Growth Responses to Climate and Drought in Relict Cork Oak Populations as a Benchmark of the Species Tolerance
Abstract
1. Introduction
2. Materials and Methods
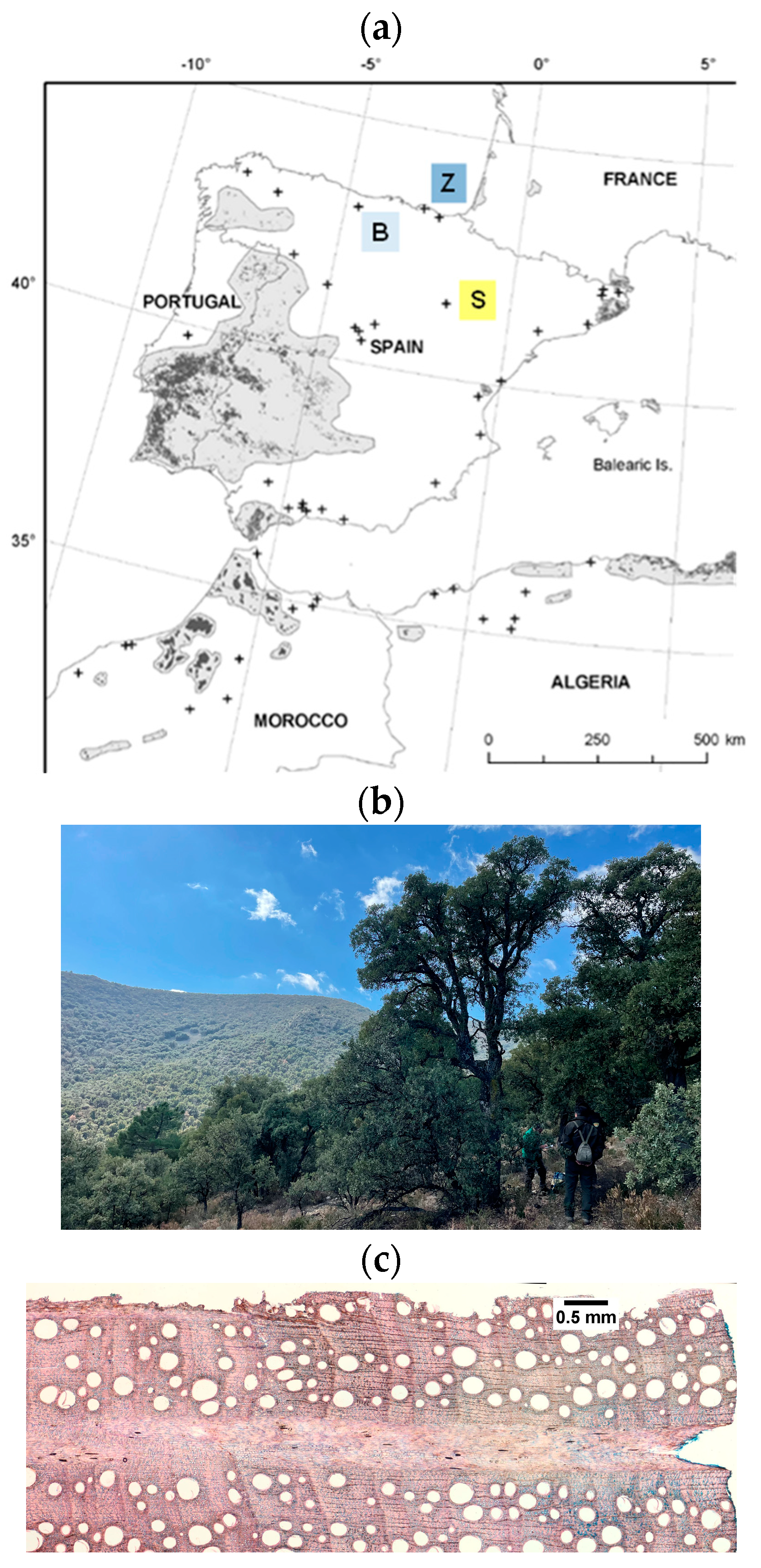
2.1. Study Sites and Field Sampling
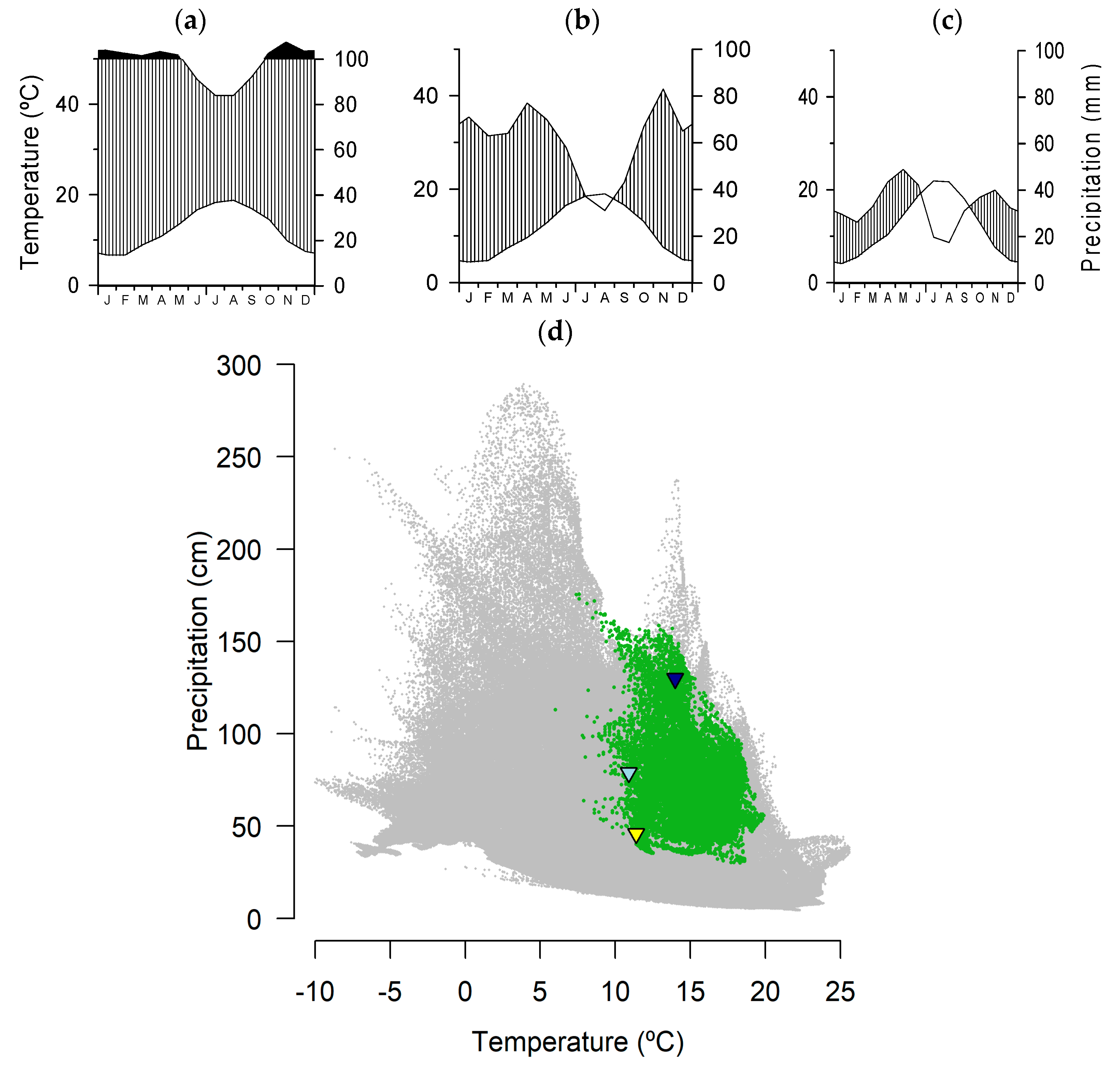
2.2. Climate Data and Drought Index
2.3. Processing Wood Samples and Tree-Ring Width Data
2.4. Statistical Analyses
3. Results
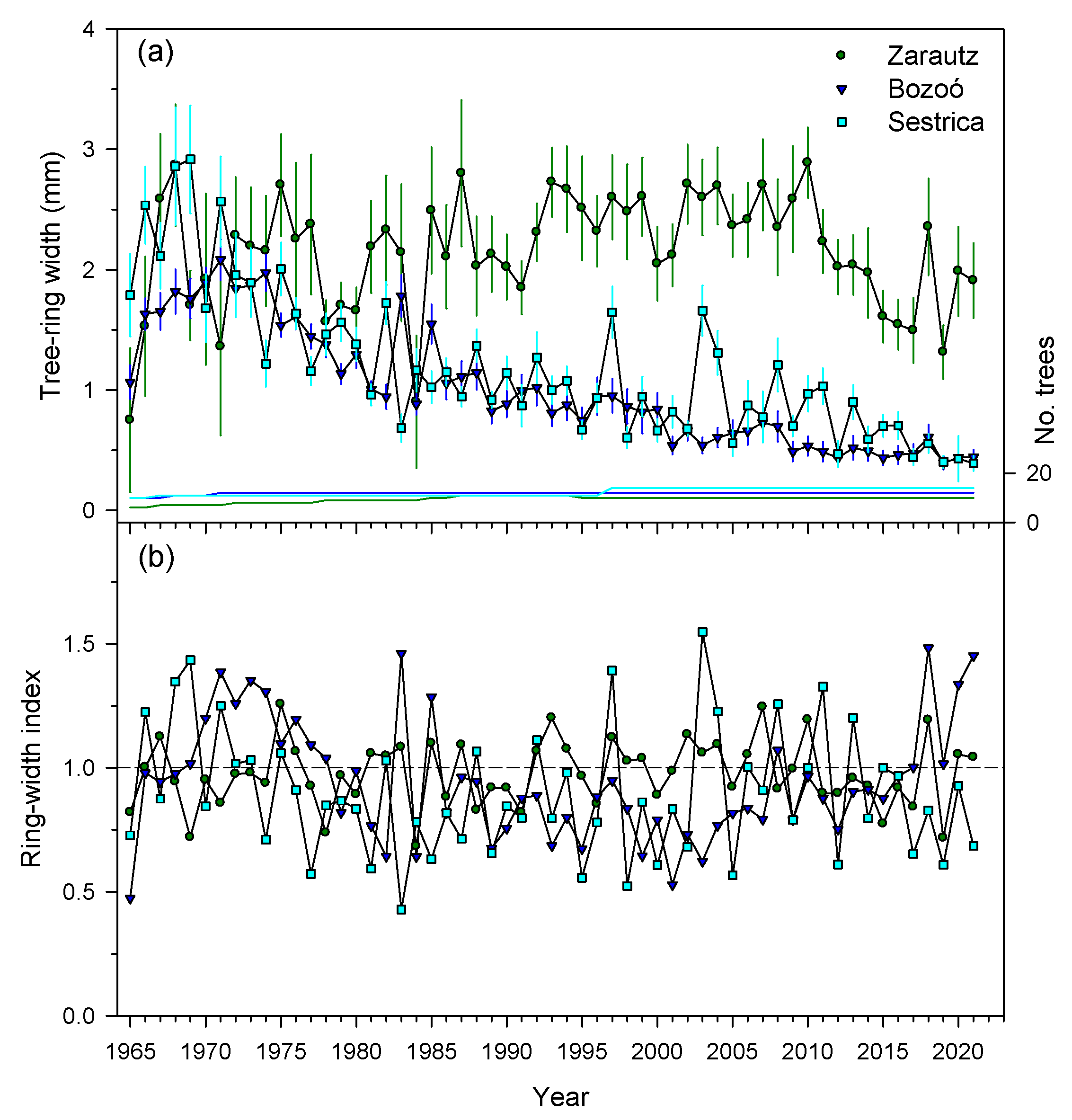
3.1. Growth Patterns and Variability
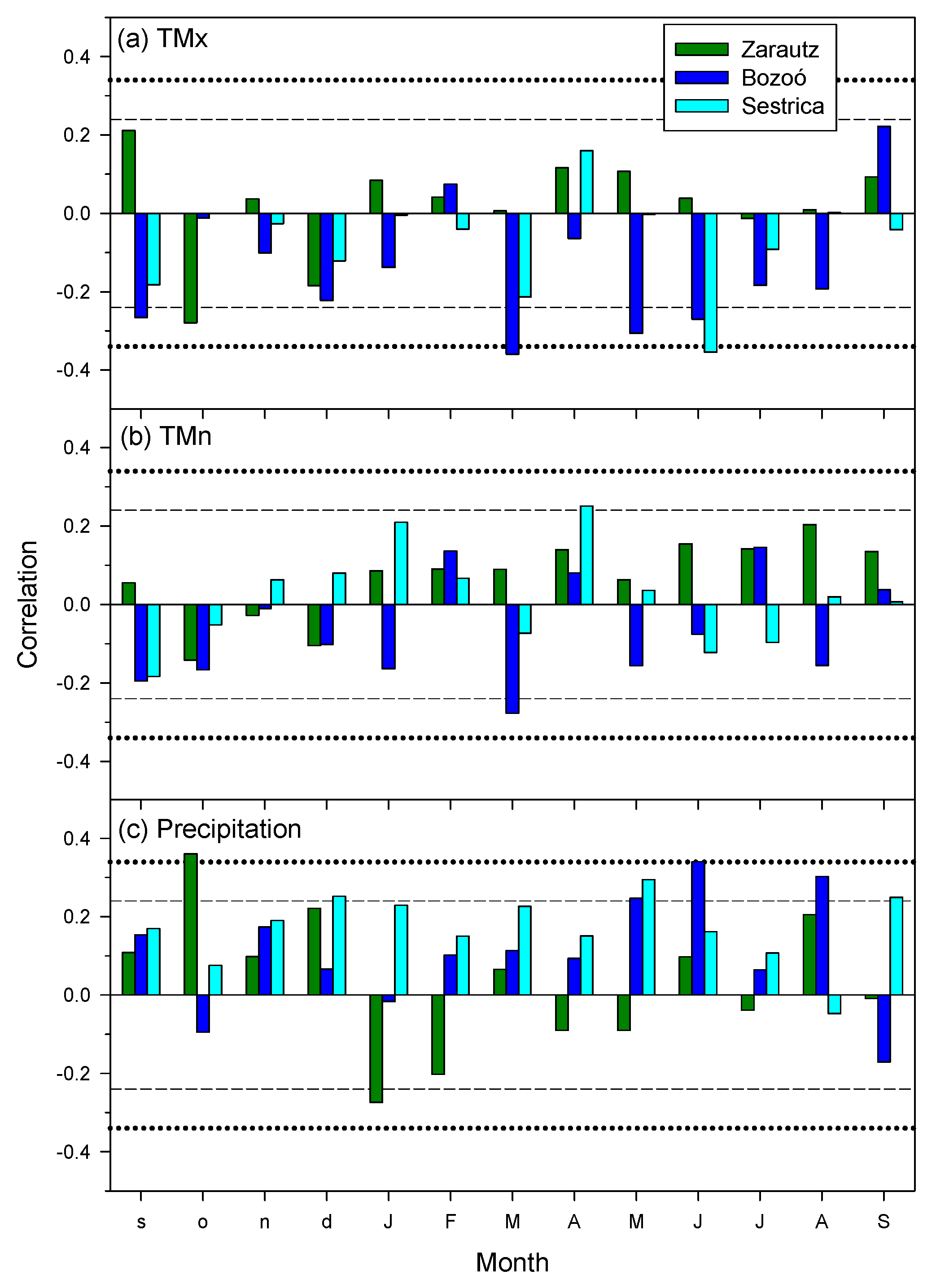
3.2. Relationships between Climate Variables, Drought and Growth Indices
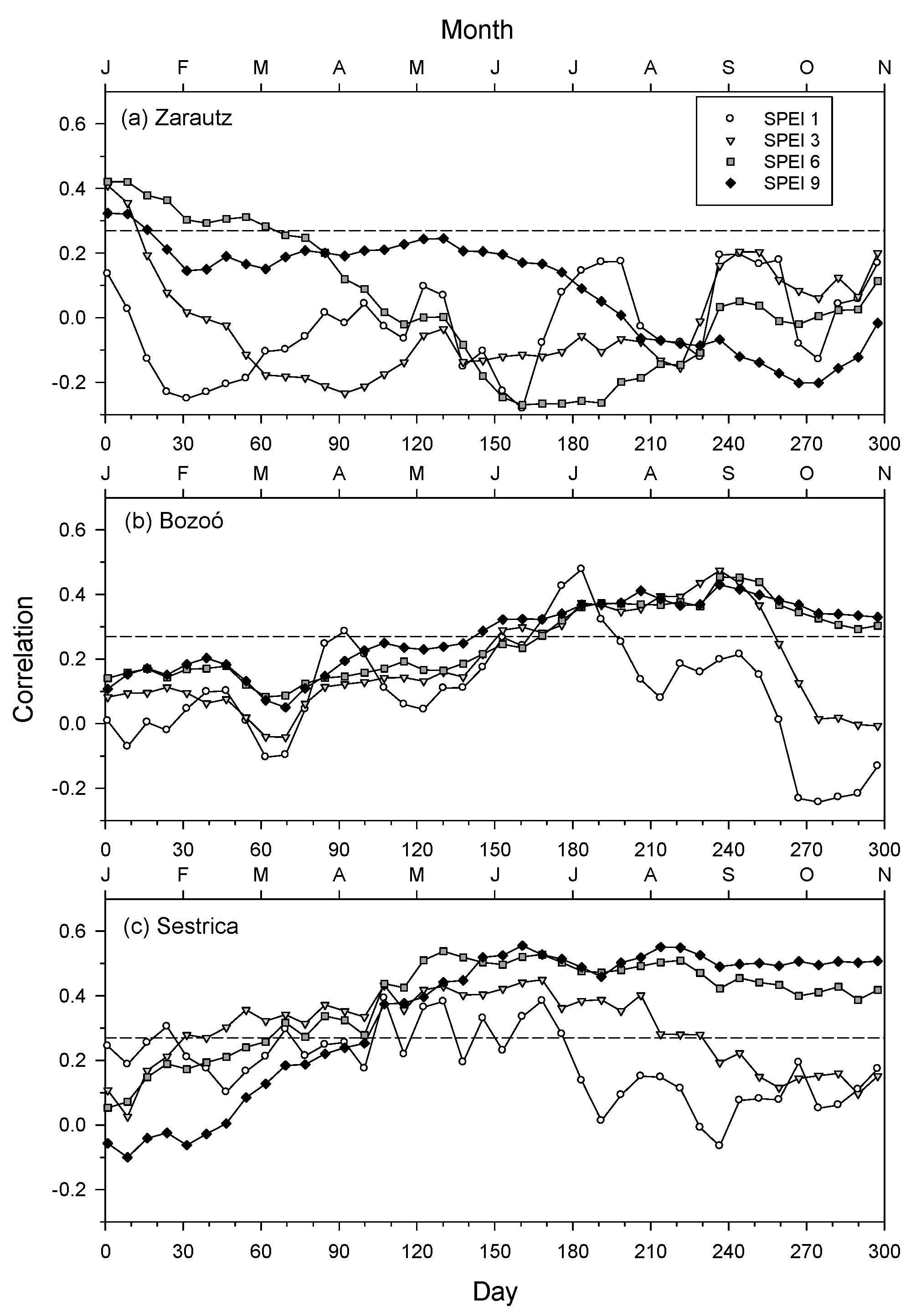
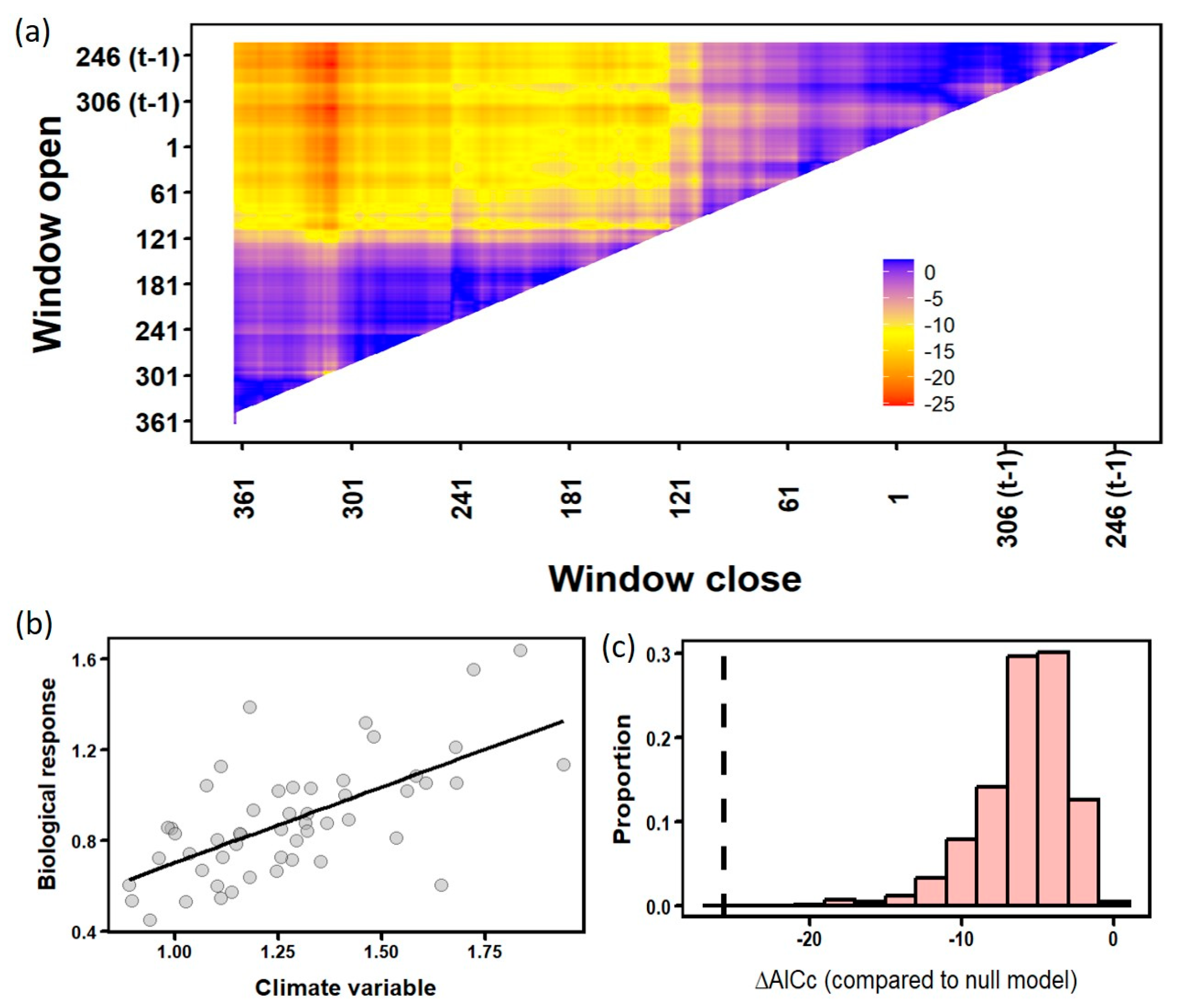
3.3. Analyses of Climate–Growth Relationships Based on Climwin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Chang. 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Aronson, J.; Pereira, J.S.; Pausas, J.G. (Eds.) Cork Oak Woodlands on the Edge: Ecology, Adaptive Management, and Restoration; Island Press: Washington, DC, USA, 2009. [Google Scholar]
- Brasier, C.M. Oak tree mortality in Iberia. Nature 1992, 360, 539. [Google Scholar] [CrossRef]
- David, T.S.; Cabral, M.T.; Sardinha, R. A mortalidade dos sobreiros e a seca. Finisterra 1992, 27, 17–24. [Google Scholar]
- Costa, A.; Pereira, H.; Madeira, M. Analysis of spatial patterns of oak decline in cork oak woodlands in Mediterranean conditions. Ann. For. Sci. 2010, 67, 204. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-induced oak decline in the western mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iForest-Biogeosci. For. 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Sánchez-Cuesta, R.; Ruiz-Gómez, F.J.; Duque-Lazo, J.; González-Moreno, P.; Navarro-Cerrillo, R.M. The environmental drivers influencing spatio-temporal dynamics of oak defoliation and mortality in dehesas of Southern Spain. For. Ecol. Manag. 2021, 485, 118946. [Google Scholar] [CrossRef]
