Effect of Fertilization on Soil Fertility and Individual Stand Biomass in Strip Cut Moso Bamboo (Phyllostachys edulis) Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experiment Design
- Spreading fertilization: Compound fertilizer was evenly spread in the plots 2–3 days after precipitation during the fertilization period.
- Cave fertilization: Twenty holes were dug within each plot (10–20 cm deep), evenly distributed in two rows, and equal amounts of fertilizer were added to each hole.
- Bamboo stump fertilization: The internodes of the bamboo stumps within the harvest area were hollowed out, and compound fertilizer was applied to the cavities, reaching the bottom. The cavities were then covered with the topsoil.
2.3. Biomass Survey
2.4. Soil Fertility Survey
2.5. Data Analyses
3. Results
3.1. Total Soil Nutrients
3.2. Available Soil Nutrients
3.3. Soil Organic Carbon
3.4. Main Enzyme Activities in the Soil
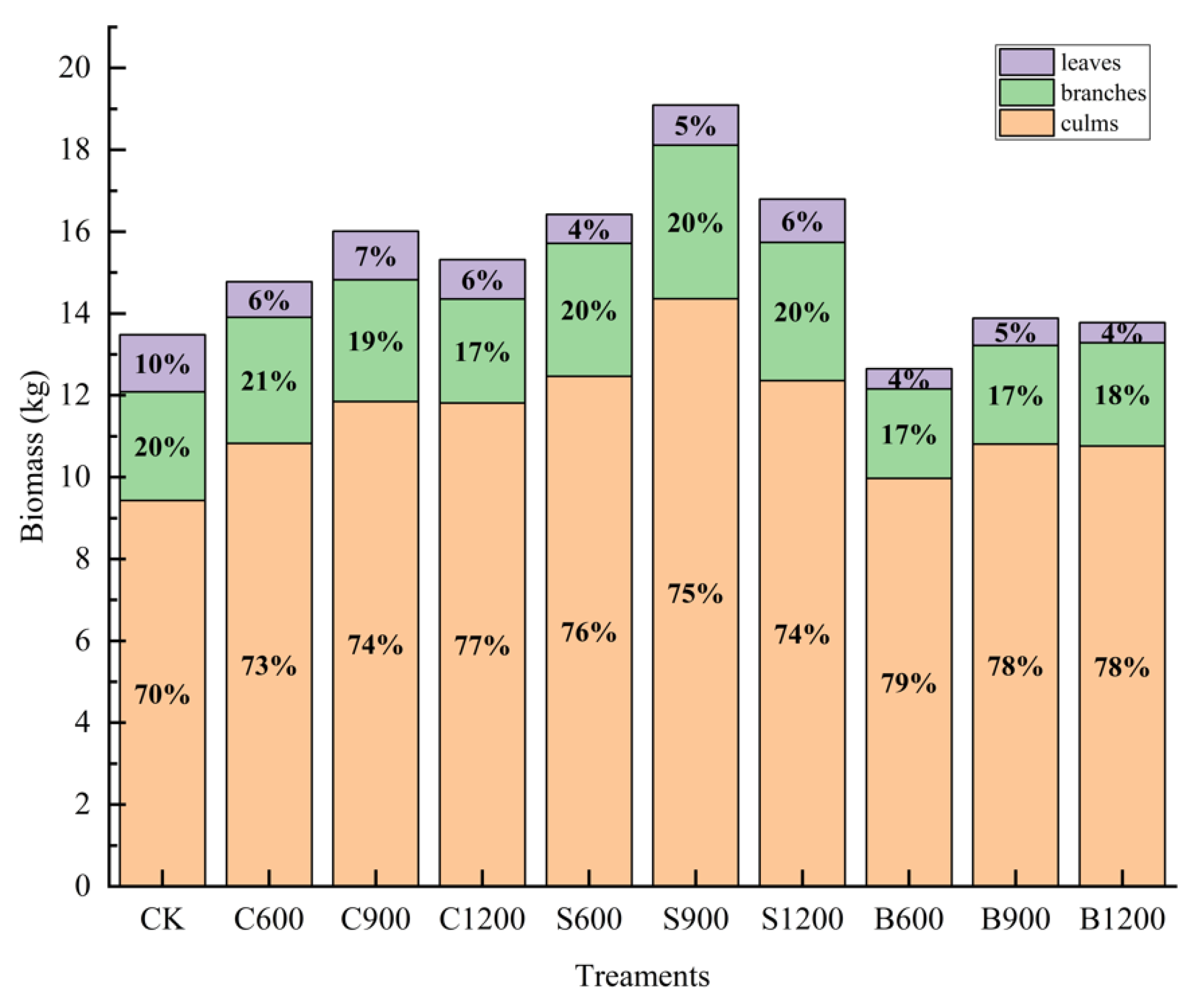
3.5. New Bamboo Stand Biomass and Allocation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, S.; Liu, G.; Su, W. Advances in research of bamboo forest cultivation. For. Res. 2018, 31, 137–144. [Google Scholar]
- Tan, H.; Tan, R.; Zhang, Y. Effect of three clear-cutting patterns on the regeneration and growth of Phyllostachys pubescens. World Bamboo Ratt. 2017, 15, 52–55. [Google Scholar]
- Wang, L.; Fan, C.; Liang, K.; Fan, Y. An approach to a labor-saving cutting model for bamboo forest management, a study based on clonal integration. Acta Agric. Univ. Jiangxiensis 2016, 38, 1110–1118. [Google Scholar]
- Bai, S.; Wang, Y. Can native clonal moso bamboo encroach on adjacent natural forest without human intervention? Sci. Rep. 2016, 6, 31504. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Su, W.; Fan, S. Qualitative recovery characteristics of moso bamboo forests under strip clearcutting. Acta Bot. Boreal.-Occident. Sin. 2019, 39, 917–924. [Google Scholar]
- Zhan, M.; Guan, F.; Yan, Y. Effects of strip harvesting on species diversity of under growth in bamboo (Phyllostachys edulis) forest. Acta Ecol. Sin. 2020, 40, 4169–4179. [Google Scholar]
- Wang, S.; Fan, S.; Xiao, X. Effects of strip cutting on aboveground biomass accumulation and allocation and allometric growth of Phyllostachys edulis. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 19–24. [Google Scholar]
- Zheng, Y.; Guan, F.; Fan, S.; Yan, X.; Huang, L. Dynamics of leaf-litter biomass, nutrient resorption efficiency and decomposition in a moso bamboo forest after strip clearcutting. Front. Plant Sci. 2021, 12, 799424. [Google Scholar] [CrossRef]
