Abstract
Soil fungi often operate through diverse functional guilds, and play critical roles in driving soil nutrient cycling, organic matter decomposition and the health of above-ground vegetation. However, fungal functional guilds at the early-stage restoration of disrupted subalpine forest soils remain elusive. In the present study, we collected 36 soil samples along an altitudinal gradient (2900 m a.s.l., 3102 m a.s.l., and 3194 m a.s.l.) from cut slopes (CS) (from Wenma highway) and natural soils (NS) at the Miyaluo of Lixian County, Southwest China. By applying nuclear ribosomal internal transcribed spacer (ITS) sequencing, this study revealed the ecological characteristics of fungal functional guild in the early-stage restoration of cut slope soils. The results showed that the predicted prevalence of ectomycorrhizal fungi decreased, while plant pathogens and arbuscular mycorrhizal fungi increased in CS. In the high-altitude regions (3102 m a.s.l. and 3194 m a.s.l.), the differences in communities between natural and cut slope soils were more pronounced for total soil fungi, soil saprotroph, litter saprotroph, arbuscular mycorrhizal fungi and ectomycorrhizal fungi, in contrast to the low altitude communities (2900 m a.s.l.). An opposite pattern was evident for plant pathogens. Variations in the differences of both soil properties (mainly soil pH) and community assembling processes (e.g., heterogeneous selection, dispersal limitation and drift) between natural and cut slope soils across the altitudinal gradient likely shaped the shifting patterns of community difference. This study provides valuable insights for devising restoration approaches for cut slopes in subalpine forest ecosystems, emphasizing the importance of taking soil fungal functional guilds into account in evaluating the restoration of cut slopes, and underscoring the necessity for increased attention to the restoration of soil fungi in cut slopes at the high-altitude ecosystems.
1. Introduction
Subalpine forests provide a diverse array of ecosystem services such as watershed protection, soil formation, erosion control, carbon sequestration, and habitat for a diverse range of species [1]. Yet, they are sensitive to disturbances and land-use changes [2]. The construction of highways in these ecosystems usually results in the formation of cut slopes, causing landscape connectivity degradation [3], biodiversity loss [4], and decreases in ecosystem quality [5]. Various initiatives are employed to rapidly stabilize cut slopes and restore above-ground landscapes, with external soil spray seeding (ESSS) emerging as a widely adopted technique that involves spraying artificially mixed soils onto cut slopes. [6]. The artificially mixed soils consist of composite materials (e.g., water-retention and bonding agents), backfill soils, soluble chemical fertilizers, and grass seeds [7,8]. Despite the nutrients introduced by the ESSS [9], the above-ground herb in cut slopes only grows for a few years [10]. The short-lived herbs may be caused by the immature below-ground functions (e.g., nutrient cycling) and their undertakers (e.g., soil bacteria and fungi) in cut slopes at the early-stage restoration. Previous studies on ESSS-restored ecosystems primarily focus on the restoration of soil quality [11], above-ground landscape [8], soil substrate [6,12,13], extracellular enzyme [14], and bacterial community [15]. Evidence indicates that fungal diversity and functional guilds are useful bioindicators of soil restoration in open-cut mined sites, and may complement more traditional vegetation-based surveys [16,17]. Yet, similar to the restoration of open-cut mined sites, we still know little about fungal communities and functional guilds at the early-stage ESSS-restoration of highway-construction disrupted soils in subalpine forest ecosystems.
Soil fungi have been recognized as key drivers for nutrient cycling, organic matter decomposition and plant health [18,19,20]. Evidence shows that fungi are more resistant to disturbances but less resilient, whereas bacteria are less resistant but more resilient [21]. A 196-day ESSS simulation experiment demonstrates that fungal diversities (decreasing from the first to 46th day and then nearly maintaining consistent) in subalpine forest soils do not show increasing patterns as in bacteria (decreasing from the first to 16th day and then increasing from the 16th to 196th day) after the declines caused by initial treatments [22]. This suggests that the restoration of fungal communities in disrupted subalpine forest soils seems to be more challenging compared to bacterial communities, highlighting a necessity to pay attention to fungal communities in these environments at the early-stage restoration. Notably, evaluating the restoration of fungal community is far more than just revealing the diversities and taxonomic compositions of the entire community. This is because, similar to macrobiota, mesobiota and other microbiota, a fungal community also operates through functional guilds such as saprotrophic, mycorrhizal and pathogenic fungi [23]. It is reported that saprotrophic fungi are the main decomposers of plant litter [24], and pathogenic fungi could cause disease, impacting the survival of their hosts [25]. Mycorrhizal fungi (e.g., arbuscular mycorrhizal and ectomycorrhizal fungi) are able to form symbiotic associations with plant roots, facilitating the nutrient uptake of plants [26]. Specifically, soil nutrients (e.g., inorganic phosphorus, and mineral or organic forms of nitrogen, such as ammonium and nitrate nitrogen, and amino acids) can be taken up by specialized transporters located on the membrane of mycorrhizal fungi in symbiotic associations; then, these nutrients can be imported from the symbiotic interface to plant cells through selective transporters [27]. Conversely, the hexose transporters of mycorrhizal fungi can import plant-derived carbon to fungal cells [27]. Under such a scenario, the composition of fungal functional guilds in cut slope soils may be closely connected to the survival and sustainability of the above-ground vegetation, thereby determining the restoration of landscapes.
Altitude is recognized as a synthetic variable affecting all aspects of an ecosystem such as climate, soil property and hydrology [28,29]. Evidence demonstrates that soil fungal communities shift significantly with the increasing altitudes in natural ecosystems [30,31]. Regarding cut slope soils in subalpine forest ecosystems, both altitude-induced effects and differences in the lifestyle, dispersal ability, and nutrient acquisition strategy of fungal functional guilds are expected to make it even more complicated to evaluate the restorations of fungal communities. Evidence indicates that the communities of arbuscular mycorrhizal and ectomycorrhizal fungi shift significantly across tropic, subtropic and temperate forests, and these responses are distinguished from the total soil fungi [32]. A more recent study conducted in an alpine area indicates that the diversity patterns induced by altitude shifts vary among total soil fungi and three functional guilds [33]. These findings highlight a necessity to take both altitude and functional guilds into account in evaluating the restoration of soil fungal communities in subalpine areas. A previous study indicates that the prevalence of ectomycorrhizal fungi decline in response to open-cut mining disturbances, but recover over time in woody-dominated sites [17]. Similarly, a more recent study also indicates that the composition of fungal functional guilds in soils subjected to open-cut mining disturbances shifts significantly across the restoration chronosequence [16]. This suggests that fungal functional guilds are useful indicators for the restoration of soils subjected to disturbances. Yet, we know little about how altitude regulates the restoration of fungal functional guilds in highway-construction disrupted subalpine forest soils.
In this study, we focused on the early-stage restoration of cut slopes to evaluate soil fungal functional guilds by using high-throughput sequencing and bioinformatics approaches. We collected 18 natural forest soils (NS) and 18 cut slope soils (CS) from three altitudes (2900 m a.s.l., 3102 m a.s.l. and 3194 m a.s.l.) along the Wenma highway (Miyaluo, Lixian, Sichuan, Southwest China). The nuclear ribosomal internal transcribed spacer (ITS) regions of soil fungi were amplified and then sequenced to address two questions: (i) the ecological characteristics of fungal functional guild at the early-stage restoration of CS in subalpine forest ecosystems, and (ii) how altitude regulates the restorations of functional guild. This study provides new insights for designing restoration strategies for cut slopes in subalpine forest ecosystems, highlighting the importance of considering soil fungal functional guilds and underscoring the necessity for increased attention to the restoration of soil fungi in cut slopes at the high-altitude ecosystems.
