Waterlogging Hardening Effect on Transplant Stress Tolerance in Pinus densiflora
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Sites and Plant Materials
2.2. Growth
2.3. Leaf Gas Exchange Measurement Growth
2.4. Leaf Nonstructural Carbohydrates Content
2.5. Proline Content
2.6. Total Chlorophyll Content
2.7. Statistical Analysis
2.8. RNA Extraction and Sequencing
2.9. Transcriptome Analysis: De Novo Assembly and Differential Expression Analysis
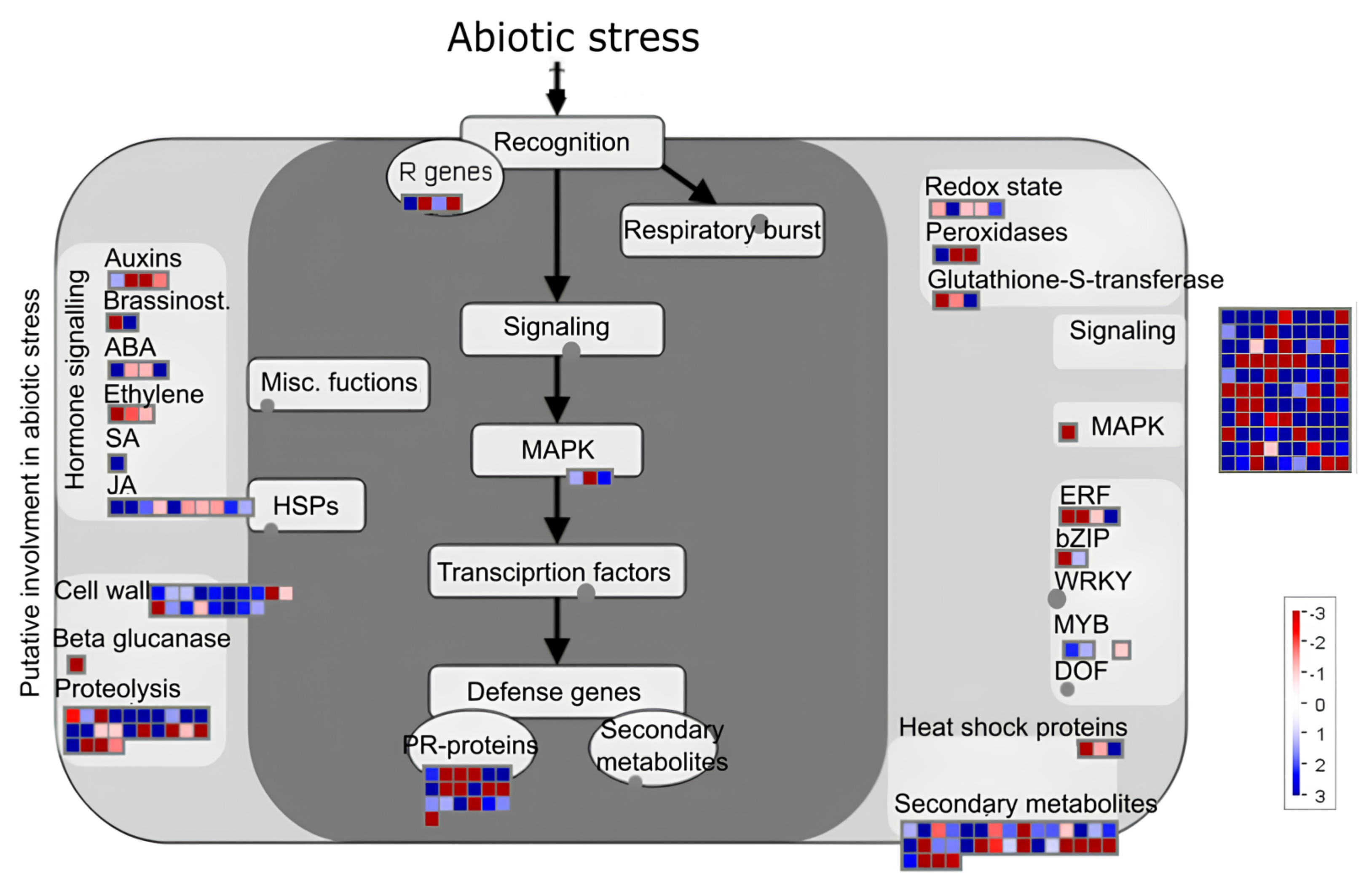
2.10. Functional Analysis and MapMan Analysis
2.11. Quantitative Real-Time (qRT)-PCR Validation
2.12. Data Deposition
3. Results
3.1. Data Deposition
3.2. Assembled Transcriptome Data
3.3. Comparison of Differentially Expressed Genes between Waterlogging and Control Seedlings
3.4. Gene Ontology (GO) Term Classification of Genes Induced by Transplant Stress
3.5. Gene Ontology (GO) Term Classification of Differentially Expressed Genes between Waterlogging and Control after Transplanting
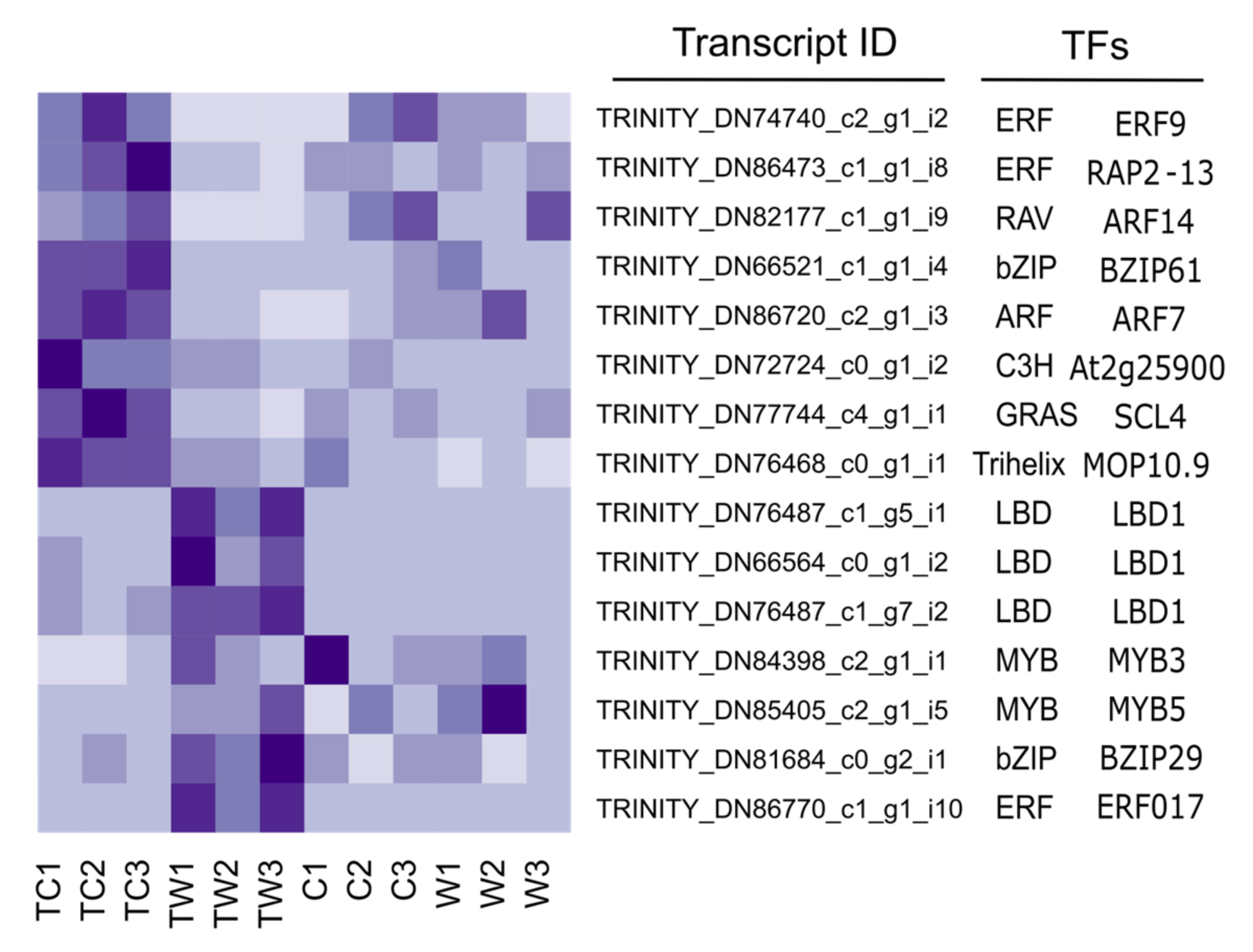
3.6. Transcription Factors and Pathway Analysis after Transplanting
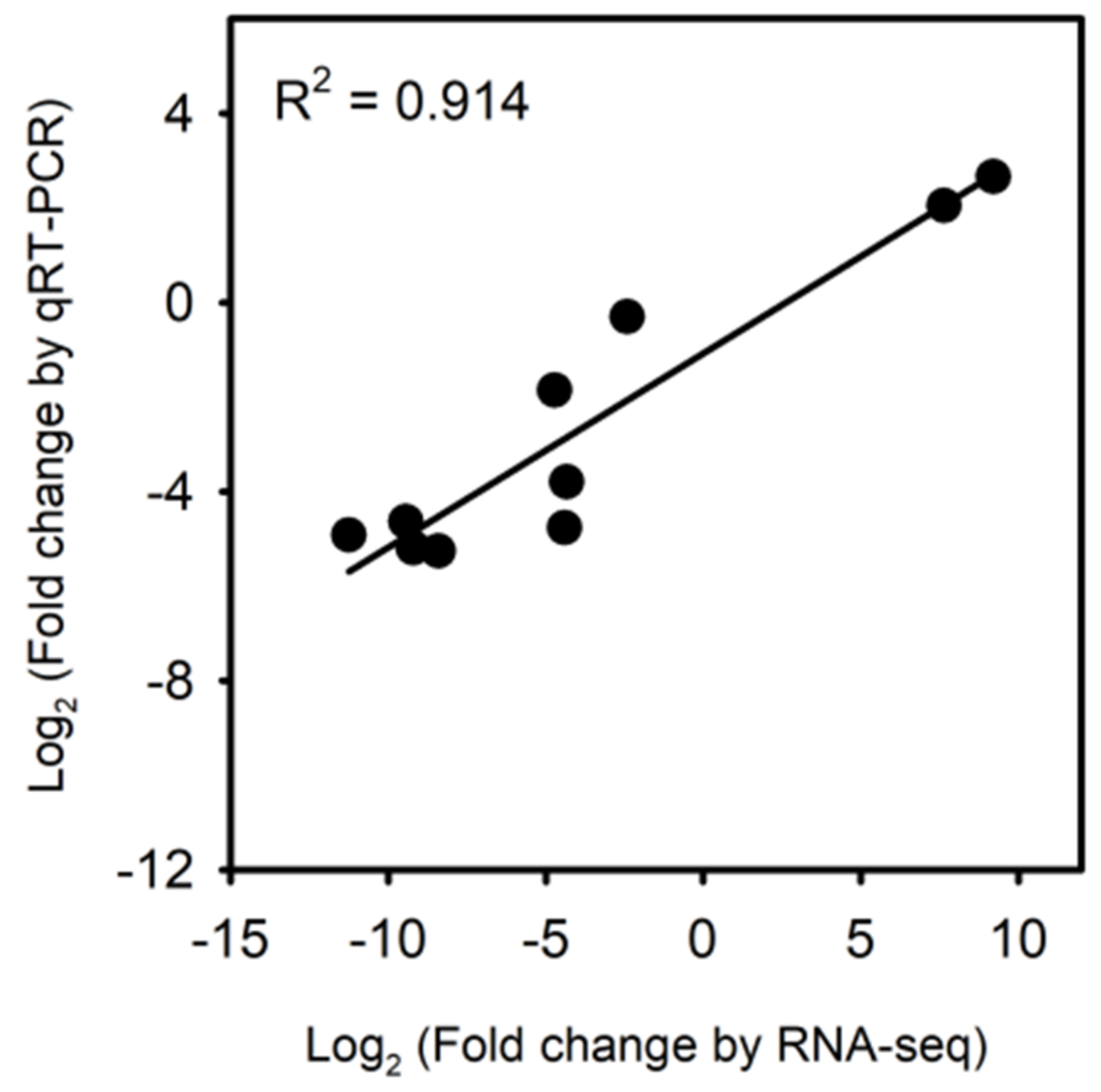
3.7. Validation of RNA-Seq Expression of Waterlogging Hardened Trees in Transplant Stress
3.8. Physiological Response of Waterlogging Hardening after Transplanting
4. Discussion
4.1. Genes Regulated by Transplant Stress
4.2. Waterlogging Hardening Effect after Transplanting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An overview of plant responses to soil waterlogging. Plant Stress 2008, 2, 20–27. [Google Scholar]
- Muhammad, A.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar]
- Urquhart, G.R. Flood-tolerance and flood-escape mechanisms for seeds and seedlings of common swamp trees of Central America. Trop. Ecol. 2004, 45, 197–208. [Google Scholar]
- Onda, Y.; Gomi, T.; Mizugaki, S.; Nonoda, T.; Sidle, R.C. An overview of the field and modelling studies on the effects of forest devastation on flooding and environmental issues. Hydrol. Process. Int. J. 2010, 24, 527–534. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The Impact of Disasters on Agriculture and Food Security; Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- Bange, M.; Milroy, S.; Thongbai, P. Growth and yield of cotton in response to waterlogging. Field Crops Res. 2004, 88, 129–142. [Google Scholar] [CrossRef]
- Hodgson, A. The effects of duration, timing and chemical amelioration of short-term waterlogging during furrow irrigation of cotton in a cracking grey clay. Aust. J. Agric. Res. 1982, 33, 1019–1028. [Google Scholar] [CrossRef]
- Stagg, C.L.; Osland, M.J.; Moon, J.A.; Feher, L.C.; Laurenzano, C.; Lane, T.C.; Jones, W.R.; Hartley, S.B. Extreme precipitation and flooding contribute to sudden vegetation dieback in a coastal salt marsh. Plants 2021, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Rodriguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef]