- Li, C.; Guan, F. Effects of fertilization application on quantity and quality recovery of restoring strip-harvested moso bamboo forests. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023, 1–8. Available online: http://kns.cnki.net/kcms/detail/32.1161.S.20230210.1421.002.html (accessed on 17 January 2024).
- Su, W. Fertilization Theory and Practice for Phyllostachys edulis Stand Based on Growth and Nutrient Accumulation Rules; Chinese Academy of Forestry: Beijing, China, 2012. [Google Scholar]
- Zheng, Y.; Guan, F.; Fan, S.; Zhou, Y.; Jing, X. Functional trait responses to strip clearcutting in a moso bamboo forest. Forests 2021, 12, 793. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, S.; Guan, F.; Xia, W.; Wang, S.; Xiao, X. Strip clearcutting drives vegetation diversity and composition in the moso bamboo forests. For. Sci. 2022, 68, 27–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, L.; Wang, M. Effects of strip clear cutting in Phyllostachys edulis forests on soil physical and chemical properties and enzyme activities. J. For. Environ. 2020, 40, 234–242. [Google Scholar]
- Shen, J.; Fan, S.; Liu, G. Effects of strip clearcutting width on soil C, N, P and stoichiometry of Phyllostachys edulis forests. Chin. J. Ecol. 2023, 42, 1851–1857. [Google Scholar]
- Zheng, J.; Hu, J.; Wei, Y. Effects of green manure and smash ridging coupling on topsoil aggregate structure in paddy field. J. South. Agric. 2020, 51, 2653–2664. [Google Scholar]
- Li, Q.; Wang, F.; Lin, C. Effects of long-term fertilization on soil microbial community structure and aggregate composition in yellow clayey paddy field. J. Plant Nutr. Fert. 2015, 21, 1599–1606. [Google Scholar]
- Zhang, Y.; Fan, L.; Huang, X. Effects of eclamation and fertilization on growth and soil nutrient content after strip clear cutting in Phyllostachys edulis forests. Chin. J. Trop. Crops 2021, 42, 1047–1054. [Google Scholar]
- Ni, H. Effects of Management Intensities on Fine Root Growth and Soil Microenvironment of Phyllostachys edulis Forests. Ying Yong Sheng Tai Xue Bao 2023, 34, 928–936. [Google Scholar]
- Xu, M. Effects of Long-Term Manure and Fertilizers on Soil Activeorganic Carbon Fractions in Typical Cropland of China; Chinese Academy of Agricultural Science: Beijing, China, 2012. [Google Scholar]
