The Addition of an Invasive Plant Alters the Home-Field Advantage of Native Leaf Litter Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design, Litterbag Preparation and Sampling
2.3. Soil Sampling and Analysis
2.4. Taxa Measurement and Chemical Analyses
2.5. Data Analysis
3. Results
3.1. Initial Litter and Soil Properties
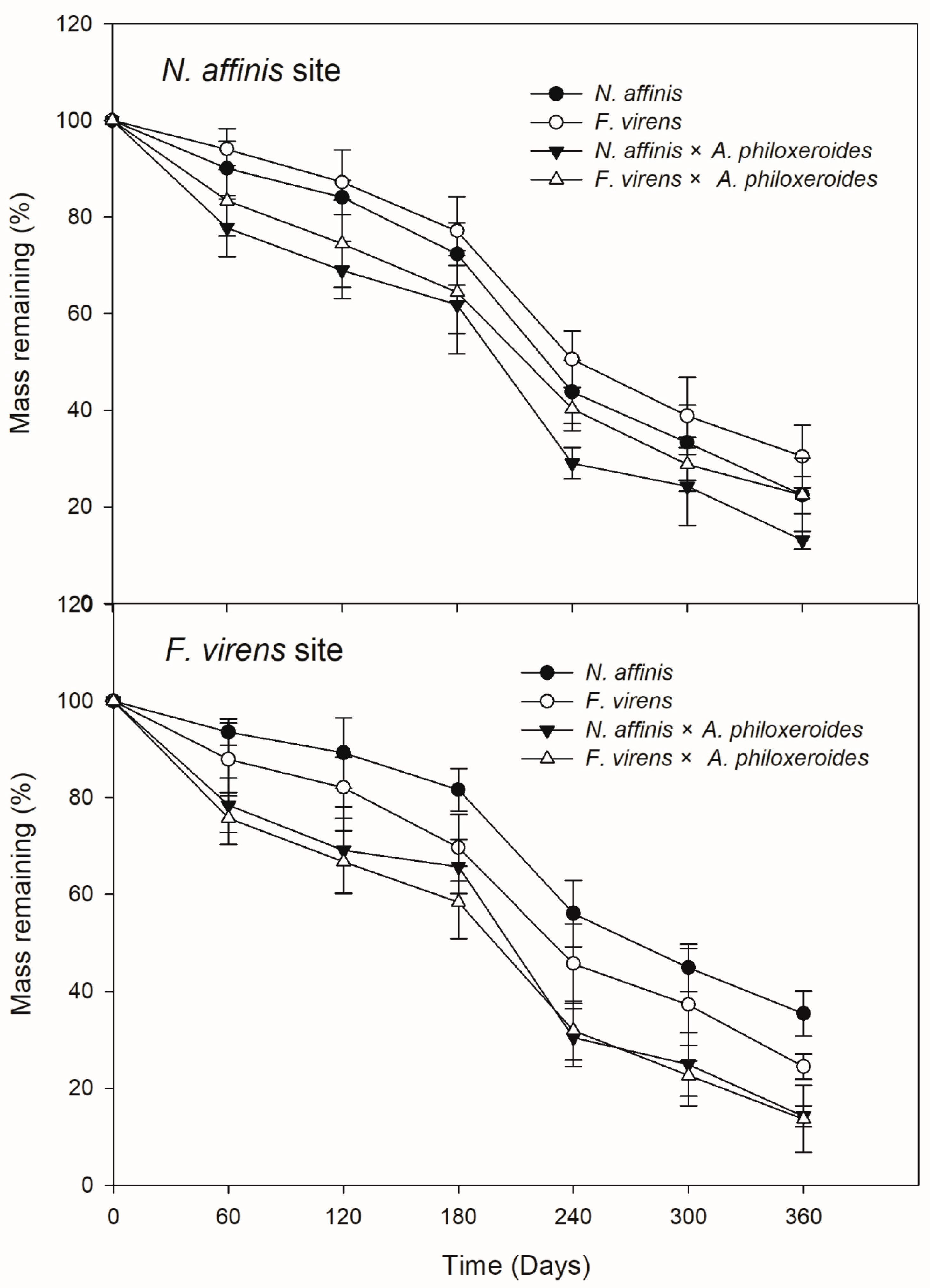
3.2. Litter Decomposition and HFA
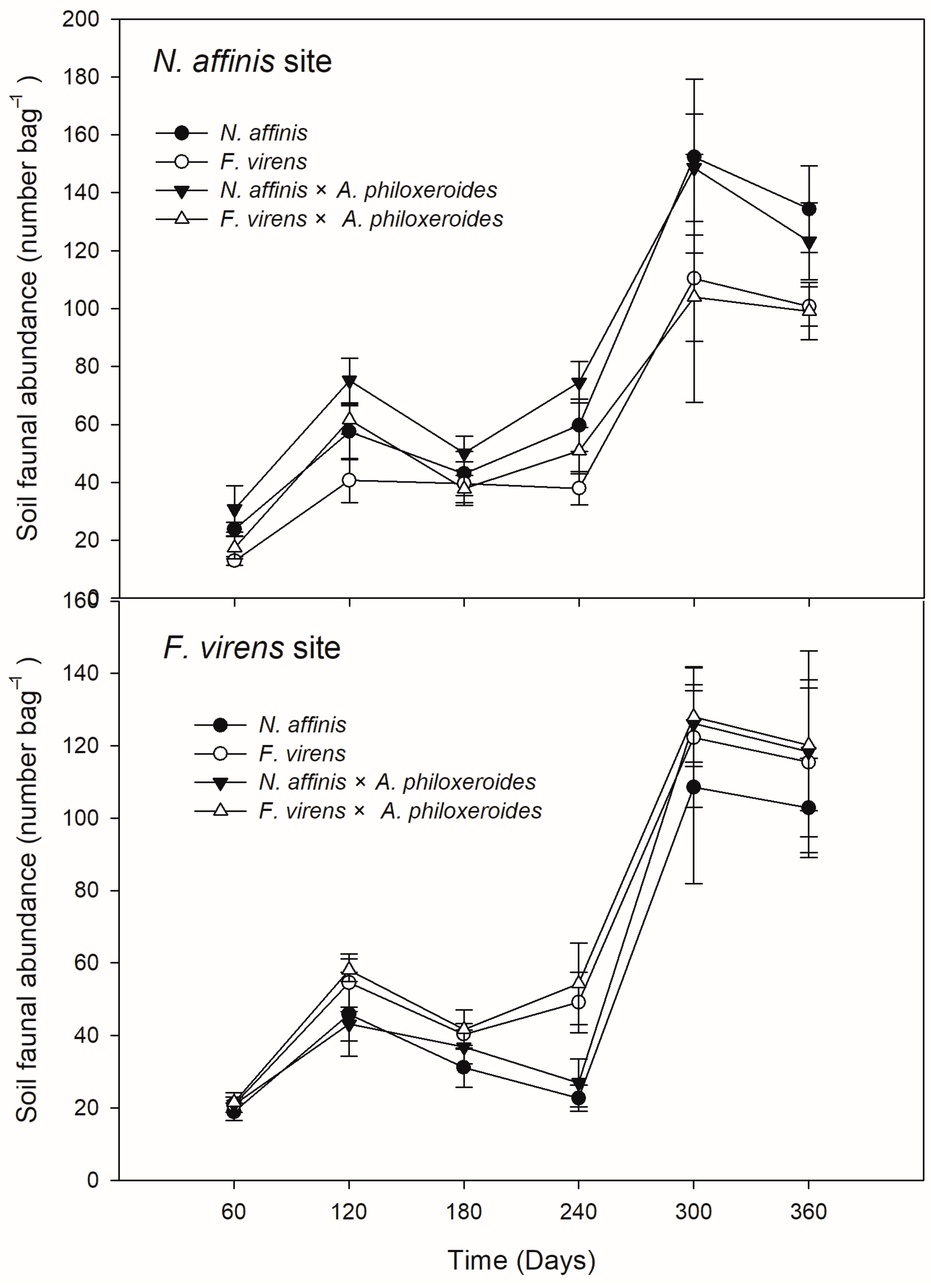
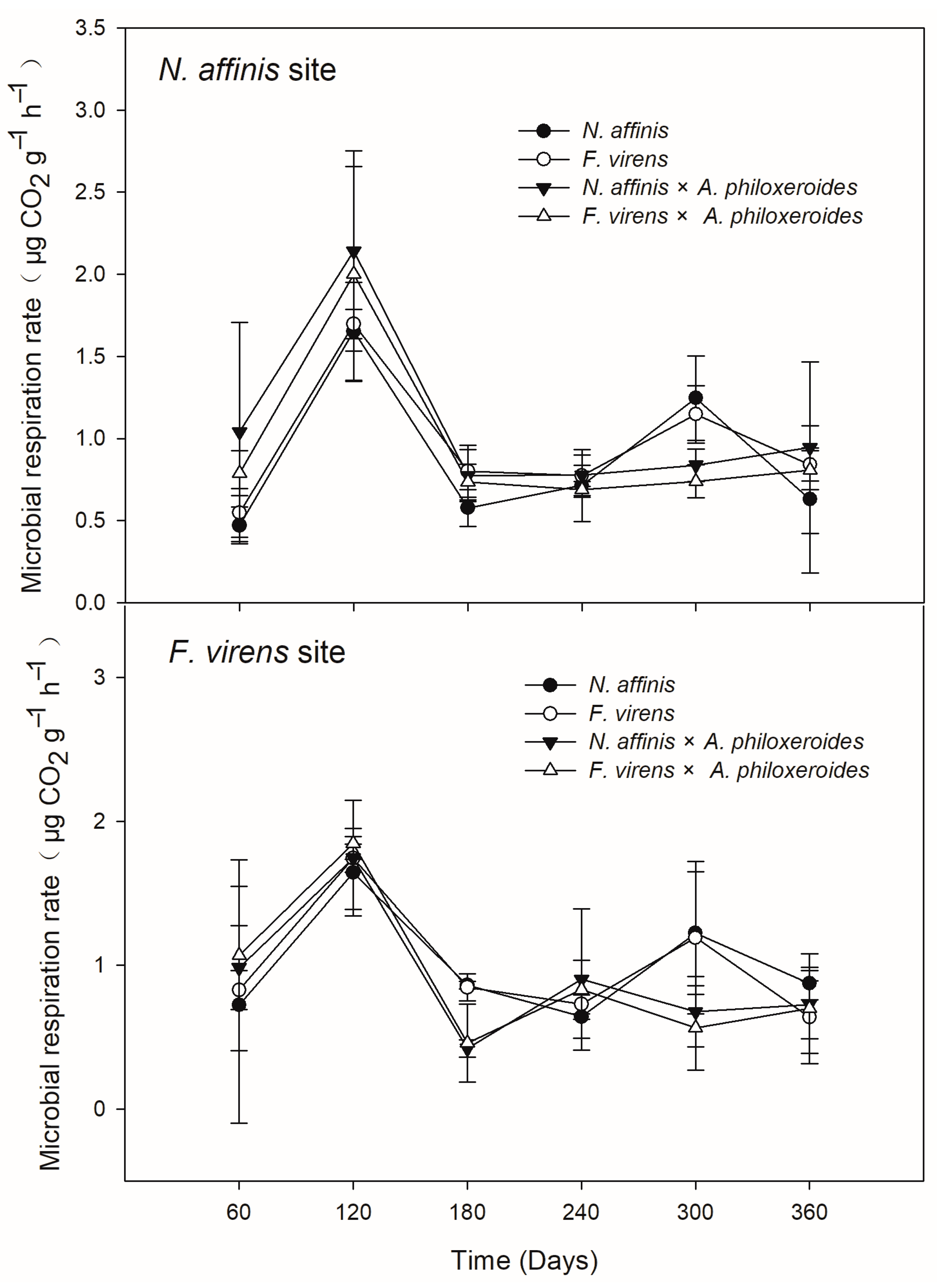
3.3. Leaf-Associated Faunal Community and Microbial Respiration Rate
4. Discussion
4.1. The Impact of Initial Quality and Environmental Conditions on HFA
4.2. HFA Effects in Response to the Addition of A. philoxeroides
4.3. The Role of Soil Fauna Groups and Microbes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, D.P.; Manrubia, M.; De Boer, W.; Zweers, H.; Veen, G.F.; Van der Wal, A. Relationship between home-field advantage of litter decomposition and priming of soil organic matter. Soil Biol. Biochem. 2018, 126, 49–56. [Google Scholar] [CrossRef]
- Pastorelli, R.; Costagli, V.; Forte, C.; Viti, C.; Rompato, B.; Nannini, G.; Certini, G. Litter decomposition: Little evidence of the “home-field advantage” in a mountain forest in Italy. Soil Biol. Biochem. 2021, 159, 108300. [Google Scholar] [CrossRef]
- Duenas, M.A.; Hemming, D.J.; Roberts, A.; Diaz-Soltero, H. The threat of invasive species to IUCN-listed critically endangered species: A systematic review. Glob. Ecol. Conserv. 2021, 26, e01476. [Google Scholar]
- Hunt, H.; Ingham, E.; Coleman, D.; Elliott, E.; Reid, C. Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology 1988, 69, 1009–1016. [Google Scholar] [CrossRef]
- Fanin, N.; Lin, D.; Freschet, G.T.; Keiser, A.D.; Augusto, L.; Wardle, D.A.; Veen, G.F. Home-field advantage of litter decomposition: From the phyllosphere to the soil. New Phytol. 2021, 231, 1353–1358. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; González-Arzac, A.; Pérez, L.I. There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Liu, Y.; Heděnec, P.; Peng, Y.; Xu, Z.; Tan, B.; Zhang, L.; Guo, L.; Wang, L.; et al. Effects of litter quality diminish and effects of vegetation type develop during litter decomposition of two shrub species in an alpine treeline ecotone. Ecosystems 2021, 24, 197–210. [Google Scholar] [CrossRef]
