 ± 5.14 a | 31.27 ± 2.24 a |
| 8 L h−1 | 8.43 ± 1.25 a | 35.95 ± 2.85 b | 22.29 ± 1.25 a | 66.68 ± 6.12 a | 33.31 ± 2.35 a | |
| 4 L h−1 | 8.12 ± 1.21 a | 40.17 ± 1.89 a,b | 16.82 ± 1.24 a,b | 65.11 ± 5.12 a | 34.88 ± 2.65 a | |
| E. moorcroftii | 12 L h−1 | 8.24 ± 1.28 b | 44.42 ± 5.42 a | 14.9 ± 2.85 a | 67.56 ± 6.87 a | 32.43 ± 3.18 a |
| 8 L h−1 | 9.49 ± 1.56 b | 29.26 ± 3.48 b | 18.63 ± 2.47 a | 57.39 ± 6.49 b | 42.6 ± 3.25 a | |
| 4 L h−1 | 19.16 ± 2.36 a | 33.82 ± 3.87 a | 15.62 ± 2.34 a | 68.61 ± 6.98 a | 31.38 ± 2.98 a |
| Plant Species | Treatments | %, TRB/BG | %, FRB/BG | %, SRB/BG | %, MRB/BG | %, LRB/BG | %, VLRB/BG |
|---|---|---|---|---|---|---|---|
| U. pumila | 12 L h−1 | 48.87 ± 4.03 a | 3.76 ± 1.14 a | 9.32 ± 1.59 a | 9.89 ± 1.64 a | 12.73 ± 1.54 a | 15.39 ± 2.25 a |
| 8 L h−1 | 58.61 ± 4.65 a | 2.45 ± 1.08 ab | 8.53 ± 1.48 a,b | 8.32 ± 1.58 a | 9.18 ± 1.39 b | 16.1 ± 2.37 a | |
| 4 L h−1 | 53.67 ± 4.69 a | 1.47 ± 0.98 b | 6.39 ± 1.35 b | 10.99 ± 1.72 a | 11.06 ± 1.42 a | 16.38 ± 2.39 a | |
| T. ramosissima | 12 L h−1 | 59.11 ± 4.12 b | 1.82 ± 0 | 1.08 ± 0.12 a,b | 4.15 ± 0.85 a | 16.93 ± 2.45 a | 25.13 ± 2.24 a,b |
| 8 L h−1 | 63.76 ± 4.54 a,b | - | 2.7 ± 0.01 a | 2.9 ± 0.02 a,b | 7.63 ± 1.11 b | 31.13 ± 2.35 a | |
| 4 L h−1 | 72.35 ± 5.51 a | - | 0.28 ± 0.01 b | 2.11 ± 0.02 b | 12.54 ± 1.45 a | 14.19 ± 2.14 b | |
| E. moorcroftii | 12 L h−1 | 63.22 ± 7.01 ab | 0.68 ± 0.01 b | 2.35 ± 1.01 b | 13.61 ± 1.25 a | 9.6 ± 2.11 b | 15.05 ± 2.14 a |
| 8 L h−1 | 69.73 ± 6.52 a | 1.18 ± 0.02 b | 1.72 ± 0.98 b | 10.76 ± 1.12 a | 7.63 ± 1.85 b | 17.23 ± 2.16 a | |
| 4 L h−1 | 52.54 ± 6.12 b | 2.5 ± 0.02 a | 6.3 ± 1.25 a | 16.42 ± 1.36a | 13.25 ± 1.26 a | 13.42 ± 2.05 a |
| Plant Species | Treatments | pH | CaCO3 | Organic Matter | EC2.5 | Nitrate- Nitrogen | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|
| % | % | dS/m | mg kg−1 | mg/100 g | ||||
| U. pumila | 12 L h−1 | 8.14 ± 0.03 a | 4.25 ± 0.13 b | 1.27 ± 0.03 b | 0.109 ± 0.007 b | 0.66 ± 0.03 c | 4.39 ± 0.08 a | 9.2 ± 0.11 a |
| 8 L h−1 | 8.09 ± 0.05 a | 7.28 ± 0.04 a,b | 1.32 ± 0.03 a,b | 0.138 ± 0.008 a,b | 3.18 ± 0.06 a | 1.08 ± 0.10 b | 8.0 ± 0.05 a | |
| 4 L h−1 | 8.42 ± 0.13 a | 9.67 ± 0.17 a | 1.41 ± 0.03 a | 0.241 ± 0.04 a | 1.35 ± 0.05 b | 1.66 ± 0.04 a,b | 7.4 ± 0.1 a | |
