Climate Change Threatens the Habitat of Pinus massoniana in China
Abstract
:1. Introduction
2. Materials and Methods
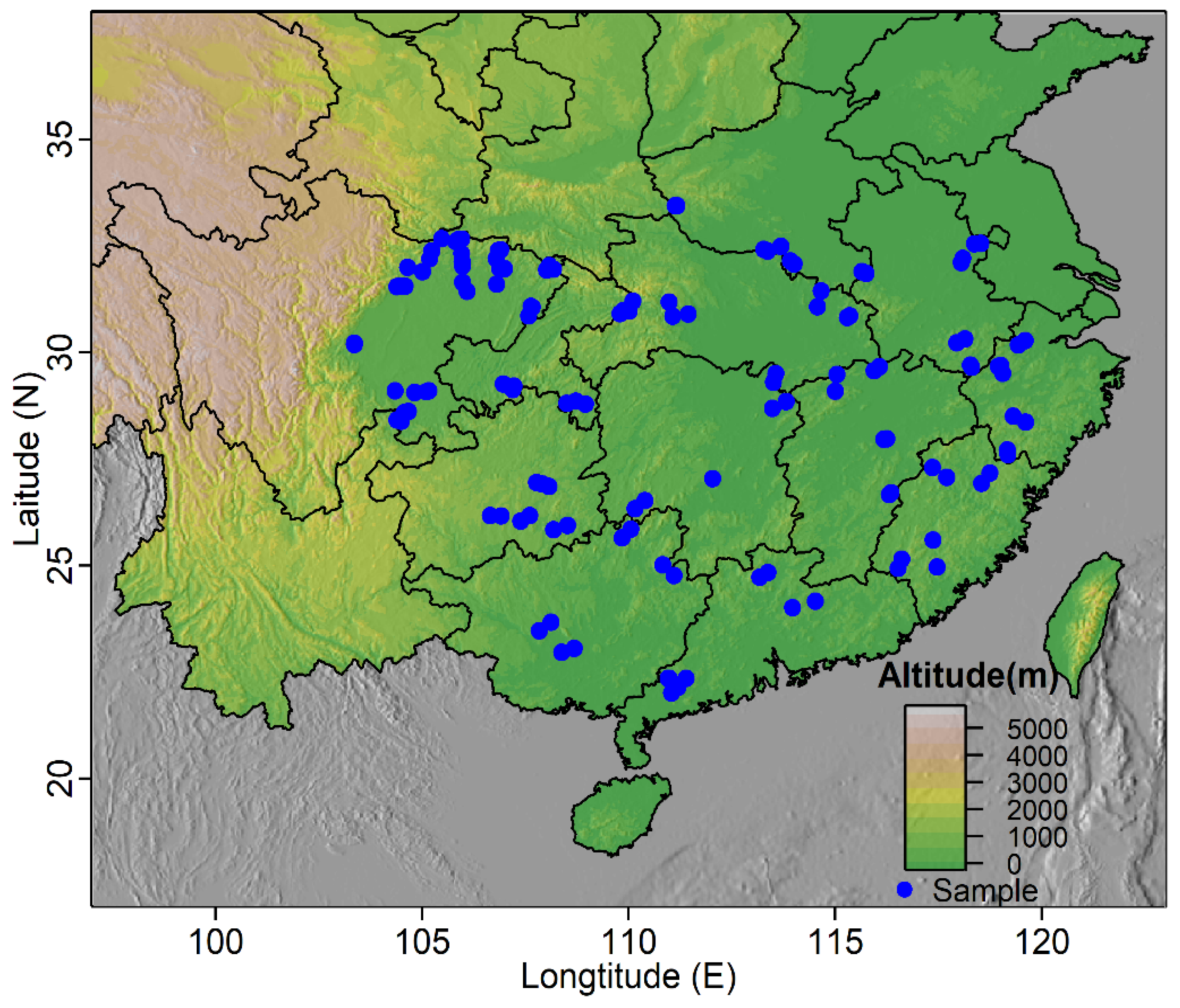
2.1. Species Occurrence Data
2.2. Environmental Variables
2.3. Model Evaluation
2.3.1. Climate Suitability Model
2.3.2. Distribution Limitation Model
2.3.3. Comprehensive Habitat Suitability Model
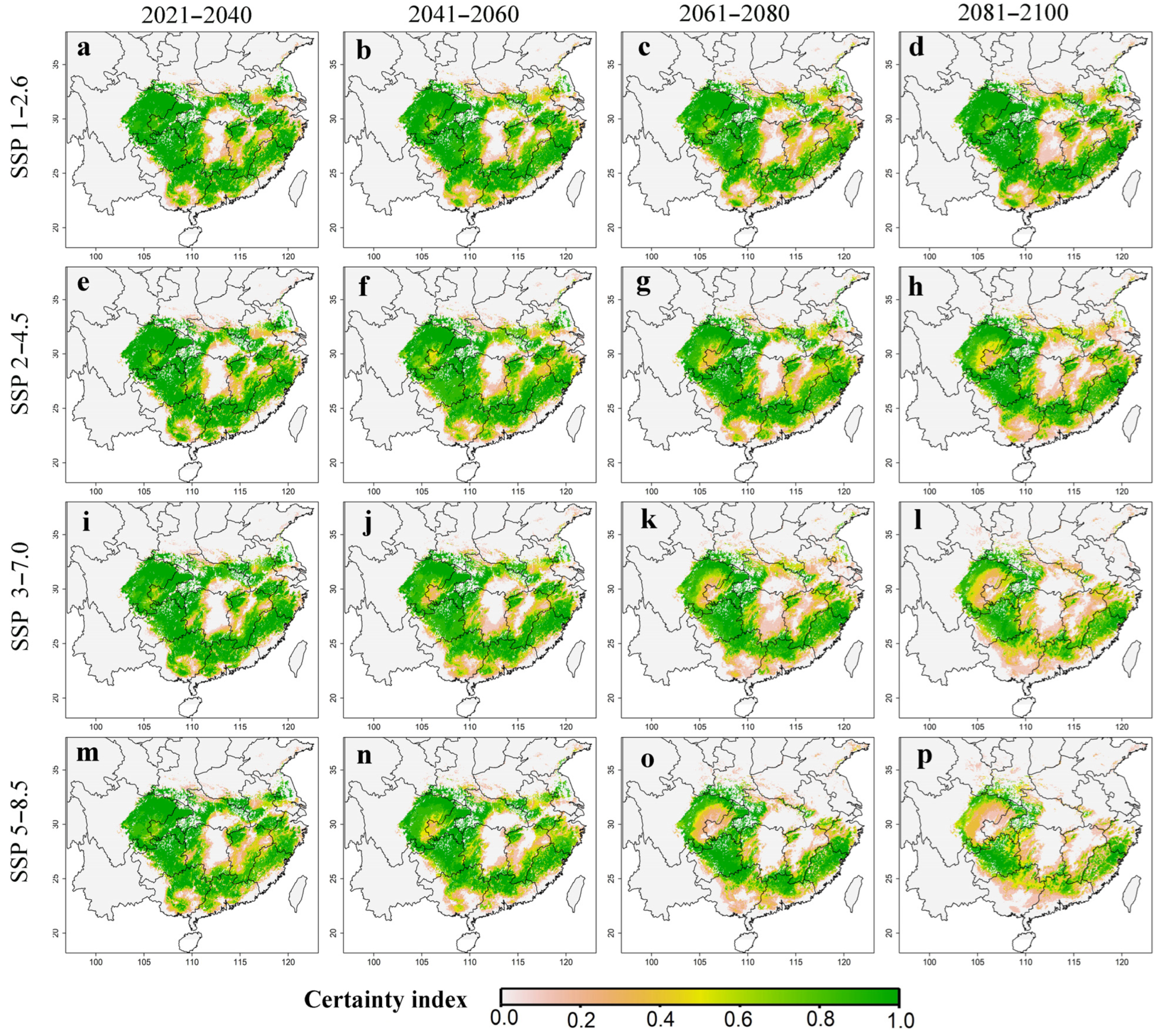
2.4. The Certainty Index of the Suitable Habitat of P. massoniana in the Future
3. Results
3.1. Model Precision
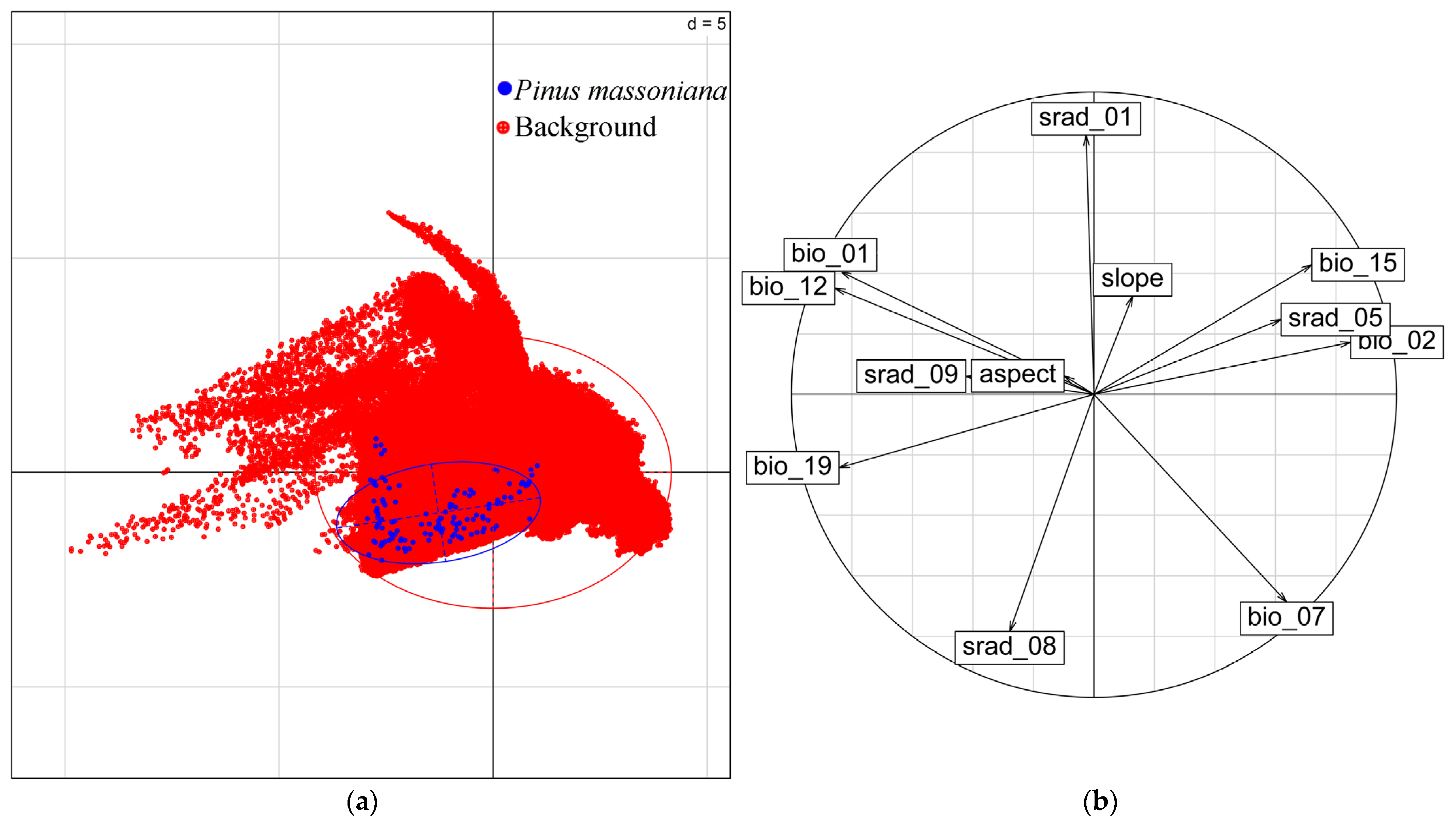
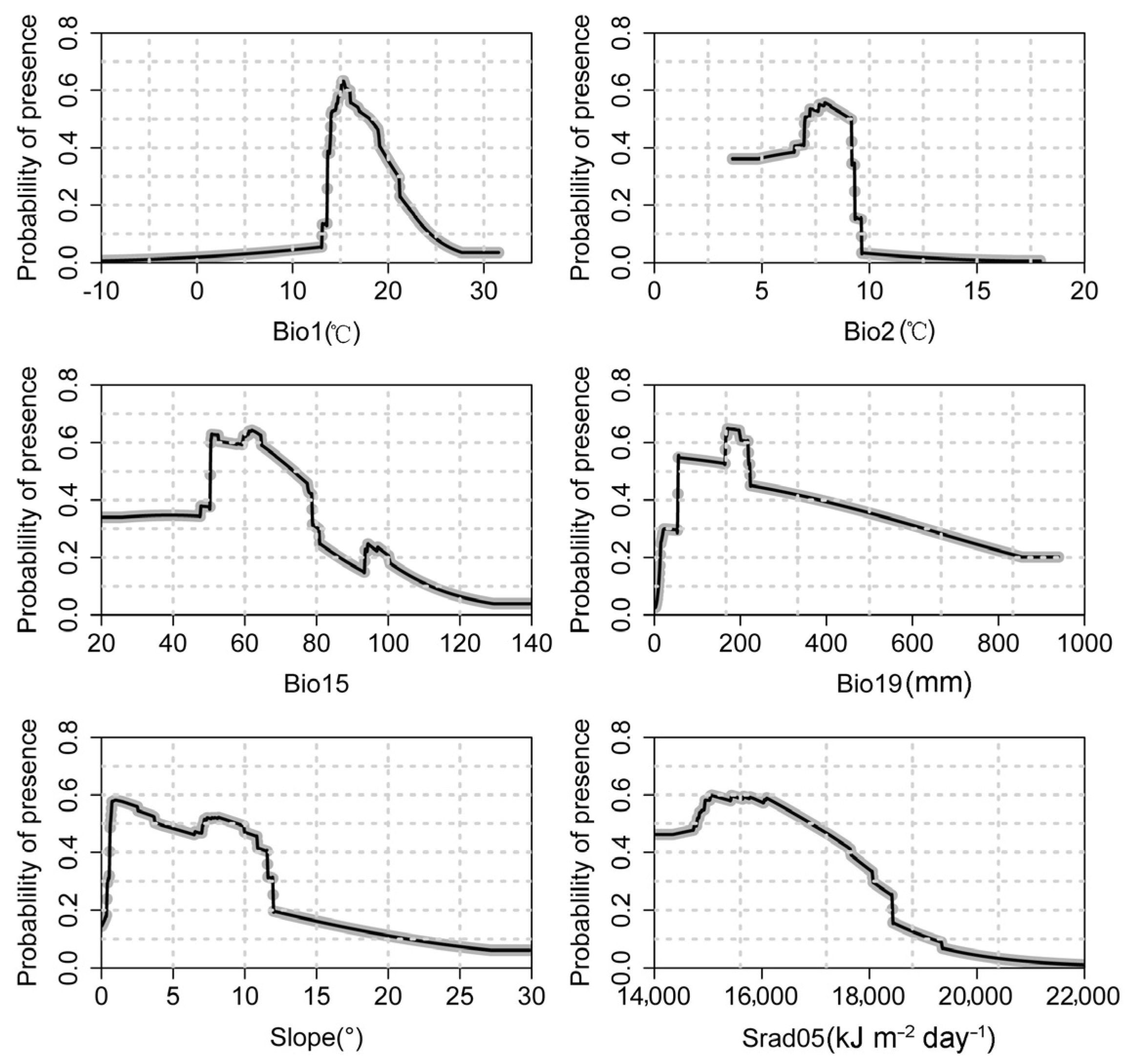
3.2. Dominant Environmental Variables Affecting the Distribution of P. massoniana
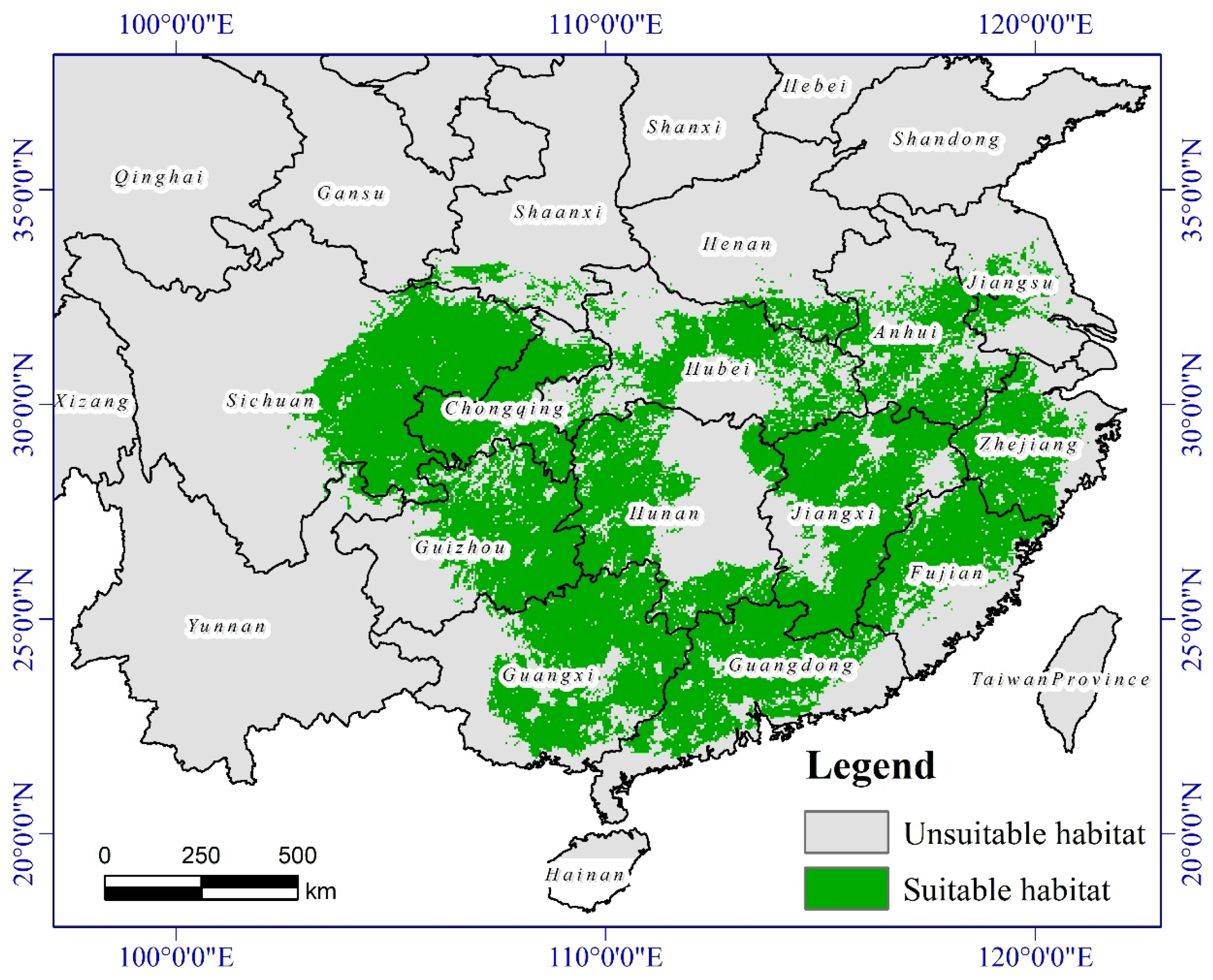
3.3. The Suitable Habitat Distribution of P. massoniana under Climate Change
4. Discussion
4.1. Uncertainty of Model Results
4.2. Management Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Woodward, F.I.; Williams, B. Climate and plant distribution at global and local scales. Vegetatio 1987, 69, 189–197. [Google Scholar] [CrossRef]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; De Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gégout, J.-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Jacob, D.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.; Engelbrecht, F.; Guiot, J. Impacts of 1.5 C global warming on natural and human systems. In Global Warming of 1.5 °C; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Lenoir, J.; Gégout, J.