- McCary, M.A.; Mores, R.; Farfan, M.A.; Wise, D.H. Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: A meta-analysis. Ecol. Lett. 2016, 19, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Little, M.N.; Custer, K.W.; Borth, E.B.; Chapman, J.I.; Kukla, M.J.; Kuminecz, C.; Maloney, M.E.; Woods, M.J.; McEwan, R.W. The influence of riparian invasion by the terrestrial shrub Lonicera maackii on aquatic macroinvertebrates in temperate forest headwater streams. Biol. Invasions 2020, 23, 25–35. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Shao, H.; Tang, S. Selected aspects of invasive Solidago canadensis with an emphasis on its allelopathic abilities: A review. Chem. Biodivers. 2022, 19, e202200728. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Halvorson, H.M.; Barry, J.R.; Lodato, M.B.; Findlay, R.T.; Francoeur, S.N.; Kuehn, K.A. Periphytic algae decouple fungal activity from leaf litter decomposition via negative priming. Funct. Ecol. 2019, 33, 188–201. [Google Scholar] [CrossRef]
- Hu, D.; Wang, M.; Zheng, Y.; Lv, M.; Zhu, G.; Zhong, Q.; Cheng, D. Leaf litter phosphorus regulates the soil meso-and micro-faunal contribution to home-field advantage effects on litter decomposition along elevation gradients. Catena 2021, 207, 105673. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, H.; Xie, X.; Liu, Y.; Liu, Q.; Zhang, B.; Deng, Y. Invasive plant mats promoted the decomposition of native leaf litter by micro-, meio-, and macroinvertebrates in an eutrophic freshwater lake in the Three Gorges Reservoir area, China. Hydrobiologia 2022, 849, 215–227. [Google Scholar] [CrossRef]
- Osburn, E.D.; Hoch, P.J.; Lucas, J.M.; McBride, S.G.; Strickland, M.S. Evaluating the roles of microbial functional breadth and home-field advantage in leaf litter decomposition. Funct. Ecol. 2022, 36, 1258–1267. [Google Scholar] [CrossRef]