- Liu, Q.; Liang, X.; Dong, P. Effects of different fertilization methods on farmland soil active organic carbon and carbon pool management indicators in loess hilly area. Soils 2023, 55, 446–452. [Google Scholar]
- Huang, Y.; An, S.; Qu, D. Responses and evolution of soil enzymatic activities during process of vegetation recovering. J. Soil Water Conserv. 2007, 21, 152–155. [Google Scholar]
- Yan, J.; Han, X.; Wang, S. Effects of different nitrogen forms on microbial quantity and enzymes activities in soybean field. Plant Nutr. Fert. Sci. 2010, 16, 341–347. [Google Scholar]
- Guan, S. Soil Enzymes and Their Research Methods; Agricultural Publishing House: Rome, Italy, 1986. [Google Scholar]
- Gao, G.; Li, Z.; Ge, X. Effects of nitrogen addition on biomass and root morphology of Phyllostachys edulis seedlings under drought stress. Chin. J. Ecol. 2022, 41, 858–864. [Google Scholar]
- Jiao, W. Influence of Stand Density and Soil Conditions on Carbon Allocation of Poplar Plantation Based on Improved Process Model in Northern Jiangsu. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2022; pp. 69–75. [Google Scholar]
- Guo, Q.; Guan, F.; Hui, C.; Liu, W.; Zou, X. Effects of density and fertilization on growth and biomass characteristics of newly grown Dendrocalamus sinicus. J. Beijing For. Univ. 2022, 44, 95–106. [Google Scholar]
- Gao, G.; Ge, X.; Zhou, J.; Zhou, B.; Li, Z.; Yang, N. Effect of drought stress and nitrogen addition on the biomass and root morphology of Cunninghamia lanceolata and Cyclobalanopsis glauca seedlings. Ecol. Environ. Sci. 2022, 31, 2292–2301. [Google Scholar]
- Xu, H.; Xiong, J.; Cheng, X.; Ling, G.; Zheng, W.; Hu, W.; Yu, M. Responses of leaf functional traits and biomass of Quercus acutissima and Phoebe bournei seedings to light and fertilization. Acta Ecol. Sin. 2021, 41, 2129–2139. [Google Scholar]
- Shen, R.; Bai, S.; Zhou, G. The response of root morphological plasticity to the expansion of a population of Phyllostachys edulis into a mixed needle- and broad-leaved forest. Acta Ecol. Sin. 2016, 36, 326–334. [Google Scholar]
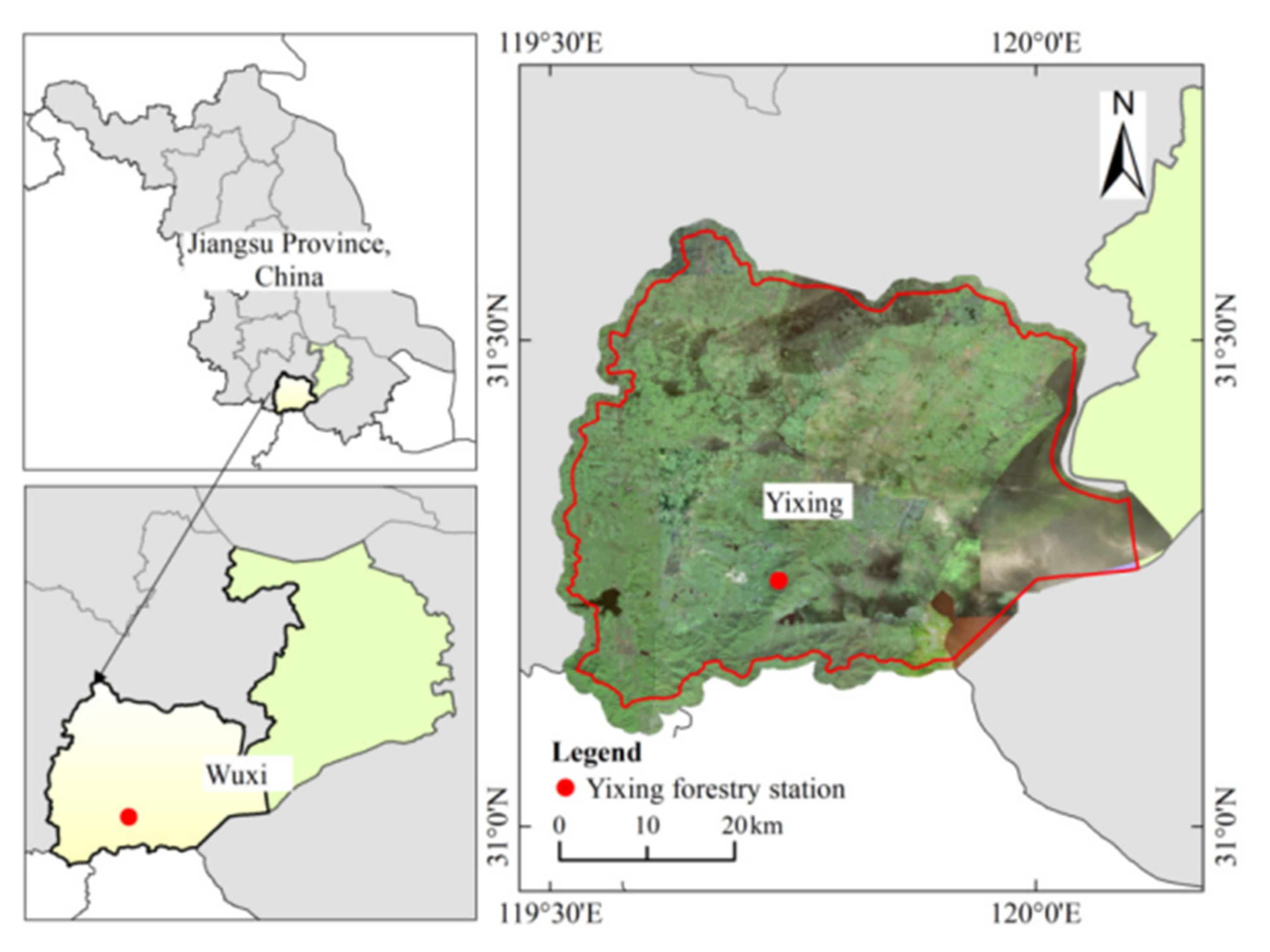


| Treatment | Longitude | Latitude | Aspect | Slope (°) | Altitude (m) |
|---|---|---|---|---|---|
| C600–C1200 | 119°45′20″ E | 31°15′37″ N | Southwest | 3 | 80 |
| S600–S1200 | 119°45′14″ E | 31°15′40″ N | Northeast | 9 | 102 |
| B600–B1200 | 119°45′12″ E | 31°15′43″ N | Southwest | 17 | 138 |
| CK1–CK3 | 119°45′10″ E | 31°15′42″ N | Northeast | 13 | 127 |
| Treatments | Soil Layer | TN | TP | TK | AN | AP | AK |
|---|---|---|---|---|---|---|---|