- Sánchez-González, M.; Beltrán, R.S.; Lanzo Palacios, R.; Prades, C. Analysis of cork quality and cork tree health in stands of western Spain. For. Ecol. Manag. 2023, 539, 121012. [Google Scholar] [CrossRef]
- Touhami, I.; Chirino, E.; Aouinti, H.; Khorchani, A.E.; Elaieb, M.T.; Khaldi, A.; Nasr, Z. Decline and dieback of cork oak (Quercus suber L.) forests in the Mediterranean basin: A case study of Kroumirie, Northwest Tunisia. J. For. Res. 2020, 31, 1461–1477. [Google Scholar] [CrossRef]
- Palma, J.H.N.; Paulo, J.A.; Faias, S.P.; Garcia-Gonzalo, J.; Borges, J.G.; Tomé, M. Adaptive management and debarking schedule optimization of Quercus suber L. stands under climate change: Case study in Chamusca, Portugal. Reg. Environ. Ch. 2015, 15, 1569–1580. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Aranda, I.; Castro, L.; Pardos, M.; Gil, L.; Pardos, J.A. Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. For. Ecol. Manag. 2005, 210, 117–129. [Google Scholar] [CrossRef]
- David, T.S.; Henriques, M.O.; Kurz-Besson, C.; Nunes, J.; Valente, F.; Vaz, M.; Pereira, J.S.; Siegwolf, R.; Chaves, M.M.; Gazarini, L.C.; et al. Water-use strategies in two co-occurring Mediterranean evergreen oaks: Surviving the summer drought. Tree Physiol. 2007, 27, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Kurz-Besson, C.; Otieno, D.; Lobo do Vale, R.; Siegwolf, R.; Schmidt, M.; Herd, A.; Nogueira, C.; David, T.S.; David, J.S.; Tenhunen, J.; et al. Hydraulic lift in cork oak trees in a savannah-type Mediterranean ecosystem and its contribution to the local water balance. Plant Soil 2006, 282, 361–378. [Google Scholar] [CrossRef]