- Riutta, T.; Slade, E.M.; Bebber, D.P.; Taylor, M.E.; Malhi, Y.; Riordan, P.; Macdonald, D.W.; Morecroft, M.D. Experimental evidence for the interacting effects of forest edge, moisture and soil macrofauna on leaf litter decomposition. Soil Biol. Biochem. 2012, 49, 124–131. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, M.; He, T. Home-field advantage of litter decomposition and nitrogen release in forest ecosystems. Biol. Fert. Soils 2013, 49, 427–434. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Bertrand, I. Functional breadth and home-field advantage generate functional differences among soil microbial decomposers. Ecology 2016, 97, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Mei, H.; Ye, L.; Wang, J.; Ma, S. Effects of water quality characteristics on the algicidal property of Alternanthera philoxeroides (Mart.) Griseb. in an aquatic ecosystem. Soil Biol. Biochem. 2012, 43, 93–100. [Google Scholar] [CrossRef]
- Schooler, S.S. Alternanthera philoxeroides (Martius) Grisebach (Alligator Weed); Taylor and Francis Group: London, UK, 2012; pp. 25–35. [Google Scholar]
- Yu, Z.; Huang, Z.; Wang, M.; Liu, R.; Zheng, L.; Wan, X.; Hu, Z.; Davis, M.R.; Lin, T.-C. Nitrogen addition enhances home-field advantage during litter decomposition in subtropical forest plantations. Soil Biol. Biochem. 2015, 90, 188–196. [Google Scholar] [CrossRef]
- Bärlocher, F. Leaf mass loss estimated by litter bag technique. In Methods to Study Litter Decomposition: A practical Guide; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 37–42. [Google Scholar]
- Yin, W.; Yang, F.; Wang, Z.; Luo, Z.; Shen, W.; Hu, S.; Wang, X.; Zhao, L.; Sun, X.; Zhang, Y.; et al. Subtropical Soil Animals in China; Science Press: Beijing, China, 1992. [Google Scholar]
- Liao, C.; Li, J. Forest Soil Animal Community Ecology in South China Tropical and Subtropical Zones; Guangdong Science and Technology Press: Guangzhou, China, 2009. [Google Scholar]
