Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China
Abstract
1. Introduction
2. Materials and Methods
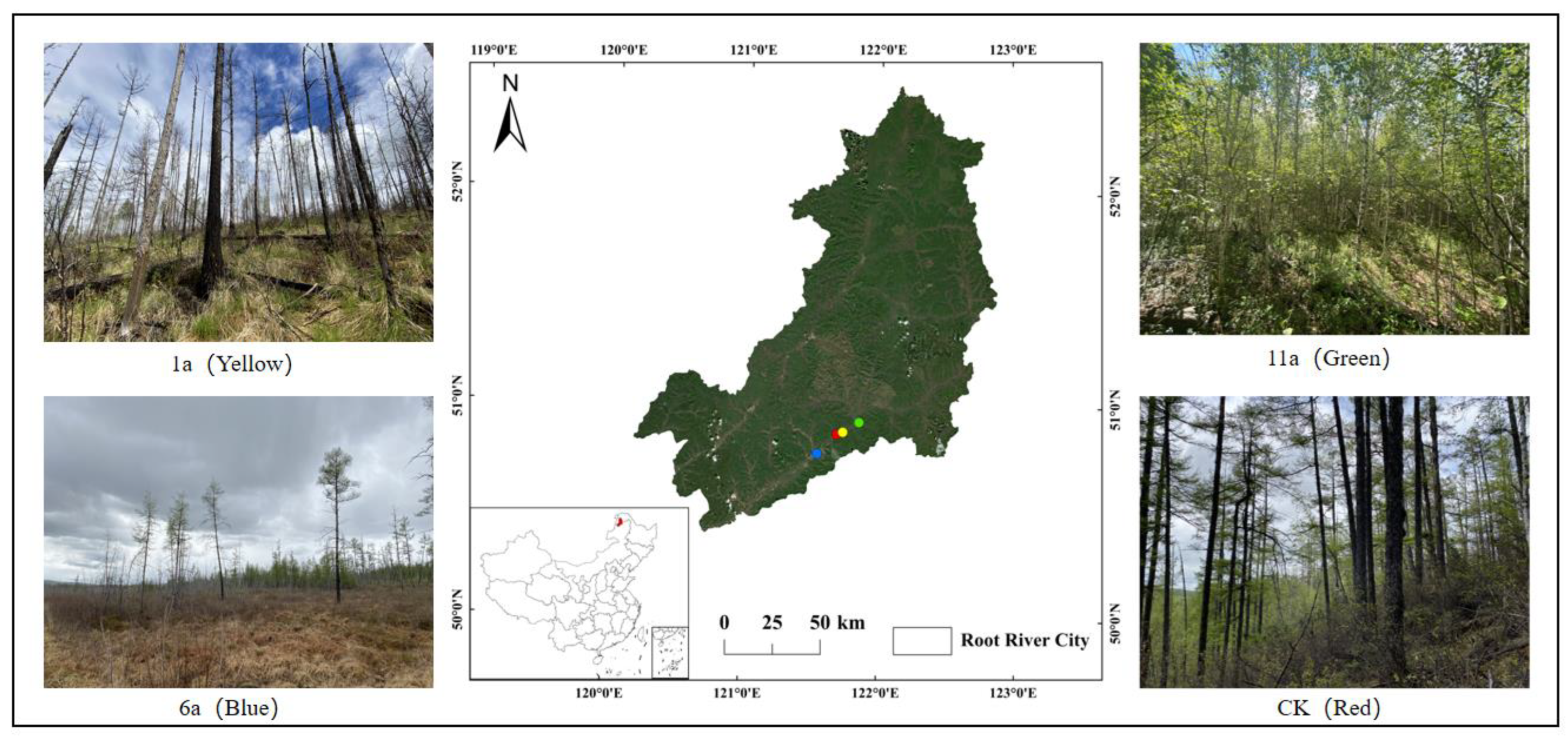
2.1. Overview of the Study Area
2.2. Placement of Sampling Points
2.3. Sample Collection and Measurement
2.3.1. Determination of Soil Physical and Chemical Properties
2.3.2. Determination of Soil Microbial Community Diversity
DNA Extraction and PCR Amplification
High-Throughput Sequencing
2.4. Statistical Analysis
3. Results
3.1. Effects of Fire Disturbance on Physical and Chemical Properties
3.2. Effects of Fire Disturbance on Soil Microbial Community Diversity
3.2.1. Soil Microbes in Fire Sites of Different Recovery Years: α-Diversity
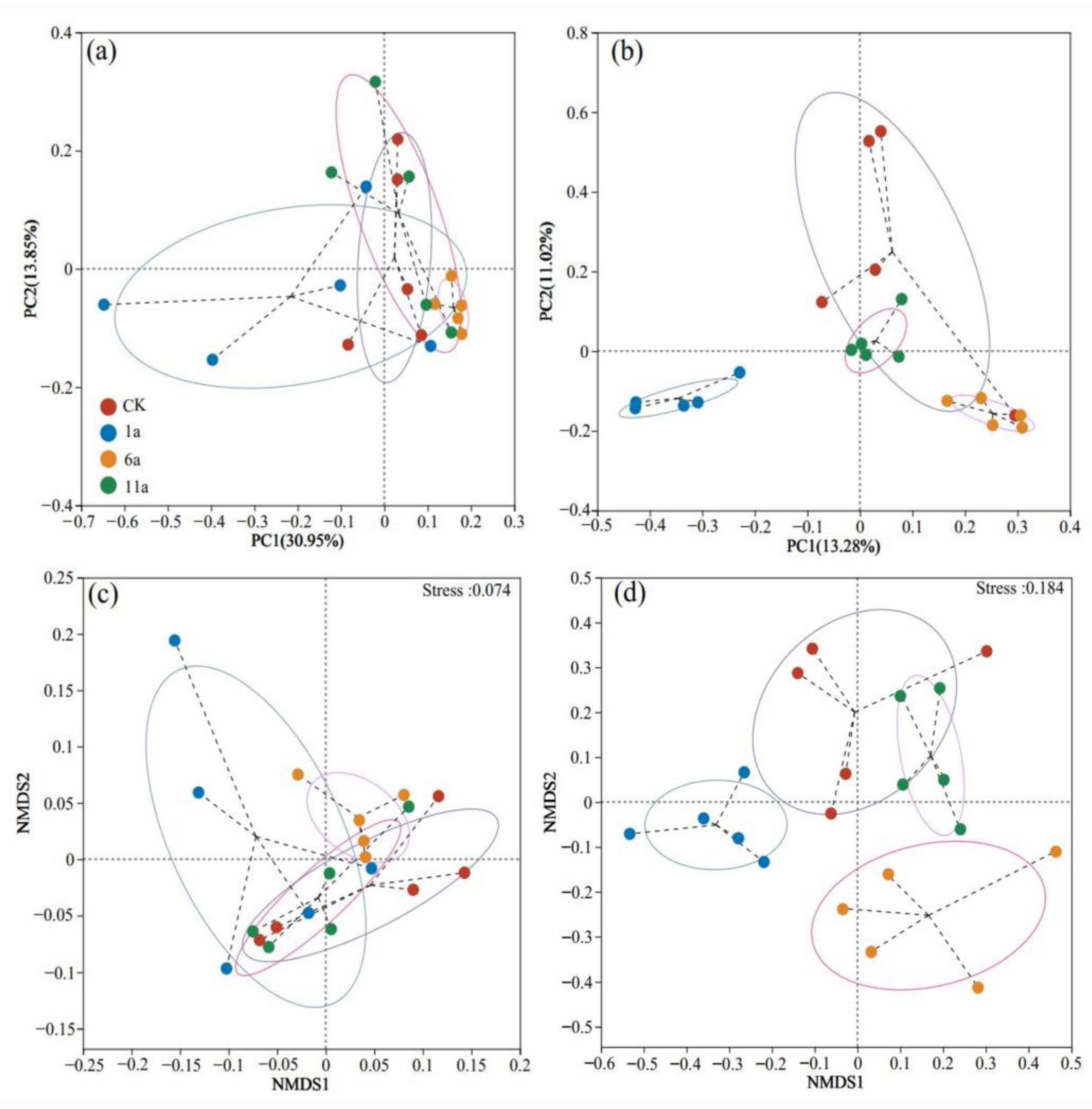
3.2.2. Soil Microbes in Fire Sites of Different Recovery Years: β-Diversity
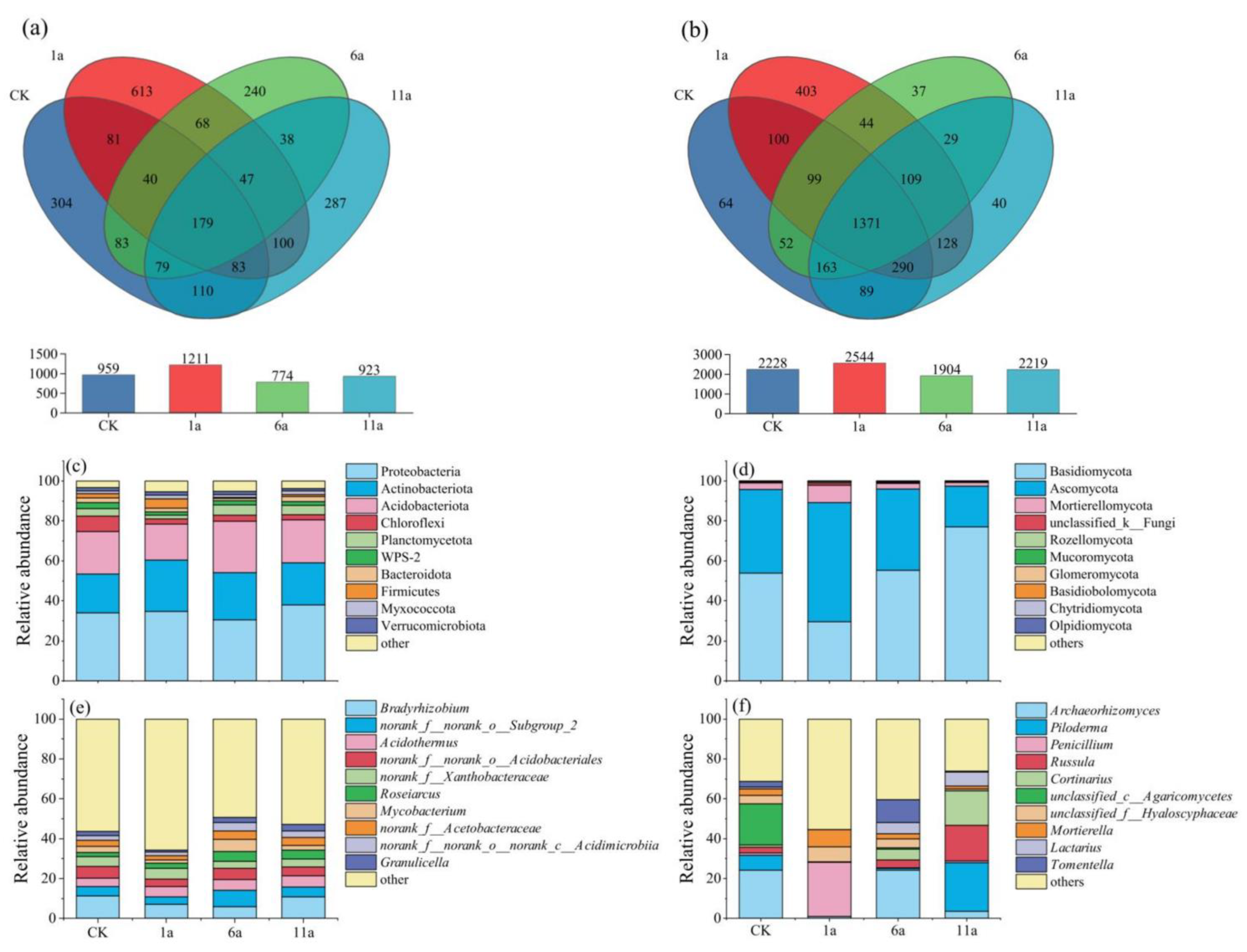
3.2.3. Effects of Fire Disturbance on Soil Microbial Community Composition
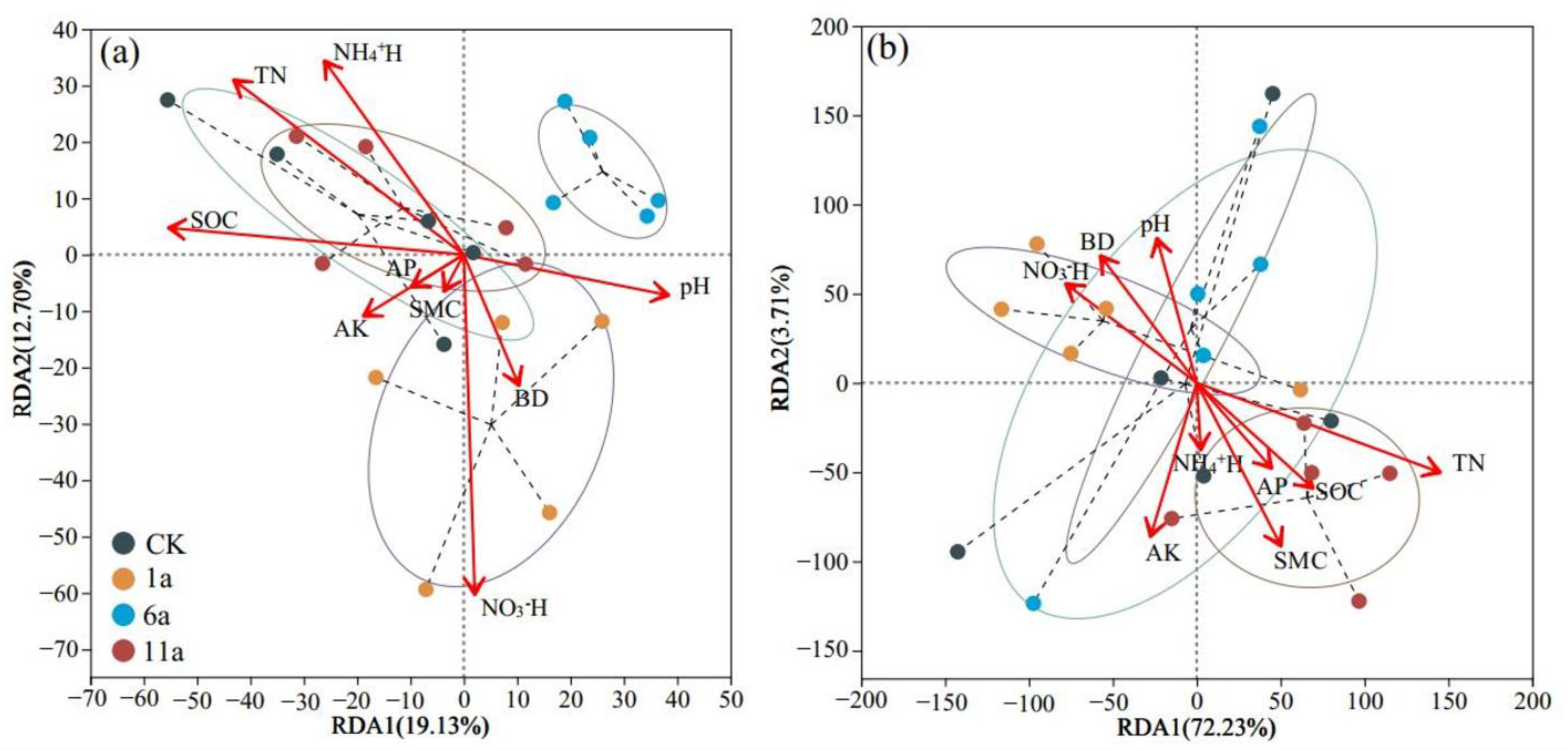
3.3. Analysis of Driving Factors of Soil Microbial Community in Fire Fields
4. Discussion
4.1. Effects of Fire on Soil Properties
4.2. Effects of Fire on Soil Bacterial Communities
4.3. Effects of Fire on Soil Fungal Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Kumara, C.; Köster, E.; Aaltonen, H.; Köster, K. How do forest fires affect soil greenhouse gas emissions in upland boreal forests? A review. Environ. Res. 2020, 184, 109328. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Farooq, I.; Kachroo, M.M.; Mahmoud, A.E.D.; Fawzy, M.; Popescu, S.M.; Alyemeni, M.N.; Sonne, C.; Rinklebe, J.; Ahmad, P. Elevation in wildfire frequencies with respect to the climate change. J. Environ. Manag. 2022, 301, 113769. [Google Scholar] [CrossRef]