2. Materials and Methods
2.1. Soil Sampling and Soil Property Measurements
Three altitudes (2900 m a.s.l., 3102 m a.s.l. and 3194 m a.s.l.) along the Wenma highway at the Miyaluo of Lixian County, Sichuan, Southwest China (31°42′38″ N–31°47′55″ N, 102°41′40″ E–102°44′23″ E) were selected as sampling sites. The mean annual temperature stands at 8.9 °C, with monthly mean temperatures fluctuating between −8.0 °C in January and 12.6 °C in July; mean annual precipitation varies between 600 and 1100 mm [34]. The period conducive to plant growth spans from late April to October. The soils in our study areas are typical brown forest soils and are classified as Cambic Umbrisols according to the IUSS Working Group (2007) [35]. According to the surveys, all cut slopes in our study areas generated and subsequently underwent ESSS-based restoration between June and October 2015, and these cut slopes have been restored for approximately three years as of the sampling date. In October 2018, we collected 36 soil samples at a depth of 0–10 cm from cut slopes (CS) and natural forest soils (NS), during which a “S-shaped” sampling strategy was employed in soil sampling [7,8,36] (Figure S1). Regarding the sampling of cut slope soils at each altitude site, we first identified six plots along an “S” route and then collected five soil cores per plot at a depth of about 0–10 cm by using a quadrat sampling (Figure S1). After removing visible rocks, plant roots, and residues, five soil cores at each plot were thoroughly mixed to ensure uniformity and the mixed soils were considered as an independent biological replicate (six biological replicates at each altitude site). Soils from natural environments adjacent to the cut slopes were collected by using the same sampling strategy. All soils were sieved through a 2.0 mm mesh and then divided into two parts. One part was stored at 4 °C to determine soil properties including conductivity (CD) and pH, moisture content (MC), soil temperature (ST, measured in situ during the sampling), total organic carbon (TOC), ammonium (-N) and nitrate nitrogen (-N), total phosphorus (TP) and nitrogen (TN), and soil available phosphorus (SAP). Another part was freeze-dried and stored at −20 °C for DNA extraction and subsequent molecular biological experiments. Details for soil property measurements are available in our previous study [15]. Summaries for these soil properties are available in Figures S2 and S3 in this study.
2.2. DNA Extraction, Fungal ITS Amplification and Sequencing
Genomic DNAs were extracted from 0.25 g fresh soil using a DNeasy® PowerSoil® kit (QIAGEN GmbH, Hilden, Germany). High-quality DNA was used for polymerase chain reaction (PCR) amplification with the primers of gITS7F (GTGARTCATCGARTCTTTG) and ITS4R (TCCTCCGCTTATTGATATGC) [37]. The PCR reaction system (25 μL) consisted of l μL DNA template (about 20 ng DNA), l μL each of 10 μM forward and reverse primers, 9.5 μL H2O, and 12.5 μL MasterMix (PCR buffer, DNA polymerase and dNTPs and Mg2+) (CWBIO, China) [15]. The amplification program was as follows: 94 °C for 5 min, 35 cycles of amplification (94 °C for 30 s, 56 °C for 30 s, 68 °C for 45 s), followed by the final extension at 72 °C for 10 min. All PCR products were checked by using a NanoDrop ND-1000 Spectrophotometer (Nano-Drop Technologies Inc., Wilmington, DE, USA), pooled at equal molar amount for each sample, and applied for sequencing using an Illumina NovoSeq platform. Given the failure of molecular experiments, only 33 soil samples were used for sequencing.
2.3. Bioinformatics Analysis
Pair-end reads were demultiplexed by using the sabre (https://github.com/najoshi/sabre; Accessed date: 22 December 2019) based on barcode sequences, resulting in two fastq files for each sample. Then, these files were converted to the format required by QIIME 2 (version 2019.10) [38] for assembly (VSEARCH algorithm) [39], quality filtering (--p-min-quality = 4 and --p-max-ambiguous = 0) and denoising (deblur algorithm) [40]. During the denoising, the sequences were initially trimmed to 235 bps, followed by a removal of the first 35 bps from the 5′ end, resulting in a final sequence length of 200 bps. The reason for such a processing is that the longer sequences can introduce more sequencing errors, leading to a significant loss of data during the denoising. Additionally, evidence shows that a sequence length of 90 bps is sufficient to reflect the patterns of microbial composition and diversity between groups [41]. Upon the completion of denoising, all amplicon sequence variants (ASVs) were taxonomically annotated to the UNITE v2019.02.02 database [42] using a classify-sklearn algorithm. ASVs occurred only once across all samples and those that cannot be annotated at the Kingdom taxonomic level were removed. Given the differences in sequencing depth among samples, we rarefied the sequence number to 8375 for each soil sample. The phylogenetic tree was constructed by using the QIIME 2 built-in tools. Firstly, ASV sequences were aligned using the MAFFT v7.310 [43]. Then, the FastTree v2.1.10 was used to construct a phylogenetic tree [44,45]. The above procedures generated an ASV abundance matrix, taxonomic classifications and phylogenetic tree for downstream statistical analysis.
2.4. Statistical Analysis
Fungal functional guilds were inferred using the microeco R package v0.1.2 [46] based on a database of FungalTraits (version: 1.2_ver_16Dec_2020V.1.2) [47]. The compositions of functional guild were visualized using the ggplot2 R package v3.5.0 [48]. To obtain robust results, only the guilds with ASV numbers greater than 150 were used for downstream analysis. The observed ASV (α-diversity) and Bray–Curtis distance (β-diversity) were calculated using microeco built-in functions [46]. β-nearest taxon index (βNTI) and community assembling processes were calculated using the iCAMP R package v1.5.12 [49]. A βNTI value above +2 and below −2 suggests that the community assembling is significantly greater and less than the null expectation, respectively, and more influenced by deterministic processes [50]. Soil properties and observed ASV were compared between NS and CS at each altitude using a Wilcoxon rank sum test (FDR < 0.05; FDR represents false discovery rate). Unique ASV numbers in NS and CS, and shared ASV numbers between NS and CS were visualized by using microeco built-in functions [46]. The overall differences in soil properties (Euclidean distance) and communities (Bray–Curtis distance) were visualized by using the principal coordinates analysis (PCoA), and subsequently tested by using the analysis of similarity (ANOSIM). To reveal how altitude regulates the restoration of fungal functional guild in cut slope soils, we compared differences in the Euclidean distance of soil properties, the Bray–Curtis distance of communities, and the βNTI between NS and CS among three altitudes using a Wilcoxon rank sum test (FDR < 0.05). A larger value means a larger difference in soil properties (Euclidean distance), community compositions (Bray–Curtis distance), or a greater community turnover (βNTI) between NS and CS at a particular altitude. To reveal key factors driving the shifts of α-/β-diversities, we correlated observed ASV and Bray–Curtis distance with soil properties using a Pearson correlation, and p-values were adjusted using FDR (psych R package v2.4.3) [51]. All statistical analyses and visualizations were performed in R v4.3.2.
3. Results
3.1. Differences in Soil Properties between Natural and Cut Slope Soils
Principal coordinates analysis indicated that there were significant differences in soil properties between NS and CS at each altitude (R ≥ 0.605, p = 0.005) (Figure S2A and Table S1), and the differences in high-altitude areas (3102 m and 3194 m) were greater than in the low altitude (2900 m) (FDR < 0.001), though the largest difference was detected at 3102 m (Figure S2B). Specifically, there were larger differences in pH and -N between NS and CS at 3102 m and 3194 m than those at 2900 m; the largest differences in MC, TN and -N were detected at 3102 m, though the differences at 3102 m and 3194 m were greater than those at 2900 m; the significant differences in CD were only detected at 3194 m, and those about TOC were only detected at 3102 m; ST showed highest difference between NS and CS at 3102 m; TP and SAP did not show any significant differences between NS and CS at all altitudes. Soil pH, MC and ST in CS were generally greater than those in NS at all altitudes, especially at 3102 m and 3194 m; the CD in CS was significantly greater than that in NS at 3194 m; the TOC in CS is significantly less than that in NS at 3102 m; TN and -N in CS were generally less than those in NS at three altitudes; the -N in CS was less than in NS at 3102 m, while an opposite trend was detected at 3194 m (Figure S3).