- Bansal, R.; Srivastava, J. Effect of waterlogging on photosynthetic and biochemical parameters in pigeonpea. Russ. J. Plant Physiol. 2015, 62, 322–327. [Google Scholar] [CrossRef]
- Folzer, H.; Dat, J.F.; Capelli, N.; Rieffel, D.; Badot, P.-M. Response of sessile oak seedlings (Quercus petraea) to flooding: An integrated study. Tree Physiol. 2006, 26, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Dai, Q.; Liu, X.; Huang, S.; Wang, Z. Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 1996, 179, 261–268. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Wang, H.; Bao, Y.; Zhang, W. Examination of the leaf proteome during flooding stress and the induction of programmed cell death in maize. Proteome Sci. 2014, 12, 33. [Google Scholar] [CrossRef]
- Ahmad, P.; Umar, S.; Sharma, S. Mechanism of free radical scavenging and role of phytohormones in plants under abiotic stresses. In Plant Adaptation Phytoremediation; Springer: Dordrecht, The Netherlands, 2010; pp. 99–118. [Google Scholar]
- Alhaithloul, H.A.S.; Abu-Elsaoud, A.M.; Soliman, M.H. Abiotic stress tolerance in crop plants: Role of phytohormones. In Abiotic Stress Plants; IntechOpen: London, UK, 2020; p. 233. [Google Scholar]
- Bashar, K.K.; Tareq, M.Z.; Amin, M.R.; Honi, U.; Tahjib-Ul-Arif, M.; Sadat, M.A.; Hossen, Q.M.M. Phytohormone-mediated stomatal response, escape and quiescence strategies in plants under flooding stress. Agronomy 2019, 9, 43. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Komatsu, S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Lu, H.; Kong, X.; Dai, J.; Li, Z.; Dong, H. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Res. 2016, 194, 83–93. [Google Scholar] [CrossRef]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2. 6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef]
- Nan, R.; Carman, J.G.; Salisbury, F.B. Water stress, CO2 and photoperiod influence hormone levels in wheat. J. Plant Physiol. 2002, 159, 307–312. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef]
- Ye, H.; Song, L.; Chen, H.; Valliyodan, B.; Cheng, P.; Ali, L.; Vuong, T.; Wu, C.; Orlowski, J.; Buckley, B. A major natural genetic variation associated with root system architecture and plasticity improves waterlogging tolerance and yield in soybean. Plant Cell Environ. 2018, 41, 2169–2182. [Google Scholar] [CrossRef]