- Senande-Rivera, M.; Insua-Costa, D.; Miguez-Macho, G. Spatial and temporal expansion of global wildland fire activity in response to climate change. Nat. Commun. 2022, 13, 1208. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.E.; Grossman, J.M. The soil habitat and soil ecology. Biol. Approaches Regen. Soil Syst. 2023, 69–84. [Google Scholar]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Guerrero, C.; García-Orenes, F.; Bárcenas, G.M.; Torres, M.P. Forest fire effects on soil microbiology. In Fire Effects on Soils and Restoration Strategies; CRC Press: Boca Raton, FL, USA, 2009; pp. 149–192. [Google Scholar]
- Gimeno-Garcıa, E.; Andreu, V.; Rubio, J.L. Spatial patterns of soil temperatures during experimental fires. Geoderma 2004, 118, 17–38. [Google Scholar] [CrossRef]
- Marfella, L.; Marzaioli, R.; Pazienza, G.; Mairota, P.; Glanville, H.C.; Rutigliano, F.A. Medium-term effects of wildfire severity on soil physical, chemical and biological properties in Pinus halepensis Mill. woodland (Southern Italy): An opportunity for invasive Acacia saligna colonization? For. Ecol. Manag. 2023, 542, 121010. [Google Scholar] [CrossRef]
- Mabuhay, J.A.; Nakagoshi, N.; Horikoshi, T. Microbial biomass and abundance after forest fire in pine forests in Japan. Ecol. Res. 2003, 18, 431–441. [Google Scholar] [CrossRef]
- Dooley, S.R.; Treseder, K.K. The effect of fire on microbial biomass: A meta-analysis of field studies. Biogeochemistry 2012, 109, 49–61. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Makoto, K.; Koike, T. Charcoal ecology: Its function as a hub for plant succession and soil nutrient cycling in boreal forests. Ecol. Res. 2021, 36, 4–12. [Google Scholar] [CrossRef]