3.2. Differences in the Composition of Fungal Functional Guilds between Natural and Cut Slope Soils
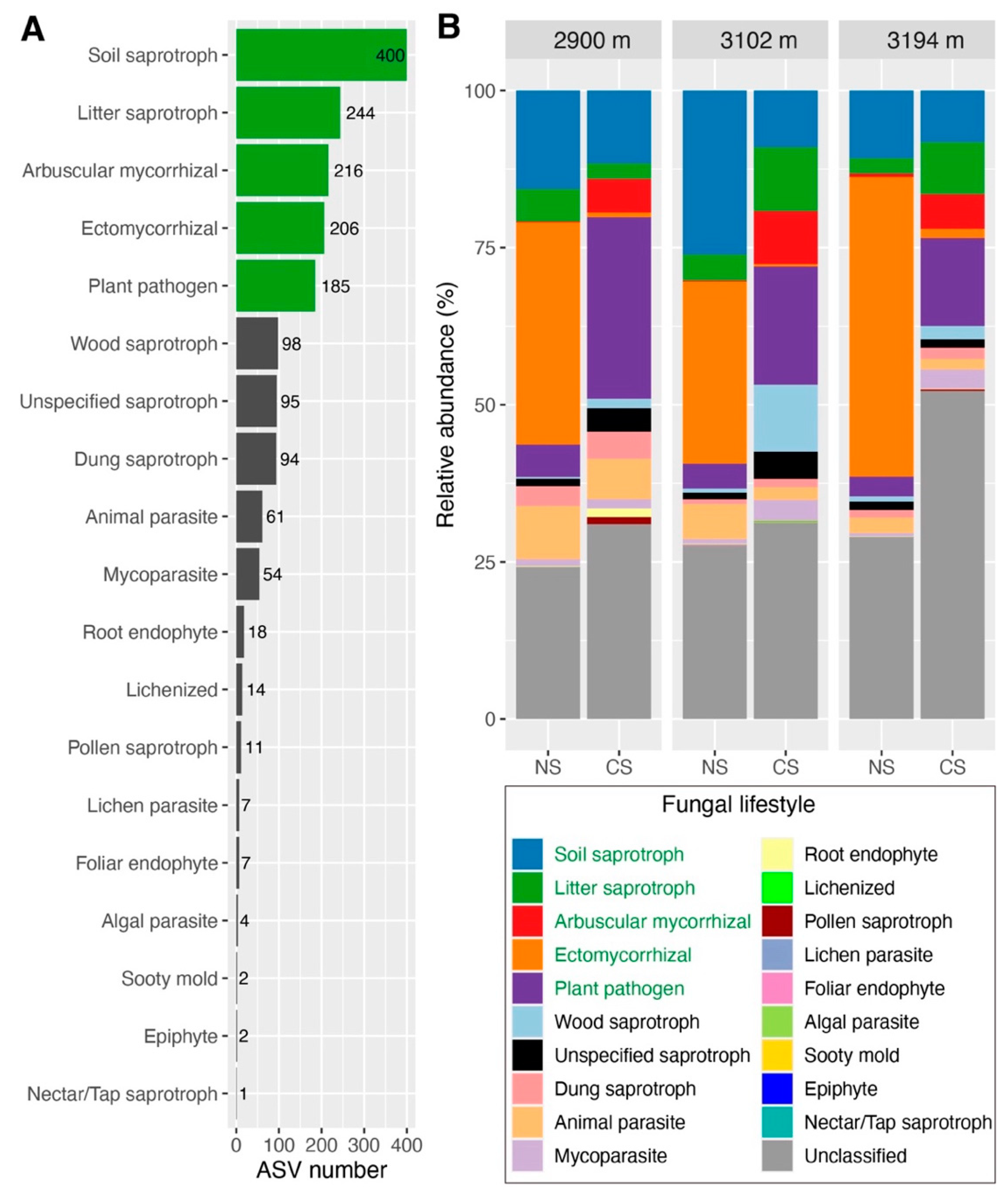
By partitioning the communities of total soil fungi according to the lifestyles, a total of 2900 ASVs were classified as 19 fungal functional guilds, of which 1181 ASVs failed to be predicted as known functional guilds (Figure 1A). The relative abundances of the predicted plant pathogen, wood and dung saprotrophs, mycoparasite and arbuscular mycorrhizal fungi were higher in CS than those in NS, whereas soil saprotroph, ectomycorrhizal fungi and animal parasites showed opposite patterns (Figure 1B). The relative abundance of litter saprotroph was higher in CS at 3102 m and 3194 m, while an opposite pattern was observed at 2900 m. The relative abundance of unspecified saprotrophs was higher in CS at 2900 m and 3102 m, whereas no remarkable difference between NS and CS was detected at 3194 m (Figure 1B). Other functional guilds including root endophyte, lichenized, pollen saprotroph, lichen parasite, foliar endophyte, algal parasite, sooty mold, epiphyte and nectar/tap saprotroph only occupied a small portion of total soil fungal communities in both NS and CS at three altitudes (Figure 1B). To reveal the robustly ecological characteristics of fungal functional guild, we further analyzed the guilds with ASVs greater than 150. The Veen diagrams showed that there were more unique ASVs of arbuscular mycorrhizal fungi and plant pathogen in CS, whereas the unique ASVs of ectomycorrhizal fungi in CS were less than those in NS at three altitudes; the unique ASVs of litter saprotroph in CS were less than those in NS at 2900 m and 3102 m, but they were similar at 3194 m (49 vs. 51); the unique ASVs of soil saprotroph in CS were more than those in NS at 2900 m and 3194 m, while an opposite pattern was observed at 3102 m (110 vs. 79) (Figure S4).
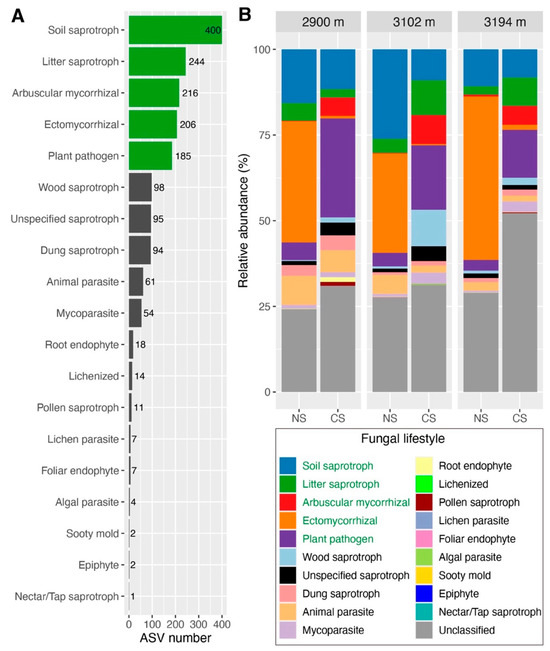
Figure 1.
The ASV number of each fungal functional guild (A) and the relative abundance of these guilds (B). Bars filled with a green color in figure (A) and the fonts with a green color in the legend of figure (B) are the functional guilds discussed in this study. Numbers in figure (A) represent the ASV numbers of functional guild. ASV: amplicon sequence variants; NS: natural soils; CS: cut slope soils.
3.3. Taxonomic Composition of Five Major Fungal Functional Guilds in Natural and Cut Slope Soils
Further analysis showed that the compositions of five major functional guilds were diverse at the taxonomic class level, especially soil and litter saprotrophs (Figure S5). Nevertheless, we still found that some class-level lineages were relatively unique for these guilds. Mucoromycetes, Tremellomycetes and Mortierellomycetes were primarily detected as soil saprotrophs (Figure S5). Mucoromycetes was more prevalent in CS at all altitudes; Mortierellomycetes was more prevalent in CS at 2900 m and 3102 m, while its prevalence in NS and CS was similar at 3194 m; Tremellomycetes was less prevalent in CS at 2900 m and 3194 m, whereas an opposite pattern was observed at 3102 m (Figure S5). Agaricomycetes and Dothideomycetes were also detected as ectomycorrhizal fungi, plant pathogens and soil saprotrophs (only for Agaricomycetes); we found that the litter saprotroph of Agaricomycetes was less prevalent while Dothideomycetes was more prevalent in CS at all altitudes (Figure S5). Besides, Leotiomycetes also occupied a considerable proportion of litter saprotrophs in both CS and NS at all altitudes; the litter saprotrophs of Pezizomycetes were more prevalent in CS at 3102 m and 3194 m, whereas the opposite trend was detected at 2900 m (Figure S5). The community of arbuscular mycorrhizal fungi consisted of Archaeosporomycetes and Glomeromycetes; Glomeromycetes was more prevalent while Archaeosporomycetes was less prevalent in CS at all altitudes (Figure S5). The community of ectomycorrhizal fungi consisted of Agaricomycetes, Pezizomycetes and Dothideomycetes; Agaricomycetes was less prevalent in CS at three altitudes, while Pezizomycetes showed an opposite pattern; Dothideomycetes was more prevalent in CS at 3102 m and 3194 m, but an opposite trend was detected at 2900 m (Figure S5). The community of plant pathogen primarily consisted of Dothideomycetes and Sordariomycetes; Sordariomycetes were less prevalent in CS at three altitudes; Dothideomycetes were more prevalent in CS at 3102 m and 3194 m, while an opposite trend was detected at 2900 m. We also found that Pucciniomycetes were more prevalent in CS at 2900 m, but they were scarce in both CS and NS at 3102 and 3194 m. Besides, the plant pathogen of Agaricomycetes was more prevalent in CS at 3102 and 3194 m, but an opposite trend was detected at 2900 m (Figure S5).
