Selecting Suitable Tree Species for Direct Seeding to Restore Forest Ecosystems in Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
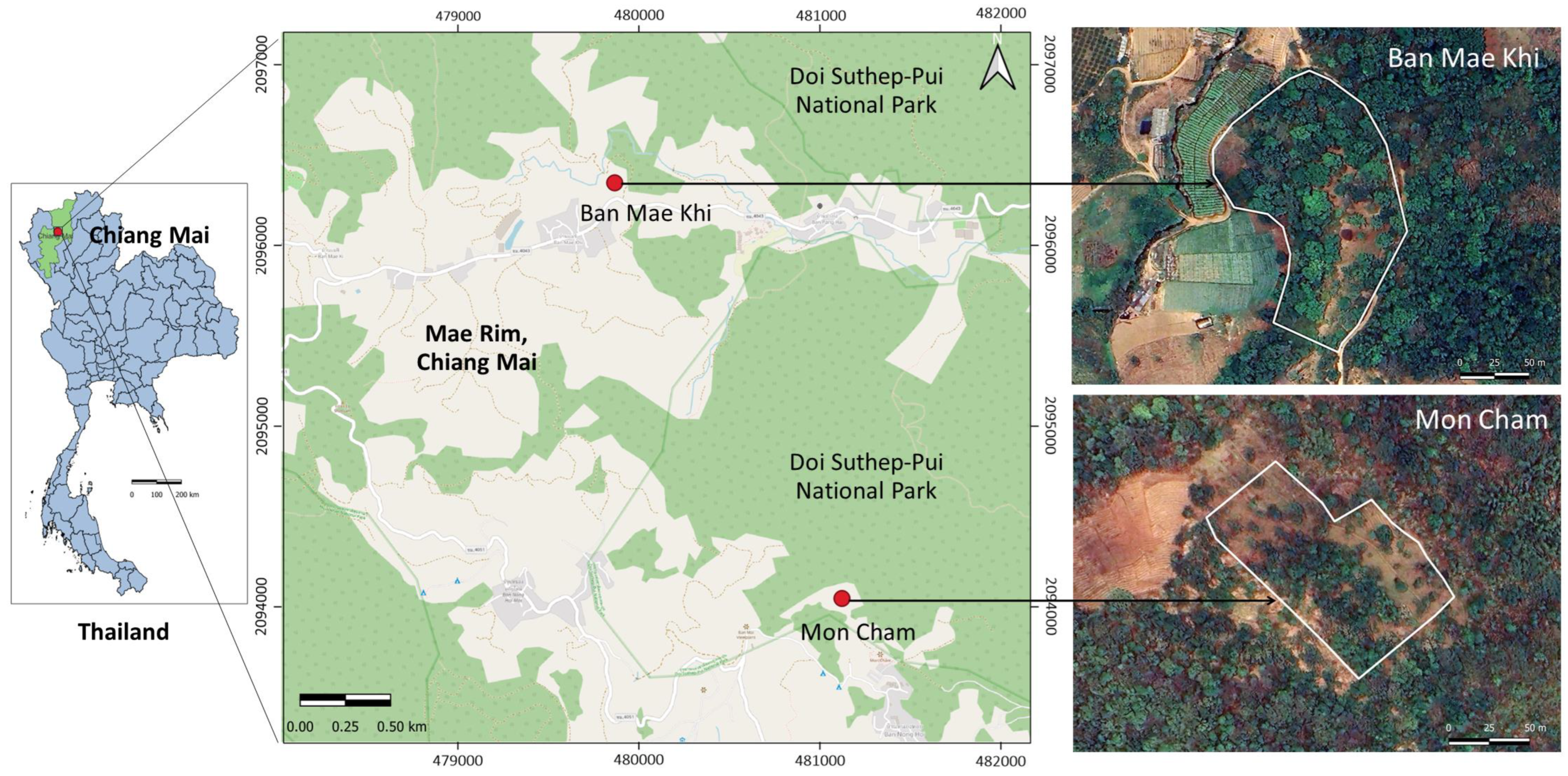
2.1. Study Sites
2.2. Studied Species
| No. | Scientific Name | Family | Propagule Mass 1 | Storage Behavior 2 | Leaf Habits 3 | Successional Guilds 4 | Collection Date | % Healthy Seed 5 | |
|---|---|---|---|---|---|---|---|---|---|
| (g) ± SD | |||||||||
| 1 | Phyllanthus emblica L. | Euphorbiaceae | 0.017 ± 0.005 | Small | Orthodox | Deciduous | P | Mar 2019 | 95 |
| 2 | Hovenia dulcis Thunb. | Rhamnaceae | 0.028 ± 0.005 | Small | Orthodox | Deciduous | P 4 | Oct 2018 | 90 |
| 3 | Acrocarpus fraxinifolius Wight & Arn. | Leguminosae | 0.034 ± 0.005 | Small | Orthodox | Deciduous | P | Jun 2019 | 100 |
| 4 | Magnolia baillonii Pierre | Magnoliaceae | 0.047 ± 0.014 | Small | Orthodox | Deciduous | C | Sep 2019 | 100 |
| 5 | Melia azedarach L. | Meliaceae | 0.050 ± 0.007 | Small | Orthodox | Deciduous | P 4 | Mar 2019 | 100 |
| 6 | Balakata baccata (Roxb.) Esser | Euphorbiaceae | 0.055 ± 0.005 | Small | Recalcitrant | Evergreen | P | Jul 2019 | 100 |