- Weninger, T.; Filipović, V.; Mešić, M.; Clothier, B.; Filipović, L. Estimating the extent of fire induced soil water repellency in Mediterranean environment. Geoderma 2019, 338, 187–196. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth-Sci. Review. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, M.H.; Lee, Y.O. Effect of pH on soil bacterial diversity. J. Ecol. Environ. 2016, 40, 10. [Google Scholar] [CrossRef]
- Hu, T.; Zhao, B.; Li, F.; Dou, X.; Hu, H.; Sun, L. Effects of fire on soil respiration and its components in a Dahurian larch (Larix gmelinii) forest in northeast China: Implications for forest ecosystem carbon cycling. Geoderma 2021, 402, 115273. [Google Scholar] [CrossRef]
- Holden, S.R.; Berhe, A.A.; Treseder, K.K. Decreases in soil moisture and organic matter quality suppress microbial decomposition following a boreal forest fire. Soil Biol. Biochem. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Xiao-Ying, W.; Chun-Yu, Z.; Qing-Yu, J. Impacts of climate change on forest ecosystems in Northeast China. Adv. Clim. Chang. Res. 2013, 4, 230–241. [Google Scholar] [CrossRef]
- Kong, J.J.; Zhang, H.Y.; Jing, S. Dynamic characteristics of forest soil phosphorus in the early succession after fire in Great Xing’an Mountains. Chin. J. Ecol. 2017, 36, 1515–1523. [Google Scholar]
- Tian, Y.; Zhang, Q.; Liu, X.; Meng, M. Stem radius variation in response to hydro-thermal factors in Larch. Forests 2018, 9, 602. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and AgrIcultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Tshering, K.; Miotlinski, K.; Blake, D.; Boyce, M.C.; Bath, A.; Carvalho, A.; Horwitz, P. Effect of fire on characteristics of dissolved organic matter in forested catchments in the Mediterranean biome: A review. Water Res. 2023, 230, 119490. [Google Scholar] [CrossRef] [PubMed]