-C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, H.; Mi, Z.; Zhang, Z.; Wang, Y.; Xu, W.; Jiang, L.; He, J.-S. Climate warming reduces the temporal stability of plant community biomass production. Nat. Commun. 2017, 8, 15378. [Google Scholar] [CrossRef]
- Paquette, A.; Vayreda, J.; Coll, L.; Messier, C.; Retana, J. Climate change could negate positive tree diversity effects on forest productivity: A study across five climate types in Spain and Canada. Ecosystems 2018, 21, 960–970. [Google Scholar] [CrossRef]
- Randin, C.F.; Engler, R.; Normand, S.; Zappa, M.; Zimmermann, N.E.; Pearman, P.B.; Vittoz, P.; Thuiller, W.; Guisan, A. Climate change and plant distribution: Local models predict high-elevation persistence. Glob. Chang. Biol. 2009, 15, 1557–1569. [Google Scholar] [CrossRef]
- Leng, W.; He, H.S.; Bu, R.; Dai, L.; Hu, Y.; Wang, X. Predicting the distributions of suitable habitat for three larch species under climate warming in Northeastern China. For. Ecol. Manag. 2008, 254, 420–428. [Google Scholar] [CrossRef]
- Austin, M.P.; Van Niel, K.P. Improving species distribution models for climate change studies: Variable selection and scale. J. Biogeogr. 2011, 38, 1–8. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Felton, A.M.; Felton, A.; Foley, W.; Lindenmayer, D.B. The role of timber tree species in the nutritional ecology of spider monkeys in a certified logging concession, Bolivia. For. Ecol. Manag. 2010, 259, 1642–1649. [Google Scholar] [CrossRef]
- Wilson, L.F. China’s Masson pine forests: Cure or curse? J. For. 1993, 91, 30–33. [Google Scholar]
- Anderson, R.P. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef]
- Brun, P.; Thuiller, W.; Chauvier, Y.; Pellissier, L.; Wüest, R.O.; Wang, Z.; Zimmermann, N.E. Model complexity affects species distribution projections under climate change. J. Biogeogr. 2020, 47, 130–142. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Mi, C.; Huettmann, F.; Guo, Y.; Han, X.; Wen, L. Why choose Random Forest to predict rare species distribution with few samples in large undersampled areas? Three Asian crane species models provide supporting evidence. PeerJ 2017, 5, e2849. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Applying various algorithms for species distribution modelling. Integr. Zool. 2013, 8, 124–135. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Grenouillet, G.; Buisson, L.; Casajus, N.; Lek, S. Ensemble modelling of species distribution: The effects of geographical and environmental ranges. Ecography 2011, 34, 9–17. [Google Scholar] [CrossRef]