| T. ramosissima | 12 L h−1 | 7.83 ± 0.03 a | 5.45 ± 0.02 b | 1.83 ± 0.06 a | 0.163 ± 0.005 a | 0.87 ± 0.05 c | 1.39 ± 0.04 a | 9.8 ± 0.39 a |
| 8 L h−1 | 8.11 ± 0.05 a | 5.45 ± 0.12 b | 1.25 ± 0.07 c | 0.155 ± 0.02 b | 1.28 ± 0.03 b | 0.75 ± 0.07 a | 9.8 ± 0.60 a | |
| 4 L h−1 | 7.99 ± 0.05 a | 10.91 ± 0.05 a | 1.45 ± 0.02 b | 0.163 ± 0.005 a | 2.38 ± 0.03 a | 2.24 ± 0.11 a | 8.0 ± 0.20 a | |
| E. moorcroftii | 12 L h−1 | 8.14 ± 0.02 a | 11.51 ± 0.28 a | 1.23 ± 0.05 a | 0.126 ± 0.01 a | 3.58 ± 0.07 b | 1.27 ± 0.03 a,b | 6.2 ± 0.03 b |
| 8 L h−1 | 8.13 ± 0.08 a | 7.88 ± 0.01 a,b | 1.20 ± 0.05 a | 0.106 ± 0.01 a | 4.55 ± 0.12 c | 0.17 ± 0.01 b | 6.2 ± 0.23 b | |
| 4 L h−1 | 8.03 ± 0.08 a | 4.45 ± 0.24 b | 1.78 ± 0.06 a | 0.189 ± 0.01 a | 6.92 ± 0.10 a | 3.87 ± 0.03 a | 11.2 ± 0.39 a | |
| Control | - | 8.02 ± 0.07 | 10.91 ± 0.20 | 1.49 ± 0.04 | 0.132 ± 0.02 | 2.85 ± 0.07 | 3.93 ± 0.12 | 10.5 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byambadorj, S.-O.; Park, B.B.; Lkhagvasuren, S.; Tsedensodnom, E.; Byambasuren, O.; Khajid, A.; Chiatante, D.; Nyam-Osor, B. Influence of Irrigation on Biomass Partitioning in Above- and Belowground Organs of Trees Planted in Desert Sites of Mongolia. Forests 2024, 15, 46. https://doi.org/10.3390/f15010046
Byambadorj S-O, Park BB, Lkhagvasuren S, Tsedensodnom E, Byambasuren O, Khajid A, Chiatante D, Nyam-Osor B. Influence of Irrigation on Biomass Partitioning in Above- and Belowground Organs of Trees Planted in Desert Sites of Mongolia. Forests. 2024; 15(1):46. https://doi.org/10.3390/f15010046
Chicago/Turabian StyleByambadorj, Ser-Oddamba, Byung Bae Park, Sarangua Lkhagvasuren, Enkhchimeg Tsedensodnom, Otgonsaikhan Byambasuren, Altankhundaga Khajid, Donato Chiatante, and Batkhuu Nyam-Osor. 2024. "Influence of Irrigation on Biomass Partitioning in Above- and Belowground Organs of Trees Planted in Desert Sites of Mongolia" Forests 15, no. 1: 46. https://doi.org/10.3390/f15010046
APA StyleByambadorj, S.-O., Park, B. B., Lkhagvasuren, S., Tsedensodnom, E., Byambasuren, O., Khajid, A., Chiatante, D., & Nyam-Osor, B. (2024). Influence of Irrigation on Biomass Partitioning in Above- and Belowground Organs of Trees Planted in Desert Sites of Mongolia. Forests, 15(1), 46. https://doi.org/10.3390/f15010046










