Model Exploration and Application of Near-Infrared Spectroscopy for Species Separation and Quantification during Mixed Litter Decomposition in Subtropical Forests of China
Abstract
1. Introduction
- The four component tree species—Pinus massoniana Lamb., Choerospondias axillaris (Roxb.) Burtt et Hill, Cyclobalanopsis glauca (Thunb.) Oerst. and Lithocarpus glaber (Thunb.) Nakai—are different in chemical composition and spectral performance, and they are also different from the mineral soil on the surface of the forest floor in chemical composition. Therefore, the NIRS model can be used to separate and predict the composition of the foliage mixtures of four tree species.
- Litter decomposition is a dynamic and continuous process, and chemical composition will change continuously. The prediction accuracy of the NIRS model declines with the prolonged time in this 1-year foliage litter decomposition experiment.
- A certain amount of adhered soil and crude ash in plant dry matter varies with different plant species and decomposition time. The NIRS models with ash-free weight as reference value perform better than the ones with crude ash content.
2. Materials and Methods
2.1. Experimental Site and Experimental Design
2.2. Experimental Setup and Artificial Mixture Preparation
2.3. Crude Ash Content Determination
2.4. Spectral Measurement
2.5. Model Development and Evaluation
3. Results
3.1. Model Development for Undecomposed Leaf Samples
3.2. Model Prediction Ability with Ash-Containing Litter Mass
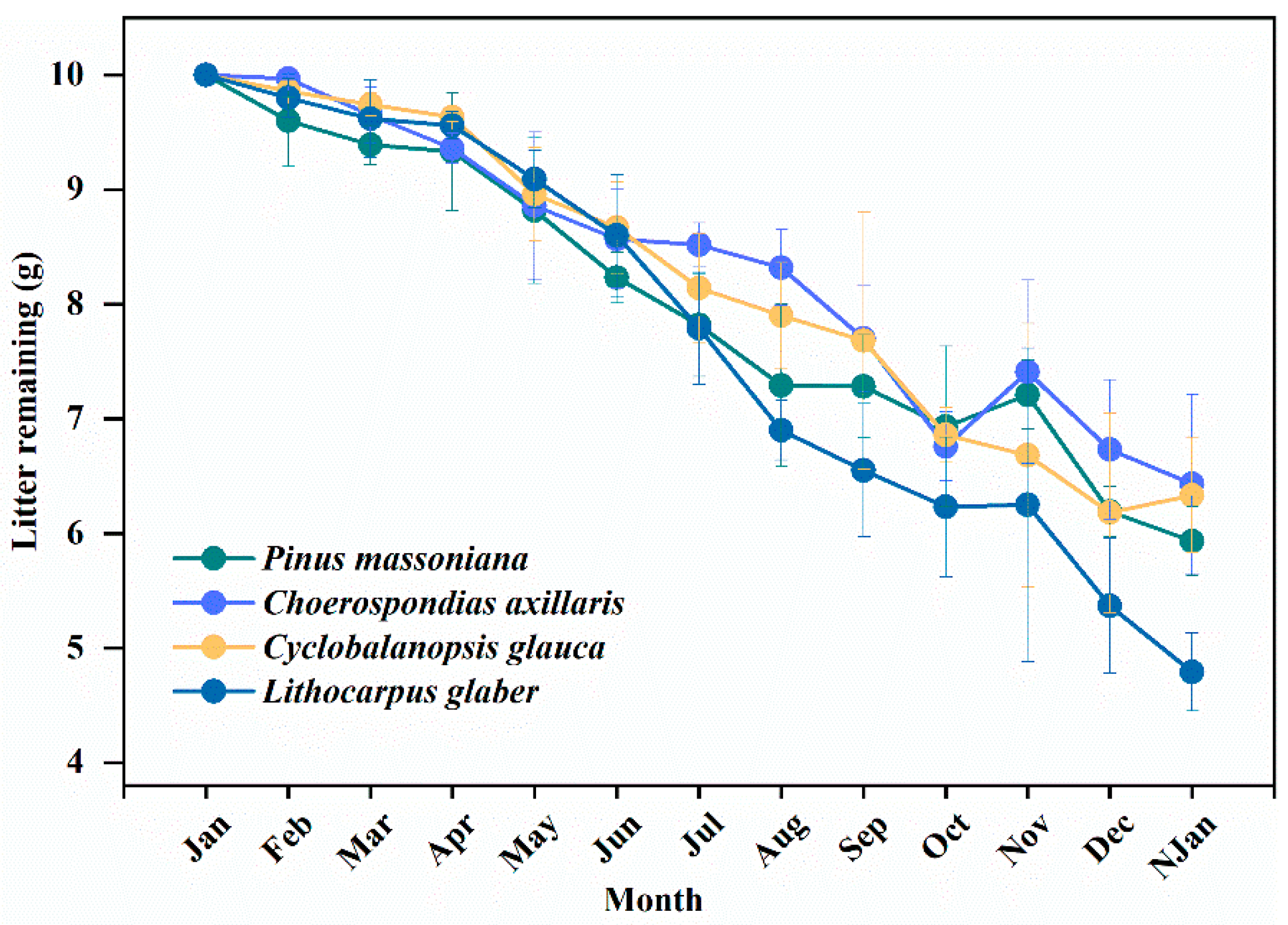
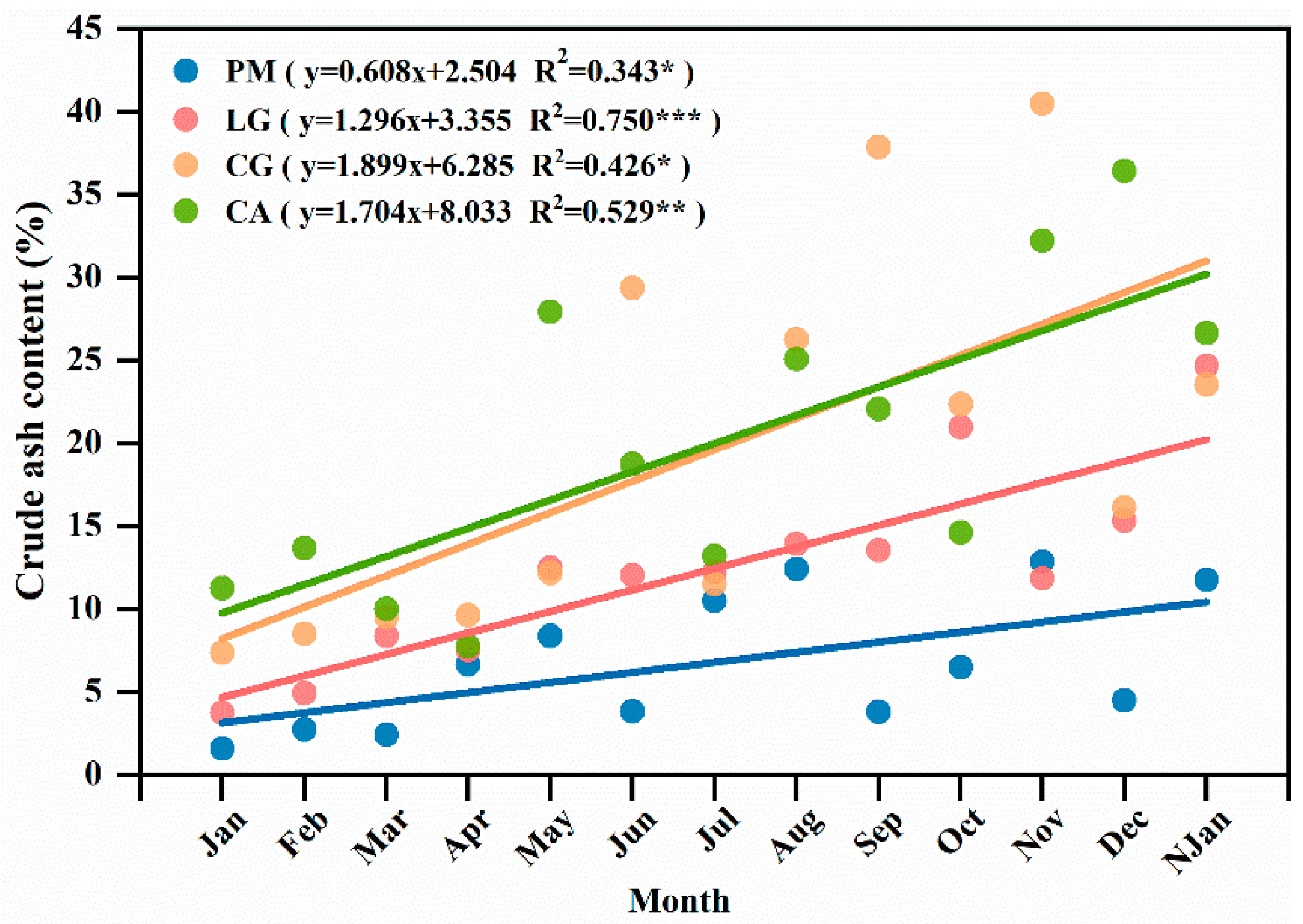
3.3. Influence of Litter Decomposition Rate and Crude Ash Content over Time
3.4. Model Development and Validation with Ash-Free Litter Mass
4. Discussion
4.1. NIRS Models to Quantify the Composition of Litter Leaves
4.2. Influence of Litter Decomposition Rate and Crude Ash Content along with Time
4.3. Model Development and Validation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Yin, X.Q.; Wang, F.B. The influence of litter mixing on decomposition and soil fauna assemblages in a Pinus koraiensis mixed broad-leaved forest of the Changbai Mountains, China. Eur. J. Soil Biol. 2013, 55, 28–39. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 2011, 32, 365–399. [Google Scholar] [CrossRef]