- Kurz-Besson, C.; Lobo do Vale, R.; Rodrigues, M.L.; Almeida, P.; Herd, A.; Grant, O.M.; David, T.S.; Schmidt, M.; Otieno, D.; Keenan, T.F.; et al. Cork oak physiological responses to manipulated water availability in a Mediterranean woodland. Agric. For. Meteorol. 2014, 184, 230–242. [Google Scholar] [CrossRef]
- Mendes, M.P.; Ribeiro, L.; David, T.S.; Costa, A. How dependent are cork oak (Quercus suber L.) woodlands on groundwater? A case study in southwestern Portugal. For. Ecol. Manag. 2016, 378, 122–130. [Google Scholar] [CrossRef]
- Otieno, D.O.; Kurz-Besson, C.; Liu, J.; Schmidt, M.W.T.; Vale do Lobo, R.; David, T.S.; Siegwolf, R.; Pereira, J.S.; Tenhunen, J.D. Seasonal variations in soil and plant water status in a Quercus suber L. stand: Roots as determinants of tree productivity and survival in the Mediterranean-type ecosystem. Plant Soil 2006, 283, 119–135. [Google Scholar] [CrossRef]
- Vaz, M.; Pereira, J.S.; Gazarini, L.C.; David, T.S.; David, J.S.; Rodrigues, A.; Maroco, J.; Chaves, M.M. Drought induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiol. 2010, 30, 946–956. [Google Scholar] [CrossRef]
- Cabral, M.T.; Ferreira, M.C.; Moreira, T.; Carvalho, E.C.; Diniz, A.C. Diagnóstico das causas da anormal mortalidade dos sobreiros a sul do Tejo. Sci. Gerund 1992, 18, 205–214. [Google Scholar]
- Camilo-Alves, C.S.P.; Vaz, M.; Da Clara, M.I.E.; Ribeiro, N.A. Chronic cork oak decline and water status: New insights. New For. 2017, 48, 753–772. [Google Scholar] [CrossRef]