3.4. Diversity and Community Assembly of Fungal Functional Guilds in Natural and Cut Slope Soils
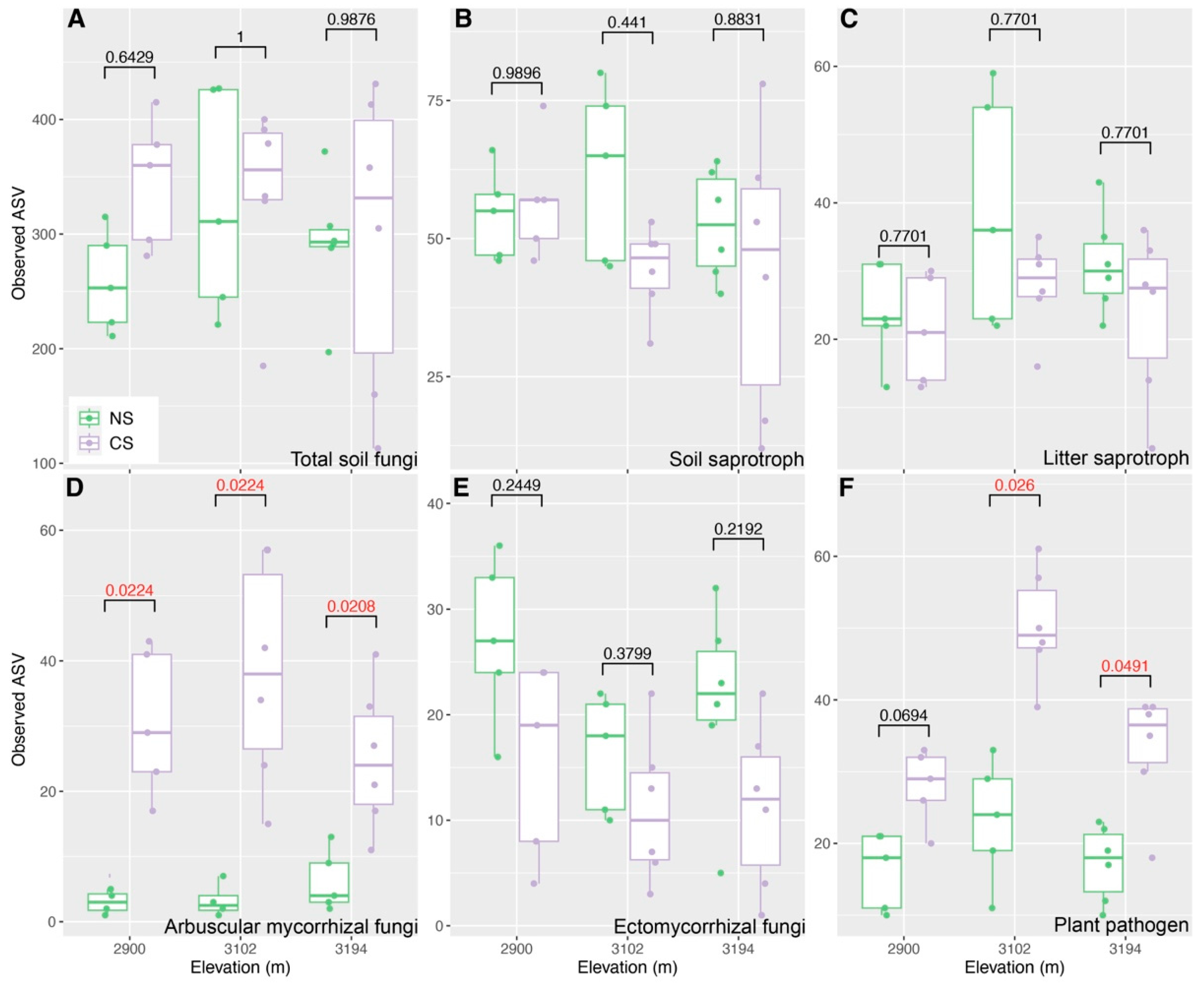
No significant differences in observed ASV (α-diversity) were detected for total soil fungi, soil and litter saprotrophs, and ectomycorrhizal fungi between NS and CS at three altitudes (FDR > 0.05); the observed ASVs of arbuscular mycorrhizal fungi and plant pathogens were higher in CS than those in NS, despite there being no significant differences of observed ASV between NS and CS for plant pathogens at 2900 m (FDR > 0.05) (Figure 2A–F). Principal coordinates analysis showed that both total soil fungal communities and the communities of five fungal functional guild (except for litter saprotroph at 2900 m) in CS significantly distinguished from those in NS at all altitudes, especially ectomycorrhizal and arbuscular mycorrhizal fungi, and plant pathogens (Figure S6 and Table S2).

Figure 2.
Variations in the observed ASV of total soil fungi (A), soil saprotroph (B), litter saprotroph (C), arbuscular mycorrhizal fungi (D), ectomycorrhizal fungi (E), and plant pathogen (F). Numbers in each subgraph represent false discovery rates (FDR) inferred using a Wilcoxon rank sum test, and a FDR with a red color represents that there is a significant difference (FDR < 0.05) between natural (NS) and cut slope (CS) soils. The subfigures A to C share the same axis titles and text with subfigures D to E. ASV: amplicon sequence variants.
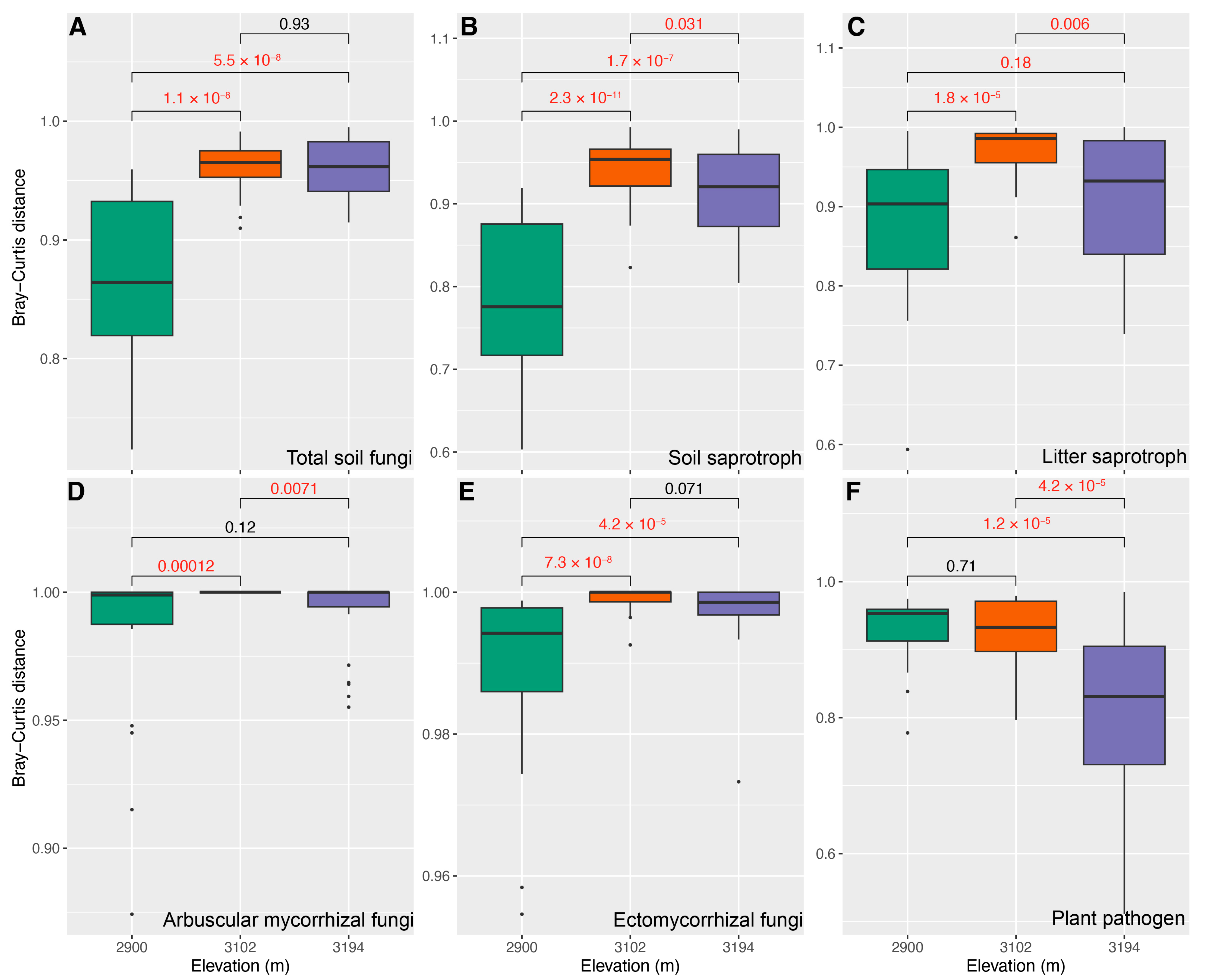
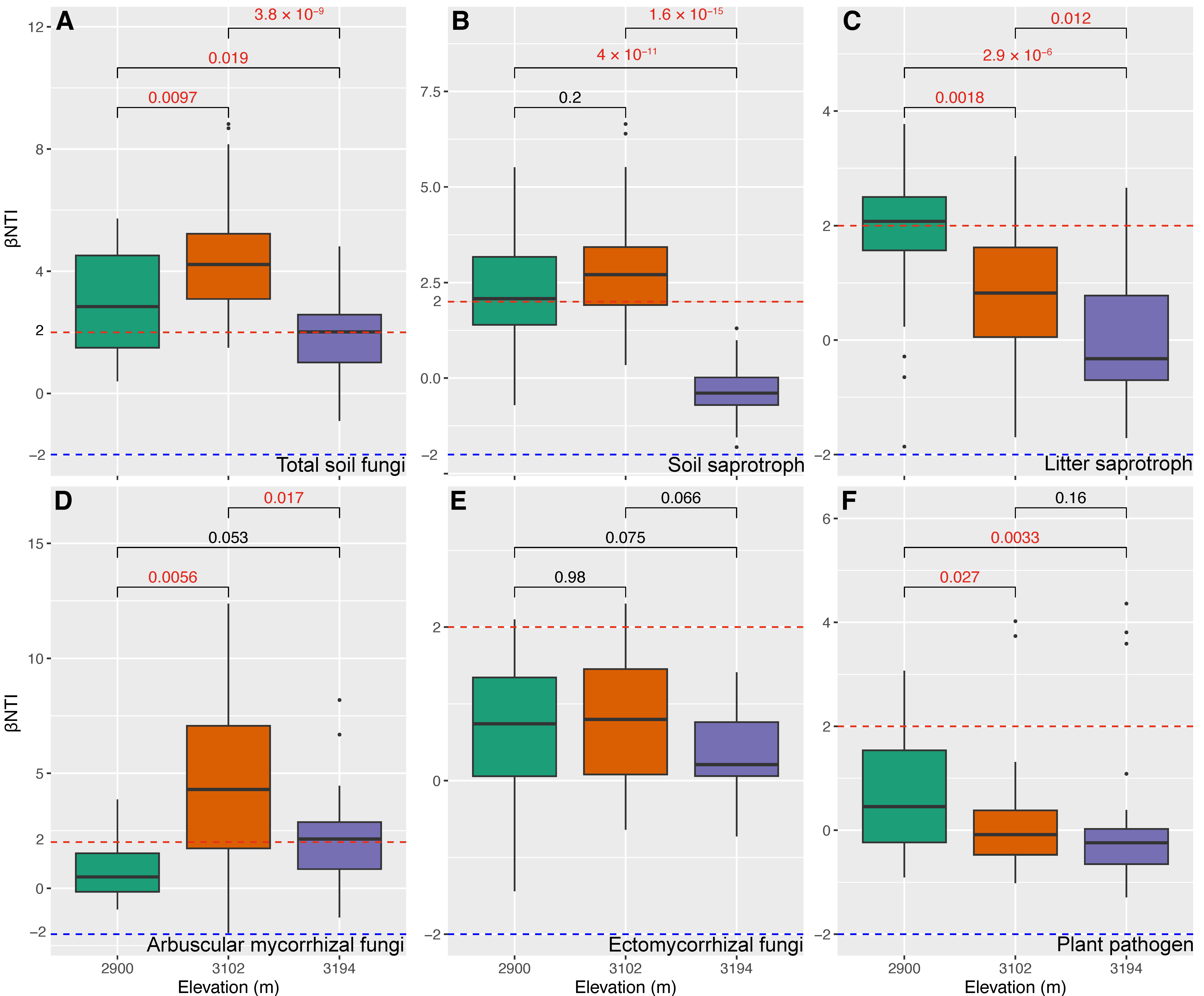
To reveal how altitude regulates the restorations of the predicted functional guild in cut slope soils, we compared the differences in Bray–Curtis distance and βNTI between NS and CS among three altitudes. The results showed that the differences in Bray–Curtis distance inferred from total soil fungi (Figure 3A), soil saprotroph (Figure 3B), litter saprotroph (Figure 3C), arbuscular mycorrhizal (Figure 3D) and ectomycorrhizal fungi (Figure 3E) were larger at 3102 m and 3194 m than those at 2900 m. An opposite trend was detected for plant pathogens (Figure 3F). The differences in βNTI inferred from total soil fungi (Figure 4A) and soil saprotroph (Figure 4B) were greater than 2 at 2900 m and 3102 m, whereas they were close to or less than 2 at 3194 m (greater than −2). The differences in βNTI inferred from arbuscular mycorrhizal fungi ranged from −2 to 2 at 2900 m, while they were greater than 2 at 3102 m and 3194 m (Figure 4D); those of litter saprotroph (Figure 4C), ectomycorrhizal fungi (Figure 4E) and plant pathogen (Figure 4F) mainly ranged from −2 to 2 at three altitudes; they generally decreased from 2 to −2 with the increasing altitude, particularly those of litter saprotroph and plant pathogen (Figure 4C,F).

Figure 3.
Comparisons for Bray–Curtis distance inferred from total soil fungi (A), soil saprotroph (B), litter saprotroph (C), arbuscular mycorrhizal fungi (D), ectomycorrhizal fungi (E), plant pathogen (F), between natural (NS) and cut slope (CS) soils among three altitudes. Numbers in each subgraph represent false discovery rates (FDR) inferred using a Wilcoxon rank sum test, and a FDR with a red color represents that there is a significant difference (FDR < 0.05) between NS and CS. The subfigures A to C share the same axis titles and text with subfigures D to E.

Figure 4.
Comparisons for β-nearest taxon index (βNTI) inferred from total soil fungi (A), soil saprotroph (B), litter saprotroph (C), arbuscular mycorrhizal fungi (D), ectomycorrhizal fungi (E), and plant pathogen (F), between natural (NS) and cut slope (CS) soils among altitudes. Numbers in each subgraph represent false discovery rates (FDR) inferred using a Wilcoxon rank sum test, and a FDR with a red color means that there is a significant difference (FDR < 0.05) between NS and CS. The subfigures A to C share the same axis titles and text with subfigures D to E.
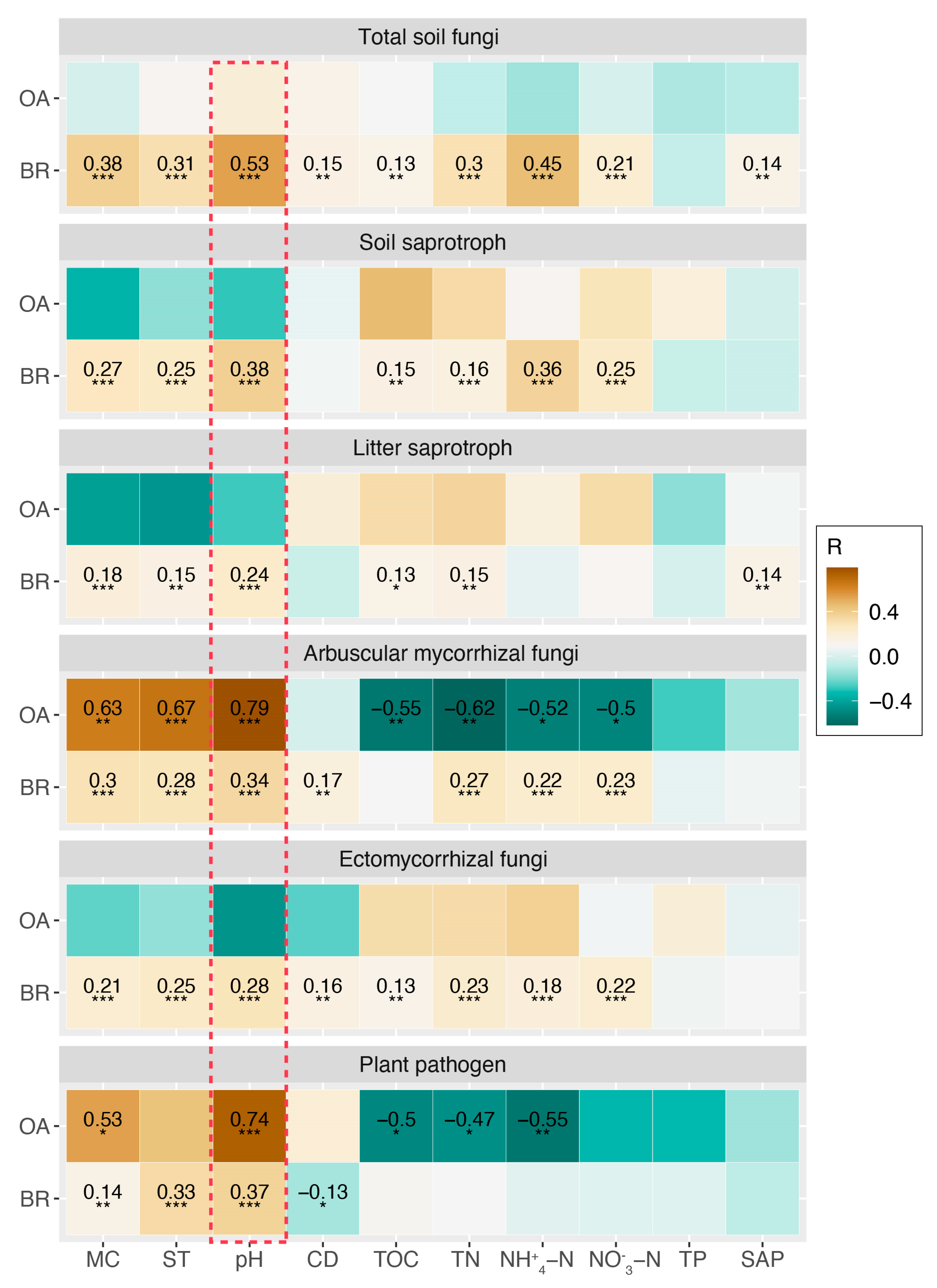
By analyzing the community assembly processes, we found that dispersal limitation, heterogeneous selection and drift mainly governed the community turnovers of total soil fungi and five functional guilds between NS and CS at three altitudes (Figure S7). Specifically, the assembly processes that can enlarge community differences (heterogeneous selection and dispersal limitation) contributed more to the community turnovers of total soil fungi between NS and CS at 3102 m and 3194 m compared to those at 2900 m (Figure S7). These two processes contributed more to the community turnovers of soil saprotroph, litter saprotroph, arbuscular mycorrhizal fungi, and plant pathogen between NS and CS at 3102 m