- Caudle, K.L.; Maricle, B.R. Effects of flooding on photosynthesis, chlorophyll fluorescence, and oxygen stress in plants of varying flooding tolerance. Trans. Kans. Acad. Sci. 2012, 115, 5–18. [Google Scholar]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Janiak, A.; Kwasniewski, M.; Sowa, M.; Gajek, K.; Żmuda, K.; Kościelniak, J.; Szarejko, I. No time to waste: Transcriptome study reveals that drought tolerance in barley may be attributed to stressed-like expression patterns that exist before the occurrence of stress. Front. Plant Sci. 2018, 8, 2212. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ma, X.; Shah, S.; Wu, X.; Shaheen, A.; Xiao, L.; Wu, Y.; Wang, S. Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. 2020, 20, 486. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Xu, X.-F.; Sun, Y.-M.; Zhang, J.-L.; Li, C.-Z. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Saiki, S.-T.; Ando, Y.; Yazaki, K.; Tobita, H. Drought hardening contributes to the maintenance of proportions of non-embolized xylem and cambium status during consecutive dry treatment in container-grown seedling of Japanese cedar (Cryptomeria japonica). Forests 2020, 11, 441. [Google Scholar] [CrossRef]
- Dong, X.; Bi, H.; Wu, G.; Ai, X. Drought-induced chilling tolerance in cucumber involves membrane stabilisation improved by antioxidant system. Int. J. Plant Prod. 2013, 7, 67–80. [Google Scholar]
- Ghanbari, F.; Sayyari, M. Controlled drought stress affects the chilling-hardening capacity of tomato seedlings as indicated by changes in phenol metabolisms, antioxidant enzymes activity, osmolytes concentration and abscisic acid accumulation. Sci. Hortic. 2018, 229, 167–174. [Google Scholar] [CrossRef]
- Cayuela, E.; Muñoz-Mayor, A.; Vicente-Agulló, F.; Moyano, E.; Garcia-Abellan, J.O.; Estañ, M.T.; Bolarín, M.C. Drought pretreatment increases the salinity resistance of tomato plants. J. Plant Nutr. Soil Sci. 2007, 170, 479–484. [Google Scholar] [CrossRef]
- Li, C.; Jiang, D.; Wollenweber, B.; Li, Y.; Dai, T.; Cao, W. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Sci. 2011, 180, 672–678. [Google Scholar] [CrossRef]
- Agualongo, D.A.P.; Da-Silva, C.J.; Garcia, N.; de Oliveira, F.K.; Shimoia, E.P.; Posso, D.A.; de Oliveira, A.C.B.; de Oliveira, D.d.S.C.; do Amarante, L. Waterlogging priming alleviates the oxidative damage, carbohydrate consumption, and yield loss in soybean (Glycine max) plants exposed to waterlogging. Funct. Plant Biol. 2022, 49, 1029–1042. [Google Scholar] [CrossRef]
- Hossain, M.A.; Li, Z.-G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: Key regulators and possible mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; González-Hernández, A.I.; Scalschi, L.; Fernández-Crespo, E.; Camañes, G.; Vicedo, B.; García-Agustín, P. Priming mediated stress and cross-stress tolerance in plants: Concepts and opportunities. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Thomas, T.D.; Dinakar, C.; Puthur, J.T. Effect of UV-B priming on the abiotic stress tolerance of stress-sensitive rice seedlings: Priming imprints and cross-tolerance. Plant Physiol. Biochem. 2020, 147, 21–30. [Google Scholar] [CrossRef]