- Parsons, S.A.; Lawler, I.R.; Congdon, R.A.; Williams, S.E. Rainforest litter quality and chemical controls on leaf decomposition with near-infrared spectrometry. J. Plant Nutr. Soil Sci. 2011, 174, 710–720. [Google Scholar] [CrossRef]
- Hui, D.F.; Jackson, R.B. Assessing interactive responses in litter decomposition in mixed species litter. Plant Soil 2009, 314, 263–271. [Google Scholar] [CrossRef]
- Sarraguca, M.C.; Matias, R.; Figueiredo, R.; Ribeiro, P.R.S.; Martins, A.T.; Lopes, J.A. Near infrared spectroscopy to monitor drug release in-situ during dissolution tests. Int. J. Pharm. 2016, 513, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.L. Research and application of near-infrared spectroscopy in rapid detection of water pollution. Desalin Water Treat. 2018, 122, 1–4. [Google Scholar] [CrossRef]
- Gehlken, J.; Nikfardjam, M.P.; Kleb, M.; Zorb, C. Near-infrared spectroscopy in process control and quality management of fruits and wine. J. Appl. Bot. Food Qual. 2021, 94, 26–38. [Google Scholar]
- Batten, G.D. Plant analysis using near infrared reflectance spectroscopy: The potential and the limitations. Anim. Prod. Sci. 1998, 38, 697–706. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xiang, J.Y.; Tang, Y.; Chen, W.J.; Xu, Y.J. A review of the application of near-infrared spectroscopy (NIRS) in forestry. Appl. Spectrosc. Rev. 2021, 57, 300–317. [Google Scholar] [CrossRef]
- Islam, K.; Singh, B.; McBratney, A. Simultaneous estimation of several soil properties by ultra-violet, visible, and near-infrared reflectance spectroscopy. Soil Res. 2003, 41, 1101–1114. [Google Scholar] [CrossRef]
- Lopo, M.; Teixeira dos Santos, C.A.; Pascoa, R.; Graca, A.R.; Lopes, J.A. Near infrared spectroscopy as a tool for intensive mapping of vineyards soil. Precis. Agric. 2018, 19, 445–462. [Google Scholar] [CrossRef]
- Card, D.H.; Peterson, D.L.; Matson, P.A.; Aber, J.D. Prediction of leaf chemistry by the use of visible and near infrared reflectance spectroscopy. Remote Sens. Environ. 1988, 26, 123–147. [Google Scholar] [CrossRef]
- Petisco, C.; Garcia-Criado, B.; Mediavilla, S.; Vazquez de Aldana, B.R.; Zabalgogeazcoa, I.; Garcia-Ciudad, A. Near-infrared reflectance spectroscopy as a fast and non-destructive tool to predict foliar organic constituents of several woody species. Anal. Bioanal. Chem. 2006, 386, 1823–1833. [Google Scholar] [CrossRef]