compared to those at 2900 m and 3194 m (Figure S7). The drift primarily governed the community turnovers of ectomycorrhizal fungi between NS and CS at three altitudes, and the contributions of drift to community turnovers at 3102 m and 3194 m were greater than at 2900 m (Figure S7). To reveal the key factors driving the variations in diversity and community, we correlated soil properties with the observed ASVs and Bray–Curtis distances of total soil fungi and five functional guilds. The results showed that the Bray–Curtis distances of total soil fungi, soil saprotroph, litter saprotroph and ectomycorrhizal fungi primarily correlated with soil pH (R ≥ 0.24, FDR < 0.001), but the observed ASVs of these populations failed to be significantly correlated with any soil properties (Figure 5). Both the observed ASVs and Bray–Curtis of arbuscular mycorrhizal fungi and plant pathogens primarily correlated with soil pH (R ≥ 0.34, FDR < 0.001) (Figure 5).

Figure 5.
Pearson correlations between fungal diversity/community and soil properties. Numbers in each cell of this figure represent correlation coefficients (the R values shown in the legend), and only those correlation coefficients with a false discovery rate (FDR) less than 0.05 are shown in the cells. The red dashed box indicates the maximal correlation coefficients in each row. MC: moisture content; ST: soil temperature; CD: conductivity; TOC: total organic carbon; TN: total nitrogen; -N: ammonium nitrogen; -N: nitrate nitrogen; TP: total phosphorus; SAP: soil available phosphorus. OA: Observed ASV; BC: Bray–Curtis distance. * FDR < 0.05, ** FDR < 0.001; *** FDR < 0.001.
4. Discussion
4.1. Ectomycorrhizal Fungi Are Depleted but Arbuscular Mycorrhizal Fungi and Plant Pathogens Are Enriched in Cut Slop Soils at the Early-Stage Restoration
In the present study, the relative abundances of predicted ectomycorrhizal fungi decreased, while those of arbuscular mycorrhizal fungi and plant pathogens increased in cut slope soils at the early-stage restoration (Figure 1B). These results are in line with previous studies if we conceptualize cut slopes as the aftermath of extreme forest destruction. For example, evidence shows that the proportion of ectomycorrhizal fungi declines in the soils of mature forests subjected to clear-cut logging, while arbuscular mycorrhizal fungi and plant pathogens exhibit opposite patterns [52]. A recent study indicates that the proportion of ectomycorrhizal fungi decreases, whereas that of plant pathogens increases in soils from clear-cut logging forests [53]. Similarly, a survey of the soils disturbed by open-cut mining also indicates that the prevalence of ectomycorrhizal fungi decline in response to disturbances [17]. These findings suggest that the functional guilds of arbuscular mycorrhizal fungi, ectomycorrhizal fungi, and plant pathogens are sensitive to anthropogenic disturbances, and they even exhibit a common response to similar disturbances. Despite that the events causing disturbances are varying (e.g., highway construction, clear-cut logging and open-cut mining), the changing patterns of these functional guilds are similar mostly because of the shifts in above-ground vegetation caused by disturbances. Regarding the ectomycorrhizal and arbuscular mycorrhizal fungi, three possible aspects can explain their relative abundances in natural and cut slope soils. First, the formation of cut slopes caused by highway constructions and subsequent ESSS-based restorations have altered naïve vegetations while ectomycorrhizal and arbuscular mycorrhizal fungi are reported to be closely coupled with above-ground vegetations [27]. Second, ectomycorrhizal and arbuscular mycorrhizal fungi compete with each other [54,55]. Third, it is reported that arbuscular mycorrhizal fungi tend to be more abundant in early successional soils, while ectomycorrhizal fungi start to proliferate and predominate with the accumulation of soil organic matter [56]. In this study, the organic carbon contents in cut slope soils were relatively low compared to those in natural soils at high-altitude areas (3102 m and 3194 m), especially at 3102 m (Figure S3). We also detected a positive correlation between soil organic carbon and the observed ASV of ectomycorrhizal fungi (Figure 5). However, such a correlation was very weak (FDR > 0.05), and the differences in soil organic carbon content between natural and cut slope soils were non-significant (FDR > 0.05) at 2900 m and 3194 m. Hence, more evidence is still needed to verify the third inference about ectomycorrhizal fungi in the future. We also found that the prevalence of litter saprotroph (3102 m and 3194 m), wood saprotroph, unspecified