- Gabbasova, I.M.; Garipov, T.T.; Suleimanov, R.R.; Komissarov, M.A.; Khabirov, I.K.; Sidorova, L.V.; Nazyrova, F.I.; Prostyakova, Z.G.; Kotlugalyamova, E.Y. The influence of ground fires on the properties and erosion of forest soils in the Southern Urals (Bashkir State Nature Reserve). Eurasian Soil Sci. 2019, 52, 370–379. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183, 80–91. [Google Scholar] [CrossRef]
- Granged, A.J.; Zavala, L.M.; Jordán, A.; Bárcenas-Moreno, G. Post-fire evolution of soil properties and vegetation cover in a Mediterranean heathland after experimental burning: A 3-year study. Geoderma 2011, 164, 85–94. [Google Scholar] [CrossRef]
- Wang, B. Variations of Soil Characteristics and Community Structure of the Burn Area in Different Restoration Periods of Gen He. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Fire effects on temperate forest soil C and N storage. Ecol. Appl. 2011, 21, 1189–1201. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Kang, J.; Cui, X. Post-Fire Evolution of Soil Nitrogen in a Dahurian Larch (Larix gmelinii) Forest, Northeast China. Forests 2023, 14, 1178. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Fernández-Guisuraga, J.M.; Taboada, A.; Suárez-Seoane, S.; Calvo, L. Impact of burn severity on soil properties in a Pinus pinaster ecosystem immediately after fire. Int. J. Wildland Fire 2019, 28, 354–364. [Google Scholar] [CrossRef]
- Moya, D.; González-De Vega, S.; Lozano, E.; García-Orenes, F.; Mataix-Solera, J.; Lucas-Borja, M.E.; de Las Heras, J. The burn severity and plant recovery relationship affect the biological and chemical soil properties of Pinus halepensis Mill. stands in the short and mid-terms after wildfire. J. Environ. Manag. 2019, 235, 250–256. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, B.; Zhang, Q.; Tian, Y. Driving force of soil microbial community structure in a burned area of Daxing’anling, China. J. For. Res. 2021, 32, 1723–1738. [Google Scholar] [CrossRef]