- Frouz, J.; Špaldoňová, A.; Lhotetáková, Z.; Cajthaml, T. Major mechanisms contributing to the macrofauna-mediated slow down of litter decomposition. Soil Biol. Biochem. 2015, 91, 23–31. [Google Scholar] [CrossRef]
- Jones, G.L.; Scullion, J.; Worgan, H.; Gwynn-Jones, D. Litter of the invasive shrub Rhododendron ponticum (Ericaceae) modifies the decomposition rate of native UK woodland litter. Ecol. Indic. 2019, 107, 105597. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Zhao, X.; Cui, J. Relationship between fresh leaf traits and leaf litter decomposition of 20 plant species in Kerqin sandy land, China. Acta Ecol. Sin. 2008, 28, 2486–2492. [Google Scholar]
- Holmroos, H.; Horppila, J.; Niemistö, J. Dynamics of dissolved nutrients among different macrophyte stands in a shallow lake. Limnology 2015, 16, 31–39. [Google Scholar] [CrossRef]
- Palozzi, J.E.; Lindo, Z. Are leaf litter and microbes team players? Interpreting home-field advantage decomposition dynamics. Soil Biol. Biochem. 2018, 124, 189–198. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Osler, G.H.; Campbell, C.D.; Burslem, D.F.; van der Wal, R. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. J. Biogeogr. 2010, 37, 1317–1328. [Google Scholar] [CrossRef]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Veen, G.F.; Keiser, A.D.; van der Putten, W.H.; Wardle, D.A. Variation in home-field advantage and ability in leaf litter decomposition across successional gradients. Funct. Ecol. 2018, 32, 1563–1574. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Smagin, A.V.; Sadovnikova, N.B.; Vasenev, V.I.; Smagina, M.V. Biodegradation of some organic materials in soils and soil constructions: Experiments, modeling and prevention. Materials 2018, 11, 1889. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Anderson, J.M. Decomposition of sun and shade leaves from three deciduous tree species, as affected by their chemical composition. Biol. Fert. Soils 2003, 37, 137–146. [Google Scholar] [CrossRef]