- Costa, A.; Pereira, H.; Oliveira, A. Variability of radial growth in cork oak mature trees under cork production. For. Ecol. Manag. 2003, 175, 239–246. [Google Scholar] [CrossRef]
- Camilo-Alves, C.S.P.; Da Clara, M.I.E.; Ribeiro, N.M.D.A. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar] [CrossRef]
- Matías, L.; Abdelaziz, M.; Godoy, O.; Gómez-Aparicio, L. Disentangling the climatic and biotic factors driving changes in the dynamics of Quercus suber populations across the species’ latitudinal range. Div. Distrib. 2019, 25, 524–535. [Google Scholar] [CrossRef]
- Morillas, L.; Leiva, M.J.; Pérez-Ramos, I.M.; Cambrollé, J.; Matías, L. Latitudinal variation in the functional response of Quercus suber seedlings to extreme drought. Sci. Total Environ. 2023, 887, 164122. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valiente, A.; Valladares, F.; Gil, L.; Aranda, I. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Interacting effects of climate and forestcover changes on mortality and growth of the southernmost European fir forests. Glob. Ecol. Biogeogr. 2009, 18, 485–497. [Google Scholar] [CrossRef]
- Camarero, J.J.; Manzanedo, R.D.; Sánchez-Salguero, R.; Navarro-Cerrillo, R.M. Growth response to climate and drought change along an aridity gradient in the southernmost Pinus nigra relict forests. Ann. For. Sci. 2013, 70, 769–780. [Google Scholar] [CrossRef]
- Cornes, R.; van der Schrier, G.; van den Besselaar, E.J.M.