- Terhoeven-Urselmans, T.; Michel, K.; Helfrich, M.; Flessa, H.; Ludwig, B. Near-infrared spectroscopy can predict the composition of organic matter in soil and litter. J. Plant Nutr. Soil Sci. 2006, 169, 168–174. [Google Scholar] [CrossRef]
- Lei, P.F.; Bauhus, J. Use of near-infrared reflectance spectroscopy to predict species composition in tree fine-root mixtures. Plant Soil 2010, 333, 93–103. [Google Scholar] [CrossRef]
- Gruselle, M.C.; Bauhus, J. Assessment of the species composition of forest floor horizons in mixed spruce-beech stands by near infrared reflectance spectroscopy (NIRS). Soil Biol. Biochem. 2010, 42, 1347–1354. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.H.; Wu, H.L.; Ouyang, S.; Zhou, B.; Zeng, Y.L.; Chen, Y.L.; Kuzyakov, Y. Tree species identity surpasses richness in affecting soil microbial richness and community composition in subtropical forests. Soil Biol. Biochem. 2019, 130, 113–121. [Google Scholar] [CrossRef]
- Zhao, L.J.; Xiang, W.H.; Li, J.X.; Lei, P.F.; Deng, X.W.; Fang, X.; Peng, C.H. Effects of topographic and soil factors on woody species assembly in a chinese subtropical evergreen broadleaved forest. Forests 2015, 6, 650–669. [Google Scholar] [CrossRef]
- Jacob, M.; Viedenz, K.; Polle, A.; Thomas, F.M. Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 2010, 164, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, J.; Todt, B.; Boča, A.; Nitschke, R.; Kohler, M.; Kühn, P.; Bauhus, J. Use of near-infrared spectroscopy to assess phosphorus fractions of different plant availability in forest soils. Biogeosciences 2015, 12, 3415–3428. [Google Scholar] [CrossRef]
- Richardson, A.D.; Reeves Iii, J.B. Quantitative reflectance spectroscopy as an alternative to traditional wet lab analysis of foliar chemistry: Near-infrared and mid-infrared calibrations compared. Can. J. For. Res. 2005, 35, 1122–1130. [Google Scholar] [CrossRef]