saprotroph (2900 m and 3102 m) and dung saprotroph increased in cut slope soils at all altitudes (Figure 1B). This is similar to those reported by Rodriguez-Ramos et al. (2021) [52] and Rähn et al. (2023) [53]. That is, the prevalence of saprotrophs increase in the soils from clear-cut logging forests. A previous study indicates that saprotrophs could release nutrients for arbuscular mycorrhizal fungi [57]. In this light, the enrichment of these saprotrophs in cut slope soils might be coupled with the nutrient acquisition strategy of arbuscular mycorrhizal fungi. Interestingly, we found that soil saprotroph was depleted in cut slope soils at three altitudes (Figure 1B). Yet, this study cannot explain such a result, and more evidence is necessary in the future. Overall, this study reveals the common responses of ectomycorrhizal and arbuscular mycorrhizal fungi, plant pathogen, and some saprotrophs to similar disturbances reported in previous studies using the highway construction as a disturbance model in a subalpine forest ecosystem.
4.2. The Prevalence Levels of Major Fungal Functional Guilds May Be Closely Related to Plant Health in Cut Slopes
A previous study indicates that arbuscular mycorrhizal and ectomycorrhizal fungi can facilitate the nutrient uptake of plants via symbiotic associations [26]. The specialized transporters located on the membrane of these fungi in symbiotic associations can take up nutrients such as inorganic phosphorus and mineral or organic forms of nitrogen (e.g., ammonium and nitrate nitrogen, and amino acids) from soils, and then import them from the symbiotic interface to plant cells through selective transporters [27]. Under such a scenario, the prevalence levels of soil arbuscular mycorrhizal and ectomycorrhizal fungi may be closely related to the health and sustainability of above-ground vegetation in cut slopes. Evidence demonstrates that ectomycorrhizal fungi are able to mineralize nutrients from organic matter and can thus, access several forms of organic nitrogen in soils directly [57]. In this light, the predominance of soil ectomycorrhizal fungi may be more beneficial to the sustainability of above-ground vegetation in cut slopes, and therefore, our findings highlight the necessity of paying more attention to the restoration of ectomycorrhizal fungi in cut slope soils at the early stage. The enrichment of plant pathogens in cut slope soils might involve the critical roles of their secreted proteins, which can function as effectors during the early phase of the plant–fungus interaction or be involved in the construction of the symbiotic interface [27]. However, plant pathogens can also cause diseases, influencing the survival and sustainability of plant hosts [25]. Therefore, the predominance of plant pathogens in cut-slope soils may increase the probability of plant disease outbreaks, thereby impacting the growth of above-ground vegetation. This could somewhat explain the previously observed phenomenon where above-ground herbs in cut-slope soils can only grow for a few years [10]. In this light, our findings also emphasize a need to pay more attention to plant pathogens in cut slope soils at the early-stage restoration. As saprophytic fungi mainly undertake the decomposition of complicated organic matters [58,59], the increases in the prevalence of some saprotrophs may be beneficial to the conversion of soil nutrients in cut slope soils. By inspecting the taxonomic composition of five major functional guilds, we found that they could be indicated by several class-level lineages, especially arbuscular mycorrhizal and ectomycorrhizal fungi, and plant pathogen (Figure S5). A previous study conducted across a restoration chronosequence of soils disturbed by open-cut mining indicates that the relative abundances of several saprotrophs belonging to the class Dothideomycetes, Eurotiomycetes, Sordariomycetes, Tremellomycetes and Agaricomycetes significantly vary with the increasing restoration years [17]. In this study, we also detected Agaricomycetes as the main ectomycorrhizal fungi, and Dothideomycetes and Sordariomycetes as the main plant pathogen (Figure S5). Such a finding highlights the need to consider these class-level lineage’s roles in plant pathogenicity, nutrient uptake by plants, and organic matter decomposition when evaluating the early-stage restoration of cut slope soils in subalpine forest ecosystems. In summary, our findings reveal the significance of several major fungal functional guilds in the ecosystem stability and plant health of cut slopes, providing valuable insights for future ecological restoration efforts in subalpine forest ecosystems.