- Landis, T.D.; Tinus, R.; Barnett, J. Chapter 4—Seedling development: The establishment, rapid growth, and hardening phases. In The Container Tree Nursery Manual; Agricultural Handbook 674; Forestry Service, U.S. Department of Agriculture: Washington, DC, USA, 1999; Volume 6—Seedling Propogation, pp. 125–163. [Google Scholar]
- Luo, N.; Grossnickle, S.C.; Li, G. The effect of nursery drought preconditioning on summer plantation performance of Pinus tabuliformis: High winter mortality mediated by trade off between seedling carbohydrate and field growth. New For. 2022, 53, 301–317. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H. The physiological basis of containerised tree seedling ‘transplant shock’: A review. Aust. For. 2005, 68, 112–120. [Google Scholar] [CrossRef]
- Rietveld, W. Transplanting stress in bareroot conifer seedlings: Its development and progression to establishment. North. J. Appl. For. 1989, 6, 99–107. [Google Scholar] [CrossRef]
- Lee, K.-C.; Kweon, H.; Sung, J.-W.; Kim, Y.S.; Song, Y.G.; Cha, S.; Koo, N. Physiological response analysis for the diagnosis of drought and waterlogging damage in Prunus yedoensis. For. Sci. Technol. 2022, 18, 14–25. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.F.; González, M. Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef]
- Szmidt, A.; Wang, X.-R. Molecular systematics and genetic differentiation of Pinus sylvestris (L.) and P. densiflora (Sieb. et Zucc.). Theor. Appl. Genet. 1993, 86, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Korea Forest Service. Statistical Year Book of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2006. (In Korean)
- Lee, S.J.; Shin, D.B.; Lee, A.R.; Oh, S.H. Characteristics of Dieback of Pinus densiflora and Risk Assessment in the Wangpicheon Ecosystem and Landscape Conservation Area in Uljin, South Korea. Forests 2023, 14, 903. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.S.; Lim, J.H. Topographic and meteorological characteristics of Pinus densiflora dieback areas in Sogwang-Ri, Uljin. Korean J. Agric. For. Meteorol. 2017, 19, 10–18. [Google Scholar] [CrossRef]
- Bhusal, N.; Adhikari, A.; Lee, M.; Han, A.; Han, A.R.; Kim, H.S. Evaluation of growth responses of six gymnosperm species under long-term excessive irrigation and traits determining species resistance to waterlogging. Agric. For. Meteorol. 2022, 323, 109071. [Google Scholar] [CrossRef]
- Press, M.C.; Scholes, J.D.; Barker, M.G. Physiological Plant Ecology; Blackwell Science Oxford: Oxford, UK, 1999. [Google Scholar]