- Hassall, M.; Edwards, D.P.; Carmenta, R.; Derhé, M.A.; Moss, A. Predicting the effect of climate change on aggregation behaviour in four species of terrestrial isopods. Behaviour 2010, 147, 151–164. [Google Scholar]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Hanamachi, Y.; Hama, T.; Yanai, T. Decomposition process of organic matter derived from freshwater phytoplankton. Limnology 2008, 9, 57–69. [Google Scholar] [CrossRef]
- Rief, A.; Knapp, B.A.; Seeber, J. Palatability of selected alpine plant litters for the decomposer Lumbricus rubellus (Lumbricidae). PLoS ONE 2012, 9, e45345. [Google Scholar] [CrossRef]
- Benito-Carnero, G.; Gartzia-Bengoetxea, N.; Arias-González, A.; Rousk, J. Low-quality carbon and lack of nutrients result in a stronger fungal than bacterial home-field advantage during the decomposition of leaf litter. Funct. Ecol. 2021, 35, 1783–1796. [Google Scholar] [CrossRef]
- Guenet, B.; Danger, M.; Abbadie, L.; Lacroix, G. Priming effect: Bridging the gap between terrestrial and aquatic ecology. Ecology 2010, 91, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Meneses, G.; Palacios-Vargas, J.G.; Delabie, J.H.C.; Zeppelini, D.; Mariano, C.S.F. Springtails (Collembola) Associated with Nests of Fungus-Growing Ants (Formicidae: Myrmicinae: Attini) in Southern Bahia, Brazil. Fla Entomol. 2017, 100, 740–742. [Google Scholar] [CrossRef]
- Le Bayon, R.C.; Bullinger-Weber, G.; Schomburg, A.; Turberg, P.; Schlaepfer, R.; Guenat, C. Earthworms as ecosystem engineers: A review. In Earthworms: Types, Roles and Research; NOVA Science Publishers: New York, NY, USA, 2017; pp. 129–178. [Google Scholar]
- Homet, P.; Gómez-Aparicio, L.; Matías, L.; Godoy, O. Soil fauna modulates the effect of experimental drought on litter decomposition in forests invaded by an exotic pathogen. J. Ecol. 2021, 109, 2963–2980. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J. The Occurrence of Springtails (Collembola) and Spiders (Araneae) as an Effectiveness Indicator of Bioremediation of Soil Contaminated by Petroleum-Derived Substances. Int. J. Environ. Res. 2016, 10, 449–458. [Google Scholar]
- Valiela, I. Marine Ecological Processes, 2nd ed.; Springer: New York, NY, USA, 1995. [Google Scholar]