- Kang, J.W.; Park, Y.D. Effects of deforestation on microbial diversity in a Siberian larch (Larix sibirica) stand in Mongolia. J. For. Res. 2019, 30, 1885–1893. [Google Scholar] [CrossRef]
- Hu, A.; Nie, Y.; Yu, G.; Han, C.; He, J.; He, N.; Liu, S.R.S.; Deng, J.; Shen, W.J.; Zhang, G. Diurnal temperature variation and plants drive latitudinal patterns in seasonal dynamics of soil microbial community. Front. Microbiol. 2019, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Sarto, M.V.; Borges, W.L.; Bassegio, D.; Pires, C.A.; Rice, C.W.; Rosolem, C.A. Soil microbial community, enzyme activity, C and N stocks and soil aggregation as affected by land use and soil depth in a tropical climate region of Brazil. Arch. Microbiol. 2020, 202, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Shi, Y.; Yang, J.; Kong, J.; Lin, X.; Zhang, H.; Zeng, J.; Chu, H. Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Sci. Rep. 2014, 4, 3829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wu, S.; Du, J.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Variations in the Diversity and Biomass of Soil Bacteria and Fungi under Different Fire Disturbances in the Taiga Forests of Northeastern China. Forests 2023, 14, 2063. [Google Scholar] [CrossRef]
- Tomao, A.; Bonet, J.A.; Castano, C.; de-Miguel, S. How does forest management affect fungal diversity and community composition? Current knowledge and future perspectives for the conservation of forest fungi. For. Ecol. Manag. 2020, 457, 117678. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Y.M.; Wang, Z.B.; Wang, H.X.; Qu, L.Y. The fungal community characteristics of rhizosphere soil of Larix gmelinii in different growth status after severe fire. Acta Ecol. Sin. 2021, 41, 9399–9409. [Google Scholar]