- Newell, E.A.; Mulkey, S.S.; Wright, J.S. Seasonal patterns of carbohydrate storage in four tropical tree species. Oecologia 2002, 131, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, G. New colorimetric methods of sugar analysis. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1966; Volume 8, pp. 85–95. [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 317–331. [Google Scholar]
- Shinano, T.; Lei, T.; Kawamukai, T.; Inoue, M.; Koike, T.; Tadano, T. Dimethylsulfoxide method for the extraction of chlorophylls a and b from the leaves of wheat, field bean, dwarf bamboo, and oak. Photosynthetica 1996, 32, 409–415. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Hao, Z.-Z.; Liu, Y.-Y.; Nazaire, M.; Wei, X.-X.; Wang, X.-Q. Molecular phylogenetics and evolutionary history of sect. Quinquefoliae (Pinus): Implications for Northern Hemisphere biogeography. Mol. Phylogenet. Evol. 2015, 87, 65–79. [Google Scholar] [CrossRef]
- Tang, S.; Lomsadze, A.; Borodovsky, M. Identification of protein coding regions in RNA transcripts. Nucleic Acids Res. 2015, 43, e78. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. PANTHER version 11: Expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017, 45, D183–D189. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, gkw982. [Google Scholar] [CrossRef]
- Lee, I.H.; Han, H.; Koh, Y.H.; Kim, I.S.; Lee, S.-W.; Shim, D. Comparative transcriptome analysis of Pinus densiflora following inoculation with pathogenic (Bursaphelenchus xylophilus) or non-pathogenic nematodes (B. thailandae). Sci. Rep. 2019, 9, 12180. [Google Scholar] [CrossRef]
- Zhu, P.; Ma, Y.; Zhu, L.; Chen, Y.; Li, R.; Ji, K. Selection of suitable reference genes in Pinus massoniana Lamb. under different abiotic stresses for qPCR normalization. Forests 2019, 10, 632. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Struve, D.K. Tree establishment: A review of some of the factors affecting transplant survival and establishment. Arboric. Urban For. 2009, 35, 10–13. [Google Scholar] [CrossRef]
- Watson, G.W.; Sydnor, T.D. The effect of root pruning on the root system of nursery trees. J. Arboric. 1987, 13, 126–130. [Google Scholar] [CrossRef]
- Arnold, M.A.; Struve, D.K. Green ash establishment following transplant. J. Am. Soc. Hortic. Sci. 1989, 114, 591–595. [Google Scholar] [CrossRef]
- Watson, G.W.; Himelick, E. Root distribution of nursery trees and its relationship to transplanting success. J. Arboric. 1982, 8, 225–229. [Google Scholar] [CrossRef]
- Abod, S.; Webster, A. The influence of root pruning on subsequent root and shoot growth of Malus, Tilia and Betula. J. Hortic. Sci. 1991, 66, 227–233. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Vélez-Bermúdez, I.-C.; Salazar-Henao, J.E.; Fornalé, S.; López-Vidriero, I.; Franco-Zorrilla, J.-M.; Grotewold, E.; Gray, J.; Solano, R.; Schmidt, W.; Pagés, M. A MYB/ZML complex regulates wound-induced lignin genes in maize. Plant Cell 2015, 27, 3245–3259. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Reiter, W.-D. Biosynthesis and properties of the plant cell wall. Curr. Opin. Plant Biol. 2002, 5, 536–542. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A.; Pierik, R. Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit. Rev. Plant Sci. 2011, 30, 548–562. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Barku, V.Y. Wound healing: Contributions from plant secondary metabolite antioxidants. In Wound Healing-Current Perspectives; IntechOpen: Rijeka, Croatia, 2019; p. 13. [Google Scholar]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, J.; Barbeta, A.; Sperlich, D.; Coll, M.; Peñuelas, J. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Front. Plant Sci. 2013, 4, 409. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Pittermann, J.; Coomes, D.A. Elegance versus speed: Examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 2012, 173, 673–694. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Ma, B.; Hou, Q.; Wan, X. Phylogeny and functions of LOB domain proteins in plants. Int. J. Mol. Sci. 2020, 21, 2278. [Google Scholar] [CrossRef]
- Ye, L.; Wang, X.; Lyu, M.; Siligato, R.; Eswaran, G.; Vainio, L.; Blomster, T.; Zhang, J.; Mähönen, A.P. Cytokinins initiate secondary growth in the Arabidopsis root through a set of LBD genes. Curr. Biol. 2021, 31, 3365–3373.e3367. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, Y.S.; Regan, S.; Busov, V. Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell 2010, 22, 3662–3677. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeon, S.J.; Yanders, S.; Park, S.C.; Kim, H.S.; Kim, S. MYB3 plays an important role in lignin and anthocyanin biosynthesis under salt stress condition in Arabidopsis. Plant Cell Rep. 2022, 41, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tang, X.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. Functional identification of MdMYB5 involved in secondary cell wall formation in apple. Fruit Res. 2021, 1, 6. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB transcription factors and its regulation in secondary cell wall formation and lignin biosynthesis during xylem development. Int. J. Mol. Sci. 2021, 22, 3560. [Google Scholar] [CrossRef]
- Ajengui, A.; Bertolini, E.; Ligorio, A.; Chebil, S.; Ippolito, A.; Sanzani, S.M. Comparative transcriptome analysis of two citrus germplasms with contrasting susceptibility to Phytophthora nicotianae provides new insights into tolerance mechanisms. Plant Cell Rep. 2018, 37, 483–499. [Google Scholar] [CrossRef]
- Alejandro, S.; Lee, Y.; Tohge, T.; Sudre, D.; Osorio, S.; Park, J.; Bovet, L.; Lee, Y.; Geldner, N.; Fernie, A.R. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr. Biol. 2012, 22, 1207–1212. [Google Scholar] [CrossRef]