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation datasets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Aronson, J.; Pereira, J.S.; Pausas, J.G. (Eds.) Introduction. In Cork Oak Woodlands on the Edge; Island Press: Washington DC, USA, 2009. [Google Scholar][Green Version]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index—SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Tomas-Burguera, M.; Beguería, S.; Reig, F.; Latorre, B.; Peña-Gallardo, M.; Luna, M.Y.; Morata, M.; González-Hidalgo, J.C. A High Resolution Dataset of Drought Indices for Spain. Data 2017, 2, 22. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R. Raster: Geographic Data Analysis and Modeling; R Package Version 3.6-21. 2023. Available online: https://rspatial.org/raster (accessed on 10 May 2023).
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Larsson, L.A.; Larsson, P.O. CDendro and CooRecorder, v. 9.3.1; Manual; Cybis Elektronik and Data AB: Saltsjöbaden, Sweden, 2018. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Gärtner, H.; Lucchinetti, S.; Schweingruber, F.H. A new sledge microtome to combine wood anatomy and tree-ring ecology. IAWA J. 2015, 36, 452–459. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer: Dordrecht, The Netherlands, 1990; pp. 137–152. [Google Scholar]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R, R Package Version 1.7.1; CRAN: Vienna, Austria, 2020. [Google Scholar]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Hurrell, J. Decadal trends in North Atlantic Oscillation and relationship to regional temperature and precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef]
- Hurrell, J.; Van Loon, H. Decadal variations in climate associated with the North Atlantic Oscillation. Clim. Chang. 1997, 36, 301–326. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, A.; Camarero, J.J.; Bosela, M. Applying climwin to dendrochronology: A breakthrough in the analyses of tree responses to environmental variability. Dendrochronologia 2022, 71, 125916. [Google Scholar] [CrossRef]