- Lienhard, P.; Terrat, S.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J.; et al. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron. Sustain. Dev. 2014, 34, 525–533. [Google Scholar] [CrossRef]
- Wu, Y.N.; Xu, N.; Wang, H.; Li, J.B.; Zhong, H.X.; Dong, H.Y.; Zeng, Z.W.; Zong, C. Variations in the diversity of the soil microbial community and structure under various categories of degraded wetland in Sanjiang Plain, northeastern China. Land Degrad. Dev. 2021, 32, 2143–2156. [Google Scholar] [CrossRef]
| Physical and Chemical Properties | CK | 1a | 6a | 11a |
|---|---|---|---|---|
| BD (g·cm−3) | 0.80 ± 0.02 a | 0.95 ± 0.05 a | 0.89 ± 0.02 a | 0.85 ± 0.03 a |
| SMC (%) | 41.27 ± 1.30 a | 35.49 ± 1.87 a | 38.20 ± 0.99 a | 40.87 ± 0.48 a |
| pH | 4.92 ± 0.18 c | 5.56 ± 0.25 ab | 5.71 ± 0.17 a | 5.28 ± 0.15 b |
| SOC (g·kg−1) | 128.95 ± 13.84 a | 94.47 ± 6.78 b | 78.98 ± 8.57 c | 116.92 ± 14.70 a |
| TN (g·kg−1) | 2.16 ± 0.33 b | 1.17 ± 0.39 c | 1.54 ± 0.28 c | 3.04 ± 0.57 a |
| NH4+-N (mg·kg−1) | 61.54 ± 6.11 a | 52.29 ± 4.71 a | 55.02 ± 3.04 a | 64.08 ± 3.12 a |
| NO3−-N (mg·kg−1) | 1.32 ± 0.57 b | 2.20 ± 0.15 a | 1.16 ± 0.18 c | 1.27 ± 0.30 bc |
| AP (mg·kg−1) | 22.72 ± 1.76 b | 29.02 ± 2.20 a | 24.38 ± 2.75 b | 30.41 ± 5.54 a |
| AK (mg·kg−1) | 239.42 ± 50.94 b | 270.47 ± 93.54 ab | 206.75 ± 14.12 c | 276.96 ± 6.96 a |
| Microbiological | Diversity Index | CK | 1a | 6a | 11a |
|---|---|---|---|---|---|
| Bacterial | Ace | 1720.05 ± 146.27 a | 1647.79 ± 233.87 a | 1432.25 ± 201.02 a | 1671.9 ± 221.32 a |