| (cm) | (g/kg) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| C600 | 0–10 | 1.92 ± 0.06 Abc | 0.33 ± 0.02 Aab | 9.13 ± 0.15 Cb | 130.50 ± 1.87 Ac | 4.66 ± 0.20 Aab | 42.97 ± 3.17 Ab |
| 10–20 | 1.52 ± 0.06 Bb | 0.34 ± 0.02 Aa | 9.87 ± 0.02 Bcd | 83.40 ± 8.79 Bb | 2.61 ± 0.29 Bb | 36.27 ± 1.45 Ba | |
| 20–40 | 1.00 ± 0.02 Cb | 0.36 ± 0.03 Aa | 10.20 ± 0.04 Abc | 38.82 ± 4.35 Cb | 1.30 ± 0.14 Cbc | 32.16 ± 1.24 Ba | |
| C900 | 0–10 | 1.90 ± 0.12 Abc | 0.34 ± 0.01 Aab | 9.40 ± 0.13 Bb | 141.79 ± 2.78 Ac | 5.02 ± 0.21 Aa | 41.38 ± 2.47 Ab |
| 10–20 | 1.42 ± 0.22 Bbc | 0.33 ± 0.02 Aa | 9.70 ± 0.06 Bcd | 90.35 ± 3.08 Bb | 2.34 ± 0.51 Bb | 33.64 ± 3.13 ABa | |
| 20–40 | 0.83 ± 0.08 Cbc | 0.34 ± 0.02 Aab | 10.81 ± 0.06 Ab | 47.06 ± 3.57 Cb | 1.43 ± 0.13 Bbc | 28.18 ± 1.78 Ba | |
| C1200 | 0–10 | 2.19 ± 0.03 Aab | 0.39 ± 0.02 Aa | 10.63 ± 0.82 Aa | 150.90 ± 1.12 Abc | 4.69 ± 0.45 Aab | 48.51 ± 2.51 Aab |
| 10–20 | 1.87 ± 0.05 Ba | 0.37 ± 0.00 Aa | 11.27 ± 0.30 Ab | 89.21 ± 10.47 Bb | 2.85 ± 0.26 Bb | 34.84 ± 4.65 Ba | |
| 20–40 | 1.28 ± 0.06 Ca | 0.38 ± 0.01 Aa | 11.87 ± 0.14 Aa | 55.76 ± 2.24 Cb | 2.09 ± 0.04 Ba | 32.60 ± 3.65 Ba | |
| S600 | 0–10 | 2.02 ± 0.05 Abc | 0.27 ± 0.02 Abc | 9.85 ± 0.39 Aab | 143.28 ± 8.53 Ac | 4.93 ± 0.06 Aab | 39.09 ± 1.53 Ab |
| 10–20 | 1.66 ± 0.22 Aab | 0.27 ± 0.01 Aab | 10.07 ± 0.37 Ac | 84.91 ± 7.44 Bb | 2.73 ± 0.24 Bb | 31.58 ± 3.21 Ba | |
| 20–40 | 1.11 ± 0.17 Bab | 0.27 ± 0.04 Abc | 10.89 ± 0.21 Ab | 48.47 ± 3.66 Cb | 1.60 ± 0.01 Cb | 19.63 ± 0.56 Ca | |
| S900 | 0–10 | 2.29 ± 0.07 Aa | 0.37 ± 0.03 Aab | 10.76 ± 0.88 Aa | 159.94 ± 2.59 Ab | 5.33 ± 0.09 Aa | 47.43 ± 1.84 Ab |
| 10–20 | 1.79 ± 0.05 Bab | 0.36 ± 0.03 Aa | 11.97 ± 0.27 Aa | 89.96 ± 14.29 Bb | 3.04 ± 0.09 Bb | 41.97 ± 1.25 Aa | |
| 20–40 | 1.18 ± 0.22 Cab | 0.37 ± 0.01 Aa | 12.12 ± 0.16 Aa | 52.71 ± 17.65 Bb | 1.25 ± 0.11 Cbc | 38.92 ± 2.73 Aa | |
| S1200 | 0–10 | 2.09 ± 0.02 Ab | 0.31 ± 0.07 Ab | 10.64 ± 0.60 Aa | 163.32 ± 6.45 Ab | 4.79 ± 0.27 Aab | 45.68 ± 1.98 Ab |
| 10–20 | 1.71 ± 0.17 Bab | 0.29 ± 0.08 Aab | 11.05 ± 0.24 Ab | 75.19 ± 14.16 Bb | 3.97 ± 0.12 Ba | 33.98 ± 0.18 Ba | |
| 20–40 | 1.12 ± 0.03 Cab | 0.29 ± 0.05 Ab | 11.26 ± 0.32 Aab | 49.28 ± 11.85 Bb | 1.17 ± 0.05 Cc | 30.04 ± 0.31 Ca | |
| B600 | 0–10 | 1.84 ± 0.06 Ac | 0.31 ± 0.01 Ab | 9.09 ± 0.09 Cb | 141.47 ± 8.56 Ac | 4.25 ± 0.30 Ab | 38.97 ± 0.97 Ab |
| 10–20 | 1.50 ± 0.11 Bb | 0.32 ± 0.02 Aab | 9.53 ± 0.15 Bcd | 77.89 ± 9.39 Bb | 3.46 ± 0.40 Aab | 34.13 ± 0.97 Ba | |
| 20–40 | 1.16 ± 0.06 Cab | 0.30 ± 0.01 Ab | 10.13 ± 0.02 Abc | 43.19 ± 0.73 Cb | 1.21 ± 0.17 Bbc | 27.04 ± 0.72 Ca | |