- Bailey, L.; van de Pol, M. Climwin: An R toolbox for climate window analysis. PLoS ONE 2016, 11, e0167980. [Google Scholar] [CrossRef]
- van de Pol, M.; Bailey, L.D.; McLean, N.; Rijsdijk, L.; Lawson, C.R.; Brouwer, L. Identifying the best climatic predictors in ecology and evolution. Methods Ecol. Evol. 2016, 7, 1246–1257. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2004. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Costa, A.; Pereira, H.; Oliveira, A. A dendroclimatological approach to diameter growth in cork-oak adult trees under cork production. Trees Struct. Funct. 2001, 15, 438–443. [Google Scholar] [CrossRef]
- Leal, S.; Nunes, E.; Pereira, H. Cork oak (Quercus suber L.) wood growth and vessel characteristics variations in relation to climate and cork harvesting. Eur. J. For. Res. 2008, 127, 3341. [Google Scholar]
- Mendes, M.P.; Cherubini, P.; Plieninger, T.; Ribeiro, L.; Costa, A. Climate effects on stem radial growth of Quercus suber L.: Does tree size matter? Forestry 2019, 92, 73–84. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in North-Eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- Nardini, A.; LoGullo, M.A.; Salleo, S. Competitive strategies for water availability in two Mediterranean Quercus species. Plant Cell Environ. 1999, 22, 109–116. [Google Scholar] [CrossRef]
- González-Adrados, J.R.; Gourlay, I. Applications of Dendrochronology to Quercus suber L. In Cork Oak and Cork. Proceedings of the European Conference on Cork Oak and Cork; Pereira, H., Ed.; Centro de Estudos Florestais: Lisboa, Portugal, 1998; pp. 162–166. [Google Scholar]