- Thibaud, E.; Petitpierre, B.; Broennimann, O.; Davison, A.C.; Guisan, A. Measuring the relative effect of factors affecting species distribution model predictions. Methods Ecol. Evol. 2014, 5, 947–955. [Google Scholar] [CrossRef]
- Du, M.; Ding, G.; Cai, Q. The transcriptomic responses of Pinus massoniana to drought stress. Forests 2018, 9, 326. [Google Scholar] [CrossRef]
- Zhao, G.-W.; Zhong, T.-L. Improving the assessment method of seed vigor in Cunninghamia lanceolata and Pinus massoniana based on oxygen sensing technology. J. For. Res. 2012, 23, 95–101. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, M.; Zhou, L.; Yang, G.; Huang, L.; Yan, C.; Huang, Q.; Ye, L.; Zhang, X.; Guo, L. Regionalization of Habitat Suitability of Masson’s Pine based on geographic information system and Fuzzy Matter-Element Model. Sci. Rep. 2016, 6, 34716. [Google Scholar] [CrossRef]
- Forster, P.M.; Richardson, T.; Maycock, A.C.; Smith, C.J.; Samset, B.H.; Myhre, G.; Andrews, T.; Pincus, R.; Schulz, M. Recommendations for diagnosing effective radiative forcing from climate models for CMIP6. J. Geophys. Res. Atmos. 2016, 121, 460–475. [Google Scholar] [CrossRef]
- Hausfather, Z. CMIP6: The Next Generation of Climate Models Explained. Available online: https://www.carbonbrief.org/cmip6-the-next-generation-of-climate-models-explained/ (accessed on 1 October 2023).