| B900 | 0–10 | 1.77 ± 0.24 Ac | 0.25 ± 0.05 Abc | 10.34 ± 0.20 Aab | 150.38 ± 2.99 Abc | 4.31 ± 0.06 Ab | 38.06 ± 0.60 Ab |
| 10–20 | 1.46 ± 0.12 Abc | 0.26 ± 0.04 Ab | 10.43 ± 0.25 Ab | 67.55 ± 5.75 Bb | 3.58 ± 0.06 Bab | 31.02 ± 1.89 Ba | |
| 20–40 | 0.93 ± 0.07 Bbc | 0.25 ± 0.02 Abc | 10.74 ± 0.03 Abc | 54.63 ± 3.40 Bb | 1.34 ± 0.13 Cbc | 30.71 ± 0.40 Ba | |
| B1200 | 0–10 | 1.84 ± 0.04 Ac | 0.26 ± 0.02 Abc | 10.70 ± 0.48 Aa | 144.37 ± 4.10 Ac | 5.31 ± 0.42 Aa | 36.02 ± 0.22 Ab |
| 10–20 | 1.14 ± 0.30 Ac | 0.27 ± 0.05 Aab | 11.32 ± 0.29 Ab | 71.09 ± 10.95 Bb | 3.98 ± 0.05 Ba | 35.70 ± 1.00 Aa | |
| 20–40 | 0.74 ± 0.19 Abc | 0.23 ± 0.04 Ac | 10.93 ± 0.43 Ab | 61.51 ± 11.37 Bab | 1.49 ± 0.09 Cbc | 30.52 ± 1.43 Ba | |
| CK | 0–10 | 1.81 ± 0.07 Ac | 0.27 ± 0.00 Abc | 9.38 ± 0.37 Ab | 179.03 ± 8.94 Aa | 0.85 ± 0.41 Ac | 58.34 ± 9.56 Aa |
| 10–20 | 1.20 ± 0.09 Bc | 0.24 ± 0.02 Bb | 9.54 ± 0.39 Ad | 122.72 ± 22.24 Ba | 0.75 ± 0.65 Ac | 42.39 ± 9.16 Ba | |
| 20–40 | 0.74 ± 0.12 Cc | 0.24 ± 0.02 Bc | 9.94 ± 0.74 Ac | 78.84 ± 14.73 Ca | 0.40 ± 0.30 Ad | 35.93 ± 9.25 Ba |
| Soil Layer | F (Methods) | p (Methods) | F (Dosages) | p (Dosages) | F (Methods × Dosages) | p (Methods × Dosages) | |
|---|---|---|---|---|---|---|---|
| (cm) | |||||||
| TN | 0–10 | 15.090 | 0.001 | 1.932 | 0.200 | 3.979 | 0.040 |
| 10–20 | 7.031 | 0.014 | 0.018 | 0.982 | 3.514 | 0.054 | |
| 20–40 | 3.776 | 0.064 | 1.256 | 0.330 | 5.888 | 0.013 | |
| TP | 0–10 | 8.389 | 0.009 | 0.314 | 0.738 | 3.496 | 0.055 |
| 10–20 | 4.491 | 0.044 | 0.119 | 0.889 | 2.556 | 0.112 | |
| 20–40 | 17.269 | 0.001 | 0.949 | 0.423 | 5.012 | 0.021 | |
| TK | 0–10 | 2.876 | 0.108 | 10.206 | 0.005 | 1.197 | 0.376 |
| 10–20 | 16.043 | 0.001 | 50.007 | 0.000 | 16.308 | 0.000 | |
| 20–40 | 23.877 | 0.000 | 36.638 | 0.000 | 11.648 | 0.001 | |
| AN | 0–10 | 12.768 | 0.002 | 14.106 | 0.002 | 2.246 | 0.144 |
| 10–20 | 3.839 | 0.062 | 0.302 | 0.747 | 0.830 | 0.538 | |
| 20–40 | 0.730 | 0.509 | 3.141 | 0.092 | 0.702 | 0.610 | |
| AP | 0–10 | 3.277 | 0.085 | 2.433 | 0.143 | 5.373 | 0.017 |
| 10–20 | 24.061 | 0.000 | 11.272 | 0.004 | 2.000 | 0.178 | |
| 20–40 | 11.699 | 0.003 | 8.548 | 0.008 | 16.532 | 0.000 | |
| AK | 0–10 | 22.731 | 0.000 | 3.869 | 0.061 | 7.657 | 0.006 |
| 10–20 | 1.329 | 0.312 | 0.639 | 0.550 | 6.213 | 0.011 | |
| 20–40 | 1.434 | 0.288 | 20.511 | 0.000 | 22.273 | 0.000 |
| Soil Layer | Dosages | Methods | TN | TP | TK | AN | AP | AK |
|---|---|---|---|---|---|---|---|---|