- Sheffer, E.; Canham, C.D.; Kigel, J.; Perevolotsky, A. Countervailing effects on pine and oak leaf litter decomposition in human-altered mediterranean ecosystems. Oecologia 2015, 177, 1039–1051. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.N.; Bruelheide, H.; Zhang, S.R.; Ren, H.B.; Zhang, N.L.; Ma, K.P. What drives leaf litter decomposition and the decomposer community in subtropical forests-The richness of the above-ground tree community or that of the leaf litter? Soil Biol. Biochem. 2021, 160, 108314. [Google Scholar] [CrossRef]
- Li, R.S.; Yang, Q.P.; Guan, X.; Chen, L.C.; Wang, Q.K.; Wang, S.L.; Zhang, W.D. High quality litters with faster initial decomposition produce more stable residue remaining in a subtropical forest ecosystem. Catena 2022, 213, 106134. [Google Scholar] [CrossRef]
- Ge, J.L.; Xie, Z.Q.; Xu, W.T.; Zhao, C.M. Controls over leaf litter decomposition in a mixed evergreen and deciduous broad-leaved forest, Central China. Plant Soil 2016, 412, 345–355. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, X.J.; He, X.B.; Song, F.Q.; Ren, L.L.; Jiang, P. Effect of litter quality on its decomposition in broadleaf and coniferous forest. Eur. J. Soil Biol. 2008, 44, 392–399. [Google Scholar] [CrossRef]
- Wang, W.B.; Chen, D.S.; Zhang, Q.; Sun, X.M.; Zhang, S.G. Effects of mixed coniferous and broad-leaved litter on bacterial and fungal nitrogen metabolism pathway during litter decomposition. Plant Soil 2020, 451, 307–323. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.L.; Wu, J.G.; Variath, M.T.; Shi, C.H. Developmental genetic analysis for crude fiber content and crude ash content of rapeseed meal in two different growing years. J. Food Qual. 2011, 34, 284–297. [Google Scholar] [CrossRef]
- Babayemi, J.O.; Dauda, K.T.; Nwude, D.O.; Kayode, A.A.A. Evaluation of the composition and chemistry of ash and potash from various plant materials-a review. J. Appl. Sci. 2010, 10, 1820–1824. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass ashes and their phosphorus fertilizing effect on different crops. Nutr. Cycling Agroecosyst. 2010, 87, 471–482. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Ge, X.G.; Zeng, L.X.; Xiao, W.F.; Huang, Z.L.; Geng, X.S.; Tan, B.W. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Li, G.L.; Liu, Y.; Li, R.S.; Guo, B.; Xu, Y. Responses of decomposition rate, nutrient return, and composition of leaf litter to thinning intensities in a Pinus tabulaeformis plantation. Front. For. China 2009, 4, 458–463. [Google Scholar] [CrossRef]