- Zhang, Z.; Jin, G.; Feng, Z.; Sun, L.; Zhou, Z.; Zheng, Y.; Yuan, C. Joint influence of genetic origin and climate on the growth of Masson pine (Pinus massoniana Lamb.) in China. Sci. Rep. 2020, 10, 4653. [Google Scholar] [CrossRef]
- Li, S.; Shi, M.; Liu, X. Precise spatial distribution of suitability of masson pine in Hubei Province. Acta Ecol. Sin. 2019, 39, 1960–1968. [Google Scholar]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Yuan, S.; Li, X. A greener Loess Plateau in the future: Moderate warming will expand the potential distribution areas of woody species. Environ. Res. Lett. 2023, 18, 034027. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.; Lei, X.; Zhao, Z.; Xiang, W.; Yan, W. Pinus massoniana productivity at different age stages in relation to climatic factors. Chin. J. Ecol. 2013, 32, 1104. [Google Scholar]
- Molloy, S.W.; Davis, R.A.; Van Etten, E.J. Species distribution modelling using bioclimatic variables to determine the impacts of a changing climate on the western ringtail possum (Pseudocheirus occidentals; Pseudocheiridae). Environ. Conserv. 2014, 41, 176–186. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef]
- Robertson, M.; Caithness, N.; Villet, M. A PCA-based modelling technique for predicting environmental suitability for organisms from presence records. Divers. Distrib. 2001, 7, 15–27. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Wei, H.; Ran, Q.; Liu, J.; Zhang, Q.; Gu, W. Potential distribution of Notopterygium incisum Ting ex HT Chang and its predicted responses to climate change based on a comprehensive habitat suitability model. Ecol. Evol. 2020, 10, 3004–3016. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Marmion, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. The performance of state-of-the-art modelling techniques depends on geographical distribution of species. Ecol. Model. 2009, 220, 3512–3520. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Glob. Change Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef]
- Dormann, C.F.; Purschke, O.; Marquez, J.R.G.; Lautenbach, S.; Schroeder, B. Components of uncertainty in species distribution analysis: A case study of the great grey shrike. Ecology 2008, 89, 3371–3386. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, M.; Liu, Y.; Liu, F. Impact of climate change on suitable habitats of Pinus massoniana in Hunan Province. J. Nanjing For. Univ. 2019, 62, 94–100. [Google Scholar]
- Zhang, L.; Liu, S.; Sun, P.; Wang, T. Comparative evaluation of multiple models of the effects of climate change on the potential distribution of Pinus massoniana. Chin. J. Plant Ecol. 2011, 35, 1091–1105. [Google Scholar] [CrossRef]
- Tuanmu, M.-N.; Viña, A.; Winkler, J.A.; Li, Y.; Xu, W.; Ouyang, Z.; Liu, J. Climate-change impacts on understorey bamboo species and giant pandas in China’s Qinling Mountains. Nat. Clim. Chang. 2013, 3, 249–253. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Wang, W.-J.; He, J.-S.; Yang, R.-H.; Wu, H.-J.; Wang, X.-L.; Jiao, L.; Tang, Z.; Yao, Y.-J. Range shifts in response to climate change of Ophiocordyceps sinensis, a fungus endemic to the Tibetan Plateau. Biol. Conserv. 2017, 206, 143–150. [Google Scholar] [CrossRef]

| Classification and Data Source | Variables |
|---|---|
| Climatic variables Data source: WorldClim database (http://www.worldclim.org/, accessed on 30 October 2023) | BIO variables: bio1: annual mean air temperature; bio2: mean diurnal temperature range; bio7: annual temperature range; bio12: annual precipitation; bio15: precipitation seasonality; bio19: precipitation of the coldest quarter Solar radiation variables: srad_01: solar radiation in January, srad_05: solar radiation in May, srad_08: solar radiation in August, srad_09: solar radiation in September Topographical variables: ASPECT, SLOPE |
| Soil variables Data source: Harmonized World Soil Database (HWSD) (http://www.fao.org/soils-portal/, accessed on 30 October 2023) | T_GRAVEL: Topsoil Gravel Content; T_SAND: Topsoil Sand Fraction; T_SILT: Topsoil Silt Fraction; T_CLAY: Topsoil Clay Fraction; T_OC: Topsoil Organic Carbon; T_PH_H2O: Topsoil pH (H2O); T_CACO3: Topsoil Calcium Carbonate; T_CASO4: Topsoil Gypsum; T_ESP: Topsoil Sodicity; T_USDA: Topsoil USDA Texture Classification |