4.3. Altitude Regulates the Early-Stage Restoration of Major Fungal Functional Guilds in Cut Slope Soils
The community differences in total soil fungi, soil and litter saprotrophs, arbuscular mycorrhizal and ectomycorrhizal fungi between natural and cut slope soils were enlarged at high altitudes (3102 m and 3194 m) though the highest differences were detected at 3102 m (Figure 3). This aligns with the findings regarding the soil bacteria in our earlier study [15], highlighting a need to consider the impacts of altitude on soil fungi at the early-stage restoration of cut slopes in subalpine forest ecosystems, especially the major functional guilds at high altitudes. Although there is evidence showing that altitude has far-reaching impacts on fungi communities [30,31], our findings demonstrate the regulation of altitude on fungal functional guilds under a restoration scenario in subalpine forest soils. Evidence indicates that fungi are less resilient [21], and our previous study also shows that soil fungal diversities and communities are sensitive to the simulated ESSS [22]. In this light, the differences in soil properties between natural and cut slope soils at each altitude may be key in driving community differences. Such an inference is supported by two pieces of evidence. First, the differences in community between natural and cut slope soils across three altitudes closely mirrored those observed in soil properties (Figure S2B). Second, the α- and (or) β-diversities (observed ASV and (or) Bray–Curtis distance) mainly correlated with soil pH (Figure 5), while the differences in soil pH between the natural and cut slope soils were highly similar to those of communities across the altitudinal gradient (Figure S3). However, only the βNTI of arbuscular mycorrhizal fungi (a βNTI greater than +2 represents heterogeneous selection that can result in community differences; see Figure S7) can fully support the above inferences (Figure 4D), suggesting additional mechanisms for community differences between natural and cut slope soils. It is reported that dispersal limitation could limit the exchanges of organism among communities, which allows drift (e.g., stochastic birth and death) to cause much greater differences in community composition than when drift acts alone [60]. In this study, we detected remarkable contributions of dispersal limitation and (or) drift to the community assembling of total soil fungi and five functional guilds in addition to heterogeneous selection (Figure S7). Such a result is consistent with previous findings that fungal community assemblies are dominated by dispersal limitation [61,62,63]. This could be a reason why the differences in soil properties alone cannot fully account for the community differences in total soil fungi, soil and litter saprotrophs, ectomycorrhizal fungi and plant pathogens between natural and cut slope soils. Additionally, the increase in solar radiation to ecosystems with altitude also probably contributed to many undetected differences (e.g., microelement composition) between natural and cut slope soils. This could also be one of the reasons why differences in soil properties alone cannot fully explain the differences in community in this study. While the community differences in plant pathogens between the two types of soils decline with altitude, it does not necessarily indicate a good restoration of this functional guild at higher altitudes due to their high diversity in cut slope soils (Figure 2F). Nevertheless, our findings highlight the challenges of restoring fungal functional guilds in cut-slope soils in high-altitude areas.
5. Conclusions
By partitioning soil fungal communities based on lifestyles, this study revealed a decrease in the prevalence of ectomycorrhizal fungi, and an increase in the prevalence of plant pathogen and arbuscular mycorrhizal fungi in cut slope soils compared to natural soils at the early-stage restoration. At higher altitudes (3102 m and 3194 m), the differences in community composition between natural and cut slope soils were particularly more pronounced for total soil fungi, soil and litter saprotrophs, and arbuscular mycorrhizal and ectomycorrhizal fungi, in contrast to the lower altitude (2900 m). An opposite pattern was evident for plant pathogens. Variations in the differences in soil conditions (mainly soil pH) and community assembly processes (e.g., heterogeneous selection, dispersal limitation and drift) between natural and cut slope soils across the altitudinal gradient likely shaped the shifting patterns of community differences. This study provides novel insights for the development of effective restoration strategies tailored for cut slopes in subalpine forest ecosystems. The significance lies in recognizing and addressing the complexities of fungal functional guild, especially highlighting the heightened attention required for the early-stage restorations of soil fungi in cut slopes situated at high altitudes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15040636/s1, Figure S1: The sampling strategies and the geographic distributions of sampling site in this study; Figure S2: Principal coordinates analysis (PCoA) based on the Euclidean distance for soil properties including conductivity (CD) and soil pH, moisture content (MC), soil temperature (ST), total organic carbon (TOC), ammonium (NH4+ -N) and nitrate nitrogen (NO3− -N), total phosphorus (TP) and nitrogen (TN), and soil available phosphorus (SAP); Figure S3: The comparisons for soil properties between natural (NS) and cut slope (CS) soils at each altitude; Figure S4: Veen diagrams showing the unique ASV number in natural (NS) and cut slope (CS) soils, respectively, and the shared ASV number between NS and CS. Numbers in each subfigure represent ASV numbers; Figure S5: Taxonomic compositions at class-level for soil and litter saprotrophs, arbuscular mycorrhizal and ectomycorrhizal fungi, and plant pathogen; Figure S6: Principal coordinates analysis (PCoA) based on Bray-Curtis distance for the communities of total soil fungi (A), soil saprotroph (B), litter saprotroph (C), arbuscular mycorrhizal (D) and ectomycorrhizal fungi (E), and plant pathogen (F); Figure S7: The ecological processes of community assembly for total soil fungi, soil and litter saprotrophs, arbuscular mycorrhizal and ectomycorrhizal fungi, and plant pathogen; Table S1: The analysis of similarity (ANOSIM) for soil properties based on Euclidean distance; Table S2: The analysis of similarity (ANOSIM) for communities based on Bray-Curtis distance.
Author Contributions
C.L., H.L. and Y.J. conceived the ideas, designed the methodology, and wrote the manuscript. D.L. revised the manuscript and contributed to the discussion. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Science and Technology Program (2023NSFSC0755, 2023ZYD0102, 2022NSFSC1175) and the Scientific Research Initiation Project of Mianyang Normal University (QD2021A23, QD2021A37, QD2023A01).
Data Availability Statement
Raw reads are available in the Sequence Read Archive (SRA) with an accession number of PRJNA1079527.
Acknowledgments
The authors thank the supporter of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Meyer, M.D. Natural Range of Variation of Subalpine Forests in the Bioregional Assessment Area; US Forest Service, Pacific Southwest Region: Vallejo, CA, USA, 2013.