| (cm) | (kg/ha) | (g/kg) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| 0–10 | 600 | C | 1.92 ± 0.06 a* | 0.33 ± 0.02 A* | 9.13 ± 0.15 A | 130.50 ± 1.87 A* | 4.66 ± 0.20 a | 42.97 ± 3.17 a* |
| S | 2.02 ± 0.05 a* | 0.27 ± 0.02 A* | 9.85 ± 0.39 A | 143.28 ± 8.53 A* | 4.93 ± 0.06 a | 39.09 ± 1.53 a* | ||
| B | 1.84 ± 0.06 a* | 0.31 ± 0.01 A* | 9.09 ± 0.09 A | 141.47 ± 8.56 A* | 4.25 ± 0.30 a | 38.97 ± 0.97 a* | ||
| 900 | C | 1.90 ± 0.12 b* | 0.34 ± 0.01 A* | 9.40 ± 0.13 A | 141.79 ± 2.78 B* | 5.02 ± 0.21 ab | 41.38 ± 2.47 b* | |
| S | 2.29 ± 0.07 a* | 0.37 ± 0.03 A* | 10.76 ± 0.88 A | 159.94 ± 2.59 A* | 5.33 ± 0.09 a | 47.43 ± 1.84 a* | ||
| B | 1.77 ± 0.24 b* | 0.25 ± 0.05 A* | 10.34 ± 0.20 A | 150.38 ± 2.99 B* | 4.31 ± 0.06 b | 38.06 ± 0.60 b* | ||
| 1200 | C | 2.19 ± 0.03 a* | 0.39 ± 0.02 A* | 10.63 ± 0.82 A | 150.90 ± 1.12 A* | 4.69 ± 0.45 a | 48.51 ± 2.51 a* | |
| S | 2.09 ± 0.02 b* | 0.31 ± 0.07 A* | 10.64 ± 0.60 A | 163.32 ± 6.45 A* | 4.79 ± 0.27 a | 45.68 ± 1.98 a* | ||
| B | 1.84 ± 0.04 b* | 0.26 ± 0.02 A* | 10.70 ± 0.48 A | 144.37 ± 4.10 A* | 5.31 ± 0.42 a | 36.02 ± 0.22 b* | ||
| 10–20 | 600 | C | 1.52 ± 0.06 A* | 0.34 ± 0.02 A* | 9.87 ± 0.02 a* | 83.40 ± 8.79 A | 2.61 ± 0.29 A* | 36.27 ± 1.45 a |
| S | 1.66 ± 0.22 A* | 0.27 ± 0.01 A* | 10.07 ± 0.37 a* | 84.91 ± 7.44 A | 2.73 ± 0.24 A* | 31.58 ± 3.21 a | ||
| B | 1.50 ± 0.11 A* | 0.32 ± 0.02 A* | 9.53 ± 0.15 a* | 77.89 ± 9.39 A | 3.46 ± 0.40 A* | 34.13 ± 0.97 a | ||
| 900 | C | 1.42 ± 0.22 A* | 0.33 ± 0.02 A* | 9.70 ± 0.06 c* | 90.35 ± 3.08 A | 2.34 ± 0.51 A* | 33.64 ± 3.13 b | |
| S | 1.79 ± 0.05 A* | 0.36 ± 0.03 A* | 11.97 ± 0.27 a* | 89.96 ± 14.29 A | 3.04 ± 0.09 A* | 41.97 ± 1.25 a | ||
| B | 1.46 ± 0.12 A* | 0.26 ± 0.04 A* | 10.43 ± 0.25 b* | 67.55 ± 5.75 A | 3.58 ± 0.06 A* | 31.02 ± 1.89 b | ||
| 1200 | C | 1.87 ± 0.05 A* | 0.37 ± 0.00 A* | 11.27 ± 0.30 a* | 89.21 ± 10.47 A | 2.85 ± 0.26 B* | 34.84 ± 4.65 a | |
| S | 1.71 ± 0.17 A* | 0.29 ± 0.08 A* | 11.05 ± 0.24 a* | 75.19 ± 14.16 A | 3.97 ± 0.12 A* | 33.98 ± 0.18 a | ||
| B | 1.14 ± 0.30 A* | 0.27 ± 0.05 A* | 11.32 ± 0.29 a* | 71.09 ± 10.95 A | 3.98 ± 0.05 A* | 35.70 ± 1.00 a | ||
| 20–40 | 600 | C | 1.00 ± 0.02 a | 0.36 ± 0.03 a* | 10.20 ± 0.04 b* | 38.82 ± 4.35 A | 1.30 ± 0.14 a* | 32.16 ± 1.24 a |
| S | 1.11 ± 0.17 a | 0.27 ± 0.04 b* | 10.89 ± 0.21 a* | 48.47 ± 3.66 A | 1.60 ± 0.01 a* | 19.63 ± 0.56 b | ||
| B | 1.16 ± 0.06 a | 0.30 ± 0.01 ab* | 10.13 ± 0.02 b* | 43.19 ± 0.73 A | 1.21 ± 0.17 b* | 27.04 ± 0.72 a | ||