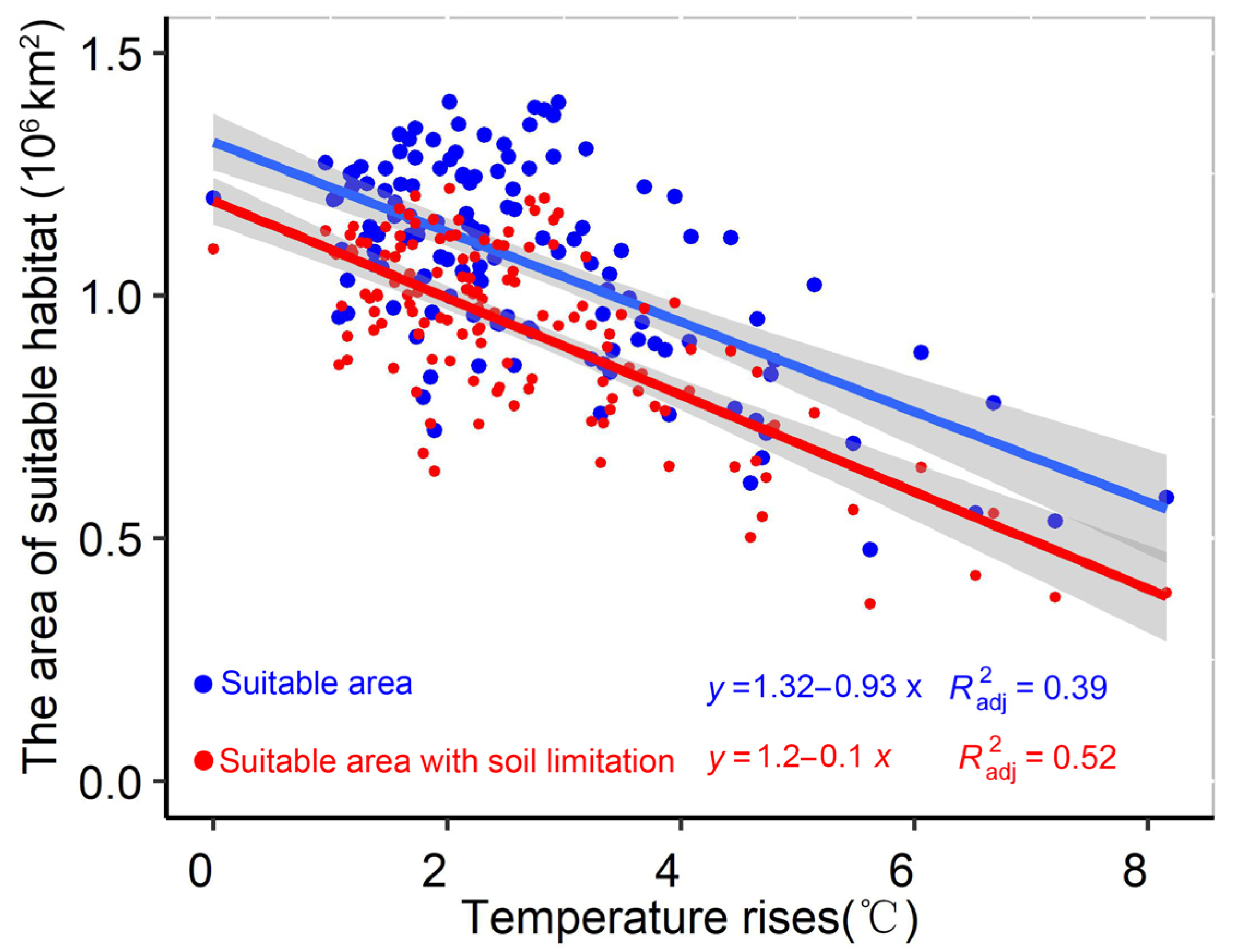
| Times | Climate Change Scenario | Average Temperature Rise (°C) | Suitable Habitat Area (106 km2) |
|---|---|---|---|
| Baseline | ----- | ------ | 1.10 |
| 2021–2040 | SSP 1–2.6 | 1.47 | 1.07 |
| SSP 2–4.5 | 1.44 | 1.06 | |
| SSP 3–7.0 | 1.30 | 1.08 | |
| SSP 5–8.5 | 1.60 | 1.02 | |
| 2041–2060 | SSP 1–2.6 | 1.96 | 1.06 |
| SSP 2–4.5 | 2.19 | 1.02 | |
| SSP 3–7.0 | 2.19 | 0.99 | |
| SSP 5–8.5 | 2.77 | 0.90 | |
| 2061–2080 | SSP 1–2.6 | 2.11 | 0.99 |
| SSP 2–4.5 | 2.79 | 0.95 | |
| SSP 3–7.0 | 3.25 | 0.86 | |
| SSP 5–8.5 | 4.16 | 0.68 | |
| 2081–2100 | SSP 1–2.6 | 2.01 | 1.05 |
| SSP 2–4.5 | 3.23 | 0.82 | |
| SSP 3–7.0 | 4.35 | 0.67 | |
| SSP 5–8.5 | 5.79 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Chao, S.; Zhao, Z.; Jing, M. Climate Change Threatens the Habitat of Pinus massoniana in China. Forests 2024, 15, 323. https://doi.org/10.3390/f15020323
Zhao Z, Chao S, Zhao Z, Jing M. Climate Change Threatens the Habitat of Pinus massoniana in China. Forests. 2024; 15(2):323. https://doi.org/10.3390/f15020323
Chicago/Turabian StyleZhao, Zefang, Shigang Chao, Zebin Zhao, and Meixiu Jing. 2024. "Climate Change Threatens the Habitat of Pinus massoniana in China" Forests 15, no. 2: 323. https://doi.org/10.3390/f15020323