| 7 | Phoebe cathia (D.Don) Kosterm. | Lauraceae | 0.083 ± 0.020 | Small | Recalcitrant | Evergreen | C | Jul 2019 | 60 |
| 8 | Adenanthera microsperma Teijsm. & Binn. | Leguminosae | 0.104 ± 0.019 | Medium | Orthodox | Deciduous | C | Mar 2019 | 100 |
| 9 | Artocarpus lacucha Wall. ex Roxb. | Moraceae | 0.173 ± 0.039 | Medium | Recalcitrant | Deciduous | C | Jun 2019 | 85 |
| 10 | Alangium kurzii Craib | Alangiaceae | 0.175 ± 0.018 | Medium | Orthodox | Evergreen | C | Jul 2019 | 100 |
| 11 | Prunus cerasoides D.Don | Rosaceae | 0.183 ± 0.039 | Medium | Orthodox | Deciduous | IP 4 | May 2019 | 100 |
| 12 | Diospyros glandulosa Lace | Ebenaceae | 0.257 ± 0.062 | Medium | Orthodox | Evergreen | IP 4 | Dec 2018 | 100 |
| 13 | Cassia bakeriana Craib | Leguminosae | 0.269 ± 0.039 | Medium | Orthodox | Deciduous | P | May 2019 | 100 |
| 14 | Syzygium fruticosum DC. | Myrtaceae | 0.375 ± 0.071 | Medium | Recalcitrant | Evergreen | C | Jul 2019 | 90 |
| 15 | Gmelina arborea Roxb. ex Sm. | Verbenaceae | 0.519 ± 0.098 | Medium | Orthodox | Deciduous | P | Apr 2019 | 90 |
| 16 | Sarcosperma arboreum Buch.-Ham. ex C.B.Clarke | Sapotaceae | 1.342 ± 0.210 | Medium | Recalcitrant | Evergreen | IC 4 | Jun 2019 | 100 |