- Gao, S.Q.; Song, Y.Y.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Gao, J.L.; Cheng, X.F.; Du, Y. Effects of temperature increase and nitrogen addition on the early litter decomposition in permafrost peatlands. Catena 2022, 209, 105801. [Google Scholar] [CrossRef]
- Moron, A.; Cozzolino, D. Determination of potentially mineralizable nitrogen and nitrogen in particulate organic matter fractions in soil by visible and near-infrared reflectance spectroscopy. J. Agric. Sci. 2004, 142, 335–343. [Google Scholar] [CrossRef]





| Mixture Components | n | R2 | RMSECV | RPD | Bias | Rank |
|---|---|---|---|---|---|---|
| Pinus massoniana | ||||||
| PM.LG | 25 | 0.960 | 5.3 | 4.98 | 0.0114 | 8 |
| PM.CG | 17 | 0.950 | 6.45 | 4.49 | −0.0148 | 7 |
| PM.CA | 21 | 0.951 | 6.25 | 4.52 | 0.00555 | 7 |
| PM.CG.LG | 12 | 0.958 | 6.41 | 4.87 | −0.00665 | 7 |
| PM.CA.LG | 11 | 0.959 | 6.34 | 4.92 | −0.00333 | 7 |
| PM.CA.CG.LG | 21 | 0.958 | 6.54 | 4.9 | −0.0172 | 7 |
| Choerospondias axillaris | ||||||
| PM.CA | 21 | 0.977 | 4.23 | 6.59 | 0.0231 | 8 |
| CA.LG | 17 | 0.976 | 4.33 | 6.47 | 0.0283 | 8 |
| CA.CG | 25 | 0.969 | 4.9 | 5.72 | 0.0134 | 7 |
| PM.CA.LG | 11 | 0.977 | 4.71 | 6.54 | 0.0292 | 7 |
| CA.CG.LG | 16 | 0.977 | 4.69 | 6.64 | 0.0205 | 7 |
| PM.CA.CG.LG | 21 | 0.979 | 4.62 | 6.9 | 0.0214 | 7 |
| Cyclobalanopsis glauca | ||||||
| PM.CG | 17 | 0.920 | 7.99 | 3.54 | −0.0235 | 7 |
| CG.LG | 21 | 0.917 | 8.24 | 3.46 | −0.0673 | 8 |
| CA.CG | 25 | 0.895 | 9.04 | 3.08 | −0.0413 | 7 |
| PM.CG.LG | 12 | 0.902 | 9.8 | 3.19 | −0.0541 | 7 |
| CA.CG.LG | 16 | 0.904 | 9.69 | 3.22 | −0.0442 | 7 |
| PM.CA.CG.LG | 21 | 0.908 | 9.84 | 3.29 | −0.0505 | 7 |
| Lithocarpus glaber | ||||||
| PM.LG | 25 | 0.864 | 9.74 | 2.71 | 0.062 | 10 |
| CG.LG | 21 | 0.899 | 8.86 | 3.15 | 0.00589 | 10 |
| CA.LG | 17 | 0.885 | 9.34 | 2.95 | 0.0541 | 10 |
| PM.CG.LG | 12 | 0.885 | 10.5 | 2.95 | 0.00977 | 10 |
| PM.CA.LG | 11 | 0.894 | 9.86 | 3.08 | 0.0163 | 10 |
| CA.CG.LG | 16 | 0.874 | 10.9 | 2.82 | 0.0421 | 10 |
| PM.CA.CG.LG | 21 | 0.886 | 10.6 | 2.96 | 0.0459 | 10 |
| Model | Species | Math.Pretreatment | n | Rank | Calibration | Validation | Test Set | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSEE | RPD | R2 | RMSECV | RPD | Bias | MD | R2 | RMSEP | RPD | |||||