- Costa, A.; Nunes, L.C.; Spiecker, H.; Graça, J. Insights into the responsiveness of cork oak (Quercus suber L.) to bark harvesting. Econ. Bot. 2015, 69, 171–184. [Google Scholar] [CrossRef]
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Ann. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]

| Site | Latitude N | Longitude W | Elevation (m a.s.l.) | Exposition | Slope (%) | MAT, Range (°C) | TAP (mm) | KOI 1 | Dbh (cm) | Height (m) | Other Tree Species |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zarautz | 43°17′20″ | 2°10′52″ | 141 | E | 15–20 | 12.4, 3.9–22.8 | 1417 | 26.3 | 34.8 ± 1.9 c | 7.5 ± 1.2 b | Arbutus unedo, Laurus nobilis |
| Bozoó | 42°44′15″ | 3°06′31″ | 759 | NE | 5–10 | 10.1, −2.5–23.4 | 789 | 21.5 | 27.3 ± 1.6 a | 5.9 ± 0.3 a | Quercus ilex, Pinus pinaster |
| Sestrica | 41°30′02″ | 1°38′11″ | 865 | E, SE | 10–45 | 11.8, −1.6–27.8 | 455 | 15.3 | 31.4 ± 1.2 b | 6.6 ± 0.9 ab | Quercus ilex, Quercus faginea |
| Site | No. Trees (No. Radii) | Period | Estimated Tree Age (yrs.) | Tree-Ring Width (mm) | First-Order Autocorrelation | Mean Sensitivity | rbar | EPS |
|---|---|---|---|---|---|---|---|---|
| Zarautz | 10 (14) | 1949–2021 | 54 ± 11 | 2.27 ± 0.22 c | 0.50 ± 0.09 a | 0.27 ± 0.04 a | 0.39 | 0.61 |
| Bozoó | 12 (21) | 1940–2021 | 64 ± 10 | 1.14 ± 0.16 a | 0.74 ± 0.11 b | 0.30 ± 0.05 a | 0.57 | 0.72 |
| Sestrica | 14 (23) | 1937–2021 | 65 ± 14 | 1.71 ± 0.18 b | 0.51 ± 0.06 a | 0.45 ± 0.09 b | 0.70 | 0.81 |
| Site | Climate Variable | Window Open (DOY) | Window Close (DOY) | Coefficient | p | R2 | ΔAICc | p AICc |
|---|---|---|---|---|---|---|---|---|