- Cummins, K.W.; Merritt, R.W.; Andrade, P.C. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 69–89. [Google Scholar] [CrossRef]
- Posch, T.; Simek, K.; Vrba, J.; Pernthaler, J.; Nedoma, J.; Sattler, B.; Sonntag, B.; Psenner, R. Predator-induced changes of bacterial size structure and productivity studied on an experimental microbial community. Aquat. Microb. Ecol. 1999, 18, 235–246. [Google Scholar] [CrossRef]


| Sites | Soil Temperature (°C) | Soil Moisture (%) | Total C (g Kg−1) | Total N (g Kg−1) | pH |
|---|---|---|---|---|---|
| N. affinis | 17.27 ± 0.072 ns | 21.04 ± 0.941 * | 52.43 ± 1.793 * | 3.38 ± 0.032 ** | 6.86 ± 1.032 ns |
| F. virens | 14.03 ± 0.089 | 23.96 ± 0.492 | 47.34 ± 1.839 | 5.54 ± 0.048 | 6.35 ± 0.932 |
| Factors | A. philoxeroides | N. affinis | F. virens |
|---|---|---|---|
| C (% DM) | 11.95 ± 0.360 a | 41.67 ± 0.001 b | 25.77 ± 0.069 c |
| N (% DM) | 4.21 ± 0.012 a | 0.48 ± 0.024 b | 1.66 ± 0.052 c |
| P (% DM) | 2.29 ± 0.076 a | 0.05 ± 0.034 b | 0.14 ± 0.076 c |
| C: N | 3.75 ± 0.025 a | 86.79 ± 0.790 b | 15.55 ± 0.025 c |
| C:P | 6.87 ± 0.065 a | 833.21 ± 0.973 b | 184.46 ± 0.065 c |
| N:P | 1.81 ± 0.022 a | 9.57 ± 0.007 b | 11.86 ± 0.022 b |
| Total phenol (% DM) | 5.43 ± 0.091 a | 1.40 ± 0.037 b | 10.10 ± 0.091 c |
| Lignin (% DM) | 2.67 ± 0.003 a | 3.16 ± 0.002 b | 8.52 ± 0.003 b |
| Cellulose (% DM) | 3.21 ± 0.008 a | 25.28 ± 1.566 b | 17.08 ± 0.008 b |
| Hemicellulose (% DM) | 8.97 ± 0.059 a | 49.72 ± 2.893 b | 12.78 ± 0.059 c |
| Source | df | Mass Remaining | Faunal Abundance | Microbial Respiration Rate | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| S | 1 | 30.70 | <10−4 | 72.41 | <10−4 | 0.15 | 0.704 |
| A | 1 | 174.93 | <10−4 | 11.15 | 0.001 | 0.16 | 0.692 |
| T | 5 | 420.36 | <10−4 | 270.35 | <10−4 | 23.59 | <10−4 |
| T × A | 1 | 1.74 | 0.190 | 0.40 | 0.530 | 0.16 | 0.692 |
| T × S | 5 | 0.23 | 0.948 | 3.46 | 0.006 | 0.69 | 0.631 |
| A × S | 5 | 0.45 | 0.813 | 0.84 | 0.525 | 5.41 | <10−4 |
| S × A × T | 5 | 0.08 | 0.995 | 0.06 | 0.998 | 0.34 | 0.889 |