| Model 1 | PM | FD + MSC | 129 | 7 | 0.944 | 6.71 | 4.22 | 0.920 | 7.78 | 3.53 | 0.0204 | 0.3 | 0.898 | 10.2 | 3.14 |
| CA | MSC | 130 | 7 | 0.965 | 5.86 | 5.33 | 0.956 | 6.36 | 4.76 | 0.0069 | 0.19 | 0.925 | 8.38 | 3.65 | |
| CG | MSC | 124 | 7 | 0.925 | 8.46 | 3.65 | 0.899 | 9.5 | 3.14 | −0.134 | 0.3 | 0.873 | 10.7 | 2.82 | |
| LG | SNV | 129 | 10 | 0.938 | 7.61 | 4 | 0.868 | 10.6 | 2.75 | 0.07 | 0.23 | 0.834 | 11.5 | 2.45 | |
| Model 2 | PM | First derivative | 124 | 6 | 0.948 | 6.15 | 4.4 | 0.932 | 6.88 | 3.83 | −0.0315 | 0.16 | 0.917 | 8.24 | 3.5 |
| CA | SNV | 128 | 9 | 0.97 | 4.22 | 5.77 | 0.959 | 4.74 | 4.93 | 0.0078 | 0.3 | 0.945 | 6.52 | 4.32 | |
| CG | First derivative | 122 | 8 | 0.933 | 6.26 | 3.85 | 0.909 | 7.01 | 3.31 | 0.055 | 0.24 | 0.888 | 7.49 | 3.01 | |
| LG | FD + MSC | 131 | 8 | 0.933 | 7.07 | 3.87 | 0.902 | 8.28 | 3.2 | −0.0213 | 0.19 | 0.864 | 10.2 | 2.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, N.; Zhang, R.; Wu, Y.; Lei, P.; Xiang, W.; Ouyang, S.; Chen, L.; Yan, W. Model Exploration and Application of Near-Infrared Spectroscopy for Species Separation and Quantification during Mixed Litter Decomposition in Subtropical Forests of China. Forests 2024, 15, 637. https://doi.org/10.3390/f15040637
Zou N, Zhang R, Wu Y, Lei P, Xiang W, Ouyang S, Chen L, Yan W. Model Exploration and Application of Near-Infrared Spectroscopy for Species Separation and Quantification during Mixed Litter Decomposition in Subtropical Forests of China. Forests. 2024; 15(4):637. https://doi.org/10.3390/f15040637
Chicago/Turabian StyleZou, Ningcan, Rong Zhang, Yating Wu, Pifeng Lei, Wenhua Xiang, Shuai Ouyang, Liang Chen, and Wende Yan. 2024. "Model Exploration and Application of Near-Infrared Spectroscopy for Species Separation and Quantification during Mixed Litter Decomposition in Subtropical Forests of China" Forests 15, no. 4: 637. https://doi.org/10.3390/f15040637
APA StyleZou, N., Zhang, R., Wu, Y., Lei, P., Xiang, W., Ouyang, S., Chen, L., & Yan, W. (2024). Model Exploration and Application of Near-Infrared Spectroscopy for Species Separation and Quantification during Mixed Litter Decomposition in Subtropical Forests of China. Forests, 15(4), 637. https://doi.org/10.3390/f15040637