| Zarautz | Tmax | 244 (t − 1) | 292 (t − 1) | −0.046 | <0.001 | 0.22 | −10.85 | 0.079 |
| Tmin | 210 | 228 | 0.041 | 0.011 | 0.12 | −4.49 | 0.661 | |
| Prec | 238 (t − 1) | 296 (t − 1) | 0.043 | <0.001 | 0.34 | −19.69 | 0.002 | |
| Bozoó | Tmax | 70 | 85 | −0.044 | <0.001 | 0.21 | −10.16 | 0.144 |
| Tmin | 360 (t − 1) | 10 | −0.048 | <0.001 | 0.20 | −9.4 | 0.167 | |
| Prec | 147 | 238 | 0.206 | <0.001 | 0.21 | −10.28 | 0.116 | |
| Sestrica | Tmax | 152 | 170 | −0.036 | 0.009 | 0.13 | −4.85 | 0.904 |
| Tmin | 104 | 126 | 0.063 | 0.007 | 0.14 | −5.4 | 0.431 | |
| Prec | 258 (t − 1) | 312 | 0.665 | <0.001 | 0.41 | −25.6 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero, J.J.; Gazol, A.; Valeriano, C.; Colangelo, M.; Rubio-Cuadrado, Á. Growth Responses to Climate and Drought in Relict Cork Oak Populations as a Benchmark of the Species Tolerance. Forests 2024, 15, 72. https://doi.org/10.3390/f15010072
Camarero JJ, Gazol A, Valeriano C, Colangelo M, Rubio-Cuadrado Á. Growth Responses to Climate and Drought in Relict Cork Oak Populations as a Benchmark of the Species Tolerance. Forests. 2024; 15(1):72. https://doi.org/10.3390/f15010072
Chicago/Turabian StyleCamarero, J. Julio, Antonio Gazol, Cristina Valeriano, Michele Colangelo, and Álvaro Rubio-Cuadrado. 2024. "Growth Responses to Climate and Drought in Relict Cork Oak Populations as a Benchmark of the Species Tolerance" Forests 15, no. 1: 72. https://doi.org/10.3390/f15010072
APA StyleCamarero, J. J., Gazol, A., Valeriano, C., Colangelo, M., & Rubio-Cuadrado, Á. (2024). Growth Responses to Climate and Drought in Relict Cork Oak Populations as a Benchmark of the Species Tolerance. Forests, 15(1), 72. https://doi.org/10.3390/f15010072