| Chao | 1707.1 ± 168.46 a | 1676.35 ± 237.73 a | 1430.34 ± 194.77 a | 1664.05 ± 236.49 a | |
| Shannon | 5.25 ± 0.22 ab | 5.1 ± 0.19 b | 5.23 ± 0.22 ab | 5.43 ± 0.26 a | |
| Fungal | Ace | 619.80 ± 159.07 a | 474.62 ± 203.76 a | 507.80 ± 150.4 a | 566.06 ± 229.86 a |
| Chao | 553.29 ± 142.72 a | 453.62 ± 213.88 a | 463.41 ± 117.79 a | 497.76 ± 183.51 a | |
| Shannon | 3.05 ± 0.24 a | 2.09 ± 0.81 a | 2.50 ± 1.13 a | 2.93 ± 0.45 a |
| Microbiological | Physical and Chemical Properties | R2 | p |
|---|---|---|---|
| Bacterial | BD | 0.1033 | 0.410 |
| SMC | 0.0094 | 0.934 | |
| pH | 0.2335 | 0.101 | |
| SOC | 0.4929 | 0.003 ** | |
| TN | 0.4150 | 0.015 * | |
| NH4+N | 0.2858 | 0.054 | |
| NO3−N | 0.6921 | 0.001 *** | |
| AP | 0.0220 | 0.838 | |
| AK | 0.0875 | 0.463 | |
| Fungal | BD | 0.1582 | 0.231 |
| SMC | 0.2046 | 0.138 | |
| pH | 0.1295 | 0.292 | |
| SOC | 0.1591 | 0.198 | |
| TN | 0.4747 | 0.003 ** | |
| NH4+N | 0.0234 | 0.802 | |
| NO3−N | 0.1778 | 0.182 | |
| AP | 0.0794 | 0.518 | |
| AK | 0.1476 | 0.260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Shu, Y.; Wei, J.; Zhao, P.; Zhou, M.; Jia, W. Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China. Forests 2024, 15, 664. https://doi.org/10.3390/f15040664
Li H, Shu Y, Wei J, Zhao P, Zhou M, Jia W. Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China. Forests. 2024; 15(4):664. https://doi.org/10.3390/f15040664
Chicago/Turabian StyleLi, Hang, Yang Shu, Jiangsheng Wei, Pengwu Zhao, Mei Zhou, and Wenjie Jia. 2024. "Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China" Forests 15, no. 4: 664. https://doi.org/10.3390/f15040664
APA StyleLi, H., Shu, Y., Wei, J., Zhao, P., Zhou, M., & Jia, W. (2024). Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China. Forests, 15(4), 664. https://doi.org/10.3390/f15040664