| 17 | Choerospondias axillaris (Roxb.) B.L.Burtt & A.W.Hill | Anacardiaceae | 1.434 ± 0.275 | Medium | Orthodox | Deciduous | I | Aug 2019 | 100 |
| 18 | Polyalthia viridis Craib | Annonaceae | 1.553 ± 0.218 | Medium | Recalcitrant | Evergreen | C | Jun 2019 | 80 |
| 19 | Garcinia cowa Roxb. ex Choisy | Guttiferae | 1.755 ± 0.364 | Medium | Recalcitrant | Evergreen | C | Jun 2019 | 100 |
| 20 | Quercus brandisiana Kurz | Fagaceae | 1.776 ± 0.536 | Medium | Recalcitrant | Evergreen | C | Jun 2019 | 100 |
| 21 | Sapindus rarak DC. | Sapindaceae | 1.946 ± 0.253 | Medium | Orthodox | Deciduous | IP 4 | Apr 2019 | 100 |
| 22 | Scleropyrum pentandrum (Dennst.) Mabb. | Santalaceae | 2.525 ± 0.672 | Large | - | Evergreen | - | Jun 2019 | 100 |
| 23 | Spondias pinnata (L.f.) Kurz | Anacardiaceae | 4.263 ± 0.678 | Large | Orthodox | Deciduous | P | Dec 2018 | 100 |
2.3. Experimental Design and Data Collection
2.4. Data Analysis
2.4.1. Seed Removal and Germination and Seedling Yield
2.4.2. Seedling Growth
2.4.3. Relative Performance Index (RPI)
2.4.4. Effect of Species Traits on Direct Seeding
3. Results
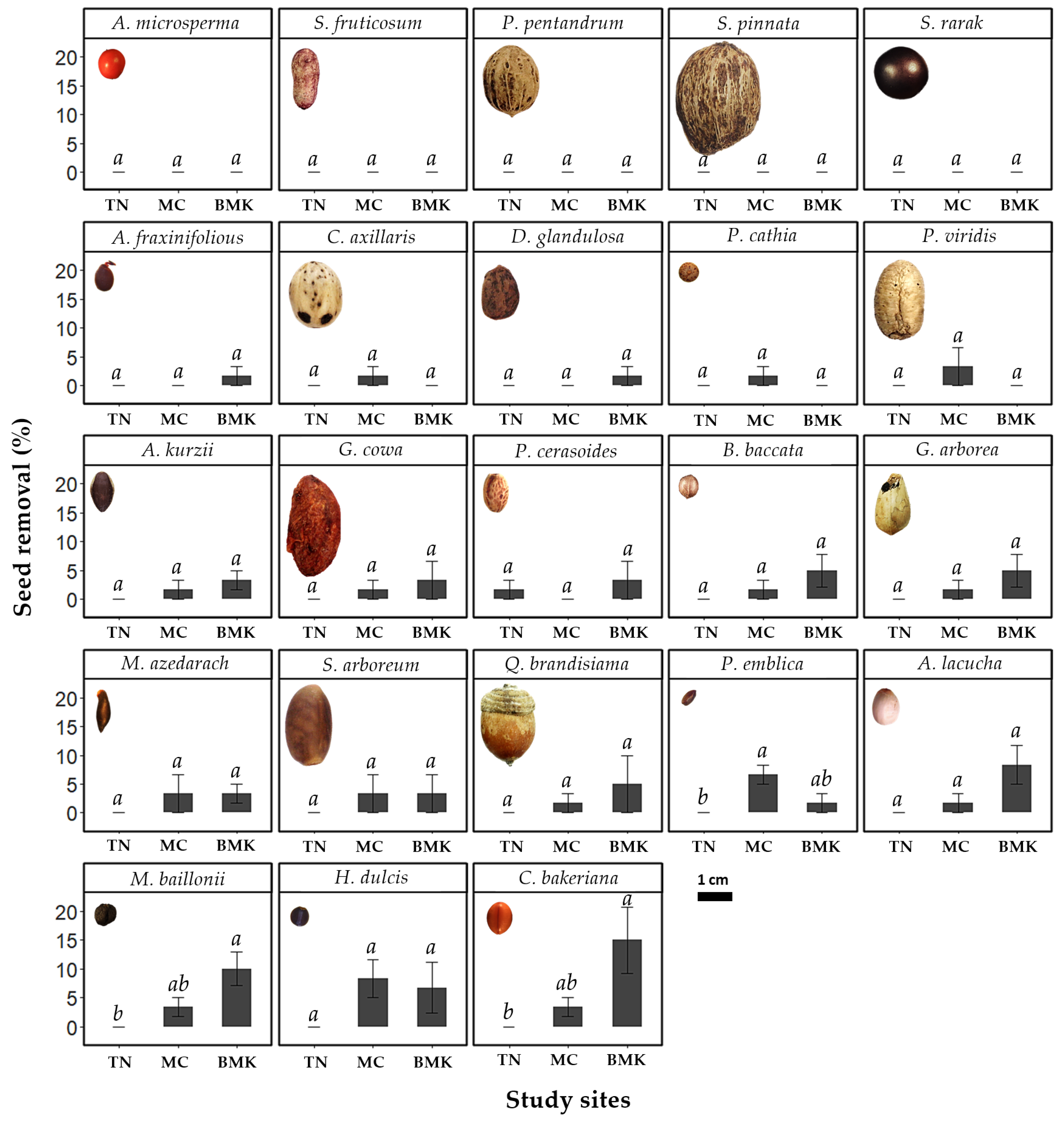
3.1. Seed Removal