| Sites | Decomposition Rate (k·Day−1) | |||
|---|---|---|---|---|
| N. affinis | F. virens | N. affinis × A. philoxeroides | F. virens × A. philoxeroides | |
| N. affinis site | 0.003 ± 0.001 a,* | 0.002 ± 0.001 b,* | 0.004 ± 0.001 c,* | 0.003 ± 0.001 a,* |
| F. virens site | 0.002 ± 0.001 a | 0.003 ± 0.001 b | 0.003 ± 0.001 b | 0.004 ± 0.001 c |
| Factors | Site | N. affinis | F. virens | N. affinis × A. philoxeroides | F. virens × A. philoxeroides | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| Mean HFA | N. affinis site | −0.652 | 0.161 | −0.842 | 0.035 | −0.014 | 0.979 | −0.655 | 0.158 |
| F. virens site | −0.677 | 0.139 | −0.817 | 0.047 | −0.043 | 0.935 | −0.606 | 0.202 | |
| HFAI | N. affinis site | −0.892 | 0.017 | −0.881 | 0.020 | −0.890 | 0.017 | −0.895 | 0.016 |
| F. virens site | −0.901 | 0.014 | −0.870 | 0.024 | −0.878 | 0.021 | −0.865 | 0.026 | |
| Invertebrate abundance | N. affinis site | 0.870 | 0.024 | 0.888 | 0.018 | 0.815 | 0.048 | 0.873 | 0.023 |
| F. virens site | 0.793 | 0.060 | 0.852 | 0.031 | 0.790 | 0.062 | 0.889 | 0.018 | |
| Microbial respiration rate | N. affinis site | −0.095 | 0.857 | −0.059 | 0.911 | −0.098 | 0.854 | −0.029 | 0.956 |
| F. virens site | −0.063 | 0.906 | −0.127 | 0.811 | −0.088 | 0.869 | −0.066 | 0.901 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xie, X.; Wen, J.; Zhai, H.; Wang, H.; Jiang, Y.; Gou, Z. The Addition of an Invasive Plant Alters the Home-Field Advantage of Native Leaf Litter Decomposition. Forests 2024, 15, 1708. https://doi.org/10.3390/f15101708
Chen S, Xie X, Wen J, Zhai H, Wang H, Jiang Y, Gou Z. The Addition of an Invasive Plant Alters the Home-Field Advantage of Native Leaf Litter Decomposition. Forests. 2024; 15(10):1708. https://doi.org/10.3390/f15101708
Chicago/Turabian StyleChen, Shaojun, Xiaohua Xie, Jie Wen, Hao Zhai, Huiqi Wang, Yuhang Jiang, and Zhanxu Gou. 2024. "The Addition of an Invasive Plant Alters the Home-Field Advantage of Native Leaf Litter Decomposition" Forests 15, no. 10: 1708. https://doi.org/10.3390/f15101708