- Pan, L.L.; Miao, H.; Wang, Q.; Walling, L.L.; Liu, S.S. Virus-induced phytohormone dynamics and their effects on plant–insect interactions. New Phytol. 2021, 230, 1305–1320. [Google Scholar] [CrossRef]
- Gho, Y.-S.; Kim, S.-j.; Jung, K.-H. Phenylalanine ammonia-lyase family is closely associated with response to phosphate deficiency in rice. Genes Genom. 2020, 42, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Huang, X.X.; Li, Q.; Cao, Y.; Bao, Y.; Meng, X.F.; Li, Y.J.; Fu, C.; Hou, B.K. UDP-glycosyltransferase 72B1 catalyzes the glucose conjugation of monolignols and is essential for the normal cell wall lignification in Arabidopsis thaliana. Plant J. 2016, 88, 26–42. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Phillips, R.P. Non-structural carbohydrate pools not linked to hydraulic strategies or carbon supply in tree saplings during severe drought and subsequent recovery. Tree Physiol. 2020, 40, 259–271. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Karst, J.; Gaster, J.; Wiley, E.; Landhäusser, S.M. Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiol. 2017, 37, 154–164. [Google Scholar] [CrossRef]
- Trifilò, P.; Kiorapostolou, N.; Petruzzellis, F.; Vitti, S.; Petit, G.; Gullo, M.A.L.; Nardini, A.; Casolo, V. Hydraulic recovery from xylem embolism in excised branches of twelve woody species: Relationships with parenchyma cells and non-structural carbohydrates. Plant Physiol. Biochem. 2019, 139, 513–520. [Google Scholar] [CrossRef]
- Brunetti, C.; Savi, T.; Nardini, A.; Loreto, F.; Gori, A.; Centritto, M. Changes in abscisic acid content during and after drought are related to carbohydrate mobilization and hydraulic recovery in poplar stems. Tree Physiol. 2020, 40, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- La Camera, S.; Gouzerh, G.; Dhondt, S.; Hoffmann, L.; Fritig, B.; Legrand, M.; Heitz, T. Metabolic reprogramming in plant innate immunity: The contributions of phenylpropanoid and oxylipin pathways. Immunol. Rev. 2004, 198, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wu, T.; Pei, T.; Wang, Z.; Yang, H.; Jiang, J.; Zhang, H.; Chen, X.; Li, J.; Xu, X. Overexpression of SlGATA17 promotes drought tolerance in transgenic tomato plants by enhancing activation of the phenylpropanoid biosynthetic pathway. Front. Plant Sci. 2021, 12, 634888. [Google Scholar] [CrossRef] [PubMed]
- Shadle, G.L.; Wesley, S.V.; Korth, K.L.; Chen, F.; Lamb, C.; Dixon, R.A. Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of L-phenylalanine ammonia-lyase. Phytochemistry 2003, 64, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, L.; Huang, S.; Yang, C.; Wang, Y.; Yuan, H.; Xu, Q.; Zhang, W.; Wang, M.; Zeng, X. Drought resistance in Qingke involves a reprogramming of the phenylpropanoid pathway and UDP-glucosyltransferase regulation of abiotic stress tolerance targeting flavonoid biosynthesis. J. Agric. Food Chem. 2021, 69, 3992–4005. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Pop, T.I.; Pamfil, D.D.; Bellini, C.C. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.-R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef]