| 900 | C | 0.83 ± 0.08 a | 0.34 ± 0.02 a* | 10.81 ± 0.06 b* | 47.06 ± 3.57 A | 1.43 ± 0.13 a* | 28.18 ± 1.78 b | |
| S | 1.18 ± 0.22 a | 0.37 ± 0.01 a* | 12.12 ± 0.16 a* | 52.71 ± 17.65 A | 1.25 ± 0.11 a* | 38.92 ± 2.73 a | ||
| B | 0.93 ± 0.07 a | 0.25 ± 0.02 b* | 10.74 ± 0.03 b* | 54.63 ± 3.40 A | 1.34 ± 0.13 a* | 30.71 ± 0.40 b | ||
| 1200 | C | 1.28 ± 0.06 a | 0.38 ± 0.01 a* | 11.87 ± 0.14 a* | 55.76 ± 2.24 A | 2.09 ± 0.04 a* | 32.60 ± 3.65 a | |
| S | 1.12 ± 0.03 a | 0.29 ± 0.05 b* | 11.26 ± 0.32 b* | 49.28 ± 11.85 A | 1.17 ± 0.05 b* | 30.04 ± 0.31 a | ||
| B | 0.74 ± 0.19 b | 0.23 ± 0.04 b* | 10.93 ± 0.43 b* | 61.51 ± 11.37 A | 1.49 ± 0.09 b* | 30.52 ± 1.43 a |
| Soil Layer | Methods | Dosages | TN | TP | TK | AN | AP | AK |
|---|---|---|---|---|---|---|---|---|
| (cm) | (kg/ha) | (g/kg) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| 0–10 | C | 600 | 1.92 ± 0.06 b | 0.33 ± 0.02 A | 9.13 ± 0.15 A* | 130.50 ± 1.87 C* | 4.66 ± 0.20 a | 42.97 ± 3.17 b |
| 900 | 1.90 ± 0.12 b | 0.34 ± 0.01 A | 9.40 ± 0.13 A* | 141.79 ± 2.78 B* | 5.02 ± 0.21 a | 41.38 ± 2.47 b | ||
| 1200 | 2.19 ± 0.03 a | 0.39 ± 0.02 A | 10.63 ± 0.82 A* | 150.90 ± 1.12 A* | 4.69 ± 0.45 a | 48.51 ± 2.51 a | ||
| S | 600 | 2.02 ± 0.05 a | 0.27 ± 0.02 A | 9.85 ± 0.39 A* | 143.28 ± 8.53 A* | 4.93 ± 0.06 a | 39.09 ± 1.53 b | |
| 900 | 2.29 ± 0.07 a | 0.37 ± 0.03 A | 10.76 ± 0.88 A* | 159.94 ± 2.59 A* | 5.33 ± 0.09 a | 47.43 ± 1.84 a | ||
| 1200 | 2.09 ± 0.02 a | 0.31 ± 0.07 A | 10.64 ± 0.60 A* | 163.32 ± 6.45 A* | 4.79 ± 0.27 a | 45.68 ± 1.98 a | ||
| B | 600 | 1.84 ± 0.06 a | 0.31 ± 0.01 A | 9.09 ± 0.09 B* | 141.47 ± 8.56 A* | 4.25 ± 0.30 b | 38.97 ± 0.97 a | |
| 900 | 1.77 ± 0.24 a | 0.25 ± 0.05 A | 10.34 ± 0.20 A* | 150.38 ± 2.99 A* | 4.31 ± 0.06 b | 38.06 ± 0.60 a | ||
| 1200 | 1.84 ± 0.04 a | 0.26 ± 0.02 A | 10.70 ± 0.48 A* | 144.37 ± 4.10 A* | 5.31 ± 0.42 a | 36.02 ± 0.22 a | ||
| 10–20 | C | 600 | 1.52 ± 0.06 A | 0.34 ± 0.02 A | 9.87 ± 0.02 ab* | 83.40 ± 8.79 A | 2.61 ± 0.29 A* | 36.27 ± 1.45 a |
| 900 | 1.42 ± 0.22 A | 0.33 ± 0.02 A | 9.70 ± 0.06 b* | 90.35 ± 3.08 A | 2.34 ± 0.51 A* | 33.64 ± 3.13 a | ||
| 1200 | 1.87 ± 0.05 A | 0.37 ± 0.00 A | 11.27 ± 0.30 a* | 89.21 ± 10.47 A | 2.85 ± 0.26 A* | 34.84 ± 4.65 a | ||
| S | 600 | 1.66 ± 0.22 A | 0.27 ± 0.01 A | 10.07 ± 0.37 c* | 84.91 ± 7.44 A | 2.73 ± 0.24 B* | 31.58 ± 3.21 b | |
| 900 | 1.79 ± 0.05 A | 0.36 ± 0.03 A | 11.97 ± 0.27 a* | 89.96 ± 14.29 A | 3.04 ± 0.09 B* | 41.97 ± 1.25 a | ||
| 1200 | 1.71 ± 0.17 A | 0.29 ± 0.08 A | 11.05 ± 0.24 b* | 75.19 ± 14.16 A | 3.97 ± 0.12 A* | 33.98 ± 0.18 b | ||