- Aurélie, G.; Xavier, M.; Sandrine, C.; Christopher, C. The function of surface fires in the dynamics and structure of a formerly grazed old subalpine forest. J. Ecol. 2009, 97, 728–741. [Google Scholar] [CrossRef]
- Forman, R.T. Estimate of the area affected ecologically by the road system in the United States. Conserv. Biol. 2000, 14, 31–35. [Google Scholar] [CrossRef]
- Banerjee, P.; Ghose, M.K.; Pradhan, R. Analytic hierarchy process based spatial biodiversity impact assessment model of highway broadening in Sikkim Himalaya. Geocarto Int. 2020, 35, 470–493. [Google Scholar] [CrossRef]
- Van Der Ree, R.; Smith, D.J.; Grilo, C. The ecological effects of linear infrastructure and traffic: Challenges and opportunities of rapid global growth. In Handbook of Road Ecology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–9. [Google Scholar]
- Xu, H.; Li, T.B.; Chen, J.N.; Liu, C.N.; Zhou, X.; Xia, L. Characteristics and applications of ecological soil substrate for rocky slope vegetation in cold and high-altitude areas. Sci. Total Environ. 2017, 609, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Chen, J.; Gao, D.; Ai, Y. Distribution patterns and drivers of artificial soil bacterial community on cut-slopes in alpine mountain area of southwest China. Catena 2020, 194, 104695. [Google Scholar] [CrossRef]
- Fu, D.; Yang, H.; Wang, L.; Yang, S.; Li, R.; Zhang, W.; Ai, X.; Ai, Y. Vegetation and soil nutrient restoration of cut slopes using outside soil spray seeding in the plateau region of southwestern China. J. Environ. Manag. 2018, 228, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, R.; Han, P.; Sun, H.; Sun, H.; Li, C.; Yang, L. Soil water repellency of the artificial soil and natural soil in rocky slopes as affected by the drought stress and polyacrylamide. Sci. Total Environ. 2018, 619, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhao, Q.; Lai, Q.; Guogang, X.U.; Chen, X.J.S. Cognition and practice on technological innovation of ecological restoration of engineering slope soil in South China. Soils 2017, 49, 643–650. [Google Scholar]
- Huang, Z.; Chen, J.; Ai, X.; Li, R.; Ai, Y.; Li, W. The texture, structure and nutrient availability of artificial soil on cut slopes restored with OSSS–Influence of restoration time. J. Environ. Manag. 2017, 200, 502–510. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Robeson, M.D.; Hu, J.; Zhu, Q. Application of erosion-resistant fibers in the recovery of vegetation on steep slopes in the Loess Plateau of China. Catena 2018, 160, 233–241. [Google Scholar] [CrossRef]
- Gao, G.; Li, Z.; Han, R. Statistical evaluation of sprayed synthetic soils amended with four additive factors used in high-and cut rock slopes. Environ. Eng. Sci. 2017, 34, 281–290. [Google Scholar] [CrossRef]
- Liao, H.; Sheng, M.; Liu, J.; Ai, X.; Li, C.; Ai, S.; Ai, Y. Soil N availability drives the shifts of enzyme activity and microbial phosphorus limitation in the artificial soil on cut slope in southwestern China. Environ. Sci. Pollut. Res. 2021, 28, 33307–33319. [Google Scholar] [CrossRef]
- Liao, H.; Li, C.; Ai, Y.; Li, X. Soil bacterial responses to disturbance are enlarged by altitude in a mountain ecosystem. J. Soils Sediments 2023, 23, 3820–3831. [Google Scholar] [CrossRef]
- Wang, K.; Bi, Y.; Cao, Y.; Peng, S.; Christie, P.; Ma, S.; Zhang, J.; Xie, L. Shifts in composition and function of soil fungal communities and edaphic properties during the reclamation chronosequence of an open-cast coal mining dump. Sci. Total Environ. 2021, 767, 144465. [Google Scholar] [CrossRef]
- Ngugi, M.R.; Fechner, N.; Neldner, V.J.; Dennis, P.G. Successional dynamics of soil fungal diversity along a restoration chronosequence post-coal mining. Restor. Ecol. 2020, 28, 543–552. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Meng, Z.; Xu, R.; Chen, J.; Zhang, Y.; Hu, T. Fertility-related interplay between fungal guilds underlies plant richness–productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Delgado Baquerizo, M.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Zhu, Y.N.; Singh, B.K. Fungal richness contributes to multifunctionality in boreal forest soil. Soil Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Delgado Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Bürgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef]
- Liao, H.; Li, C.; Ai, S.; Li, X.; Ai, X.; Ai, Y. A simulated ecological restoration of bare cut slope reveals the dosage and temporal effects of cement on ecosystem multifunctionality in a mountain ecosystem. J. Environ. Manag. 2023, 325, 116672. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- García Guzmán, G.; Heil, M. Life histories of hosts and pathogens predict patterns in tropical fungal plant diseases. New Phytol. 2014, 201, 1106–1120. [Google Scholar] [CrossRef]
- van Der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Zhu, B.; Li, C.; Wang, J.; Li, J.; Li, X. Elevation rather than season determines the assembly and co-occurrence patterns of soil bacterial communities in forest ecosystems of Mount Gongga. Appl. Microbiol. Biotechnol. 2020, 104, 7589–7602. [Google Scholar] [CrossRef]
- Sun, F.; Lü, Y.; Wang, J.; Hu, J.; Fu, B. Soil moisture dynamics of typical ecosystems in response to precipitation: A monitoring-based analysis of hydrological service in the Qilian Mountains. Catena 2015, 129, 63–75. [Google Scholar] [CrossRef]
- Sui, X.; Li, M.; Frey, B.; Dai, G.; Yang, L.; Li, M.H. Effect of elevation on composition and diversity of fungi in the rhizosphere of a population of Deyeuxia angustifolia on Changbai Mountain, northeastern China. Front. Microbiol. 2023, 14, e1087475. [Google Scholar] [CrossRef]
- Zhang, P.; Luan, M.; Li, X.; Lian, Z.; Zhao, X. The distribution of soil fungal communities along an altitudinal gradient in an alpine meadow. Glob. Ecol. Conserv. 2021, 31, e01838. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Ji, N.N.; Wang, Y.L.; Gao, C.; Jin, S.S.; Hu, H.W.; Huang, Z.; He, J.Z.; Guo, L.D. Assembly processes lead to divergent soil fungal communities within and among 12 forest ecosystems along a latitudinal gradient. New Phytol. 2021, 231, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, Z.; Liu, S.; Zhang, M.; Cao, X.; Chen, M.; Xu, G.; Xing, H.; Li, F.; Feng, Q. Altitudinal variation influences soil fungal community composition and diversity in Alpine–Gorge region on the Eastern Qinghai–Tibetan Plateau. J. Fungi 2022, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, N.; Xiao, J.; Zhao, C.; Zou, T.; Li, D.; Liu, Q.; Yin, H. Changes in plant nitrogen acquisition strategies during the restoration of spruce plantations on the eastern Tibetan Plateau, China. Soil Biol. Biochem. 2018, 119, 50–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Zhao, W.; He, H.; Li, D.; He, W.; Liu, Q.; Yin, H. Seasonal variations in the soil amino acid pool and flux following the conversion of a natural forest to a pine plantation on the eastern Tibetan Plateau, China. Soil Biol. Biochem. 2017, 105, 1–11. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Whitman, T.; Neurath, R.; Perera, A.; Chu-Jacoby, I.; Ning, D.; Zhou, J.; Nico, P.; Pett-Ridge, J.; Firestone, M. Microbial community assembly differs across minerals in a rhizosphere microcosm. Environ. Microbiol. 2018, 20, 4444–4460. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, e4717. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef]
- Revelle, W. Package ‘psych’. Compr. R Arch. Netw. 2015, 337, 338. [Google Scholar]
- Rodriguez Ramos, J.C.; Cale, J.A.; Cahill, J.F., Jr.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2021, 229, 1105–1117. [Google Scholar] [CrossRef]
- Rähn, E.; Tedersoo, L.; Adamson, K.; Drenkhan, T.; Sibul, I.; Lutter, R.; Anslan, S.; Pritsch, K.; Drenkhan, R. Rapid shift of soil fungal community compositions after clear-cutting in hemiboreal coniferous forests. For. Ecol. Manag. 2023, 544, e121211. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G. The mutualistic niche: Mycorrhizal symbiosis and community dynamics. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 143–164. [Google Scholar] [CrossRef]
- Fernández, N.; Knoblochová, T.; Kohout, P.; Janoušková, M.; Rydlová, J. Asymmetric interaction between two mycorrhizal fungal guilds and consequences for the establishment of their host plants. Front. Plant Sci. 2022, 13, e873204. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Z. The response patterns of arbuscular mycorrhizal and ectomycorrhizal symbionts under elevated CO2: A meta-analysis. Front. Microbiol. 2018, 9, e340522. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Talbot, J.M.; Bruns, T.D.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Peay, K.G. Independent roles of ectomycorrhizal and saprotrophic communities in soil organic matter decomposition. Soil Biol. Biochem. 2013, 57, 282–291. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Mao, J.; Cai, L. Dispersal limitation controlling the assembly of the fungal community in Karst Caves. J. Fungi 2023, 9, e1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Qu, M.; Li, J. Dispersal limitation dominates the community assembly of abundant and rare fungi in dryland montane forests. Front. Microbiol. 2022, 13, e929772. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, H.; Liu, S.; Yin, Y.; Yuan, Z.; Zhao, Y.; Cao, H. Distribution and assembly processes of soil fungal communities along an altitudinal gradient in Tibetan Plateau. J. Fungi 2021, 7, e1082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).