| Assembled Contigs | Number |
|---|---|
| Total Trinity genes (n) | 134,369 |
| Total Trinity transcripts (n) | 269,984 |
| GC content (%) | 41.32 |
| Contig N50 length (bp) | 1501 |
| Average contig length (bp) | 874.52 |
| Total assembled bases | 236,105,474 |
| Year | p-Value | |||||
|---|---|---|---|---|---|---|
| Factor | Treatment | 2020 | 2021 | Treatment | Year | Treatment × Year |
| Height (cm) | Control | 63.67 ± 5.89 | 79.00 ± 6.35 | 0.881 | 0.406 | 0.762 |
| Waterlogging | 63.02 ± 5.73 | 75.15 ± 5.85 | ||||
| Root collar diameter (mm) | Control | 11.25 ± 1.07 | 14.28 ± 1.38 | 0.902 | 0.424 | 0.723 |
| Waterlogging | 12.62 ± 0.91 | 15.68 ± 0.09 | ||||
| Treatment | p-Value | ||
|---|---|---|---|
| TC | TW | ||
| Height growth (cm) | 15.33 ± 1.76 (n = 6) | 12.15 ± 1.19 (n = 6) | 0.166 |
| Root collar diameter growth (mm) | 3.02 ± 0.49 (n = 6) | 3.05 ± 0.17 (n = 6) | 0.952 |
| Pmax (µmol s−1 m−2) | 15.63 ± 2.67 (n = 6) | 21.05 ± 2.98 (n = 4) | 0.217 |
| Stomatal conductance (mol s−1 m−2) | 0.19 ± 0.04 (n = 6) | 0.32 ± 0.07 (n = 4) | 0.126 |
| WUE (mmol mol−1) | 88.11 ± 10.88 (n = 6) | 70.71 ± 13.16 (n = 4) | 0.334 |
| Soluble sugars (mg g−1) | 77.60 ± 10.97 (n = 6) | 46.15 ± 7.22 (n = 4) | 0.059 |
| Starch (mg g−1) | 87.89 ± 8.86 (n = 6) | 43.48 ± 7.22 (n = 4) | 0.007 |
| Chlorophyll (mg g−1) | 0.41 ± 0.03 (n = 6) | 0.49 ± 0.05 (n = 4) | 0.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, S.; Kim, S.; Hong, J.; Kim, T.K.; Huh, W.; Kim, K.; Lee, M.; Lee, H.; Kim, S.; Park, C.; et al. Waterlogging Hardening Effect on Transplant Stress Tolerance in Pinus densiflora. Forests 2024, 15, 445. https://doi.org/10.3390/f15030445
Byeon S, Kim S, Hong J, Kim TK, Huh W, Kim K, Lee M, Lee H, Kim S, Park C, et al. Waterlogging Hardening Effect on Transplant Stress Tolerance in Pinus densiflora. Forests. 2024; 15(3):445. https://doi.org/10.3390/f15030445
Chicago/Turabian StyleByeon, Siyeon, Seohyun Kim, Jeonghyun Hong, Tae Kyung Kim, Woojin Huh, Kunhyo Kim, Minsu Lee, Hojin Lee, Sukyung Kim, Chanoh Park, and et al. 2024. "Waterlogging Hardening Effect on Transplant Stress Tolerance in Pinus densiflora" Forests 15, no. 3: 445. https://doi.org/10.3390/f15030445
APA StyleByeon, S., Kim, S., Hong, J., Kim, T. K., Huh, W., Kim, K., Lee, M., Lee, H., Kim, S., Park, C., Bhusal, N., Han, A. R., Chandrasekaran, U., & Kim, H. S. (2024). Waterlogging Hardening Effect on Transplant Stress Tolerance in Pinus densiflora. Forests, 15(3), 445. https://doi.org/10.3390/f15030445