| B | 600 | 1.50 ± 0.11 A | 0.32 ± 0.02 A | 9.53 ± 0.15 c* | 77.89 ± 9.39 A | 3.46 ± 0.40 A* | 34.13 ± 0.97 a | |
| 900 | 1.46 ± 0.12 A | 0.26 ± 0.04 A | 10.43 ± 0.25 b* | 67.55 ± 5.75 A | 3.58 ± 0.06 A* | 31.02 ± 1.89 a | ||
| 1200 | 1.14 ± 0.30 A | 0.27 ± 0.05 A | 11.32 ± 0.29 a* | 71.09 ± 10.95 A | 3.98 ± 0.05 A* | 35.70 ± 1.00 a | ||
| 20–40 | C | 600 | 1.00 ± 0.02 ab | 0.36 ± 0.03 a | 10.20 ± 0.04 c* | 38.82 ± 4.35 B | 1.30 ± 0.14 b* | 32.16 ± 1.24 a* |
| 900 | 0.83 ± 0.08 b | 0.34 ± 0.02 a | 10.81 ± 0.06 b* | 47.06 ± 3.57 AB | 1.43 ± 0.13 b* | 28.18 ± 1.78 a* | ||
| 1200 | 1.28 ± 0.06 a | 0.38 ± 0.01 a | 11.87 ± 0.14 a* | 55.76 ± 2.24 A | 2.09 ± 0.04 a* | 32.60 ± 3.65 a* | ||
| S | 600 | 1.11 ± 0.17 a | 0.27 ± 0.04 b | 10.89 ± 0.21 b* | 48.47 ± 3.66 A | 1.60 ± 0.01 a* | 19.63 ± 0.56 c* | |
| 900 | 1.18 ± 0.22 a | 0.37 ± 0.01 a | 12.12 ± 0.16 a* | 52.71 ± 17.65 A | 1.25 ± 0.11 b* | 38.92 ± 2.73 a* | ||
| 1200 | 1.12 ± 0.03 a | 0.29 ± 0.05 ab | 11.26 ± 0.32 ab* | 49.28 ± 11.85 A | 1.17 ± 0.05 b* | 30.04 ± 0.31 b* | ||
| B | 600 | 1.16 ± 0.06 a | 0.30 ± 0.01 a | 10.13 ± 0.02 b* | 43.19 ± 0.73 A | 1.21 ± 0.17 a* | 27.04 ± 0.72 a* | |
| 900 | 0.93 ± 0.07 ab | 0.25 ± 0.02 a | 10.74 ± 0.03 a* | 54.63 ± 3.40 A | 1.34 ± 0.13 a* | 30.71 ± 0.40 a* | ||
| 1200 | 0.74 ± 0.19 b | 0.23 ± 0.04 a | 10.93 ± 0.43 a* | 61.51 ± 11.37 A | 1.49 ± 0.09 a* | 30.52 ± 1.43 a* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Guan, F.; Zhou, X.; Liu, L.; Fu, D.; Zhang, X.; Li, M. Effect of Fertilization on Soil Fertility and Individual Stand Biomass in Strip Cut Moso Bamboo (Phyllostachys edulis) Forests. Forests 2024, 15, 252. https://doi.org/10.3390/f15020252
Li Z, Guan F, Zhou X, Liu L, Fu D, Zhang X, Li M. Effect of Fertilization on Soil Fertility and Individual Stand Biomass in Strip Cut Moso Bamboo (Phyllostachys edulis) Forests. Forests. 2024; 15(2):252. https://doi.org/10.3390/f15020252
Chicago/Turabian StyleLi, Zhen, Fengying Guan, Xiao Zhou, Liyang Liu, Dawei Fu, Xuan Zhang, and Minkai Li. 2024. "Effect of Fertilization on Soil Fertility and Individual Stand Biomass in Strip Cut Moso Bamboo (Phyllostachys edulis) Forests" Forests 15, no. 2: 252. https://doi.org/10.3390/f15020252
APA StyleLi, Z., Guan, F., Zhou, X., Liu, L., Fu, D., Zhang, X., & Li, M. (2024). Effect of Fertilization on Soil Fertility and Individual Stand Biomass in Strip Cut Moso Bamboo (Phyllostachys edulis) Forests. Forests, 15(2), 252. https://doi.org/10.3390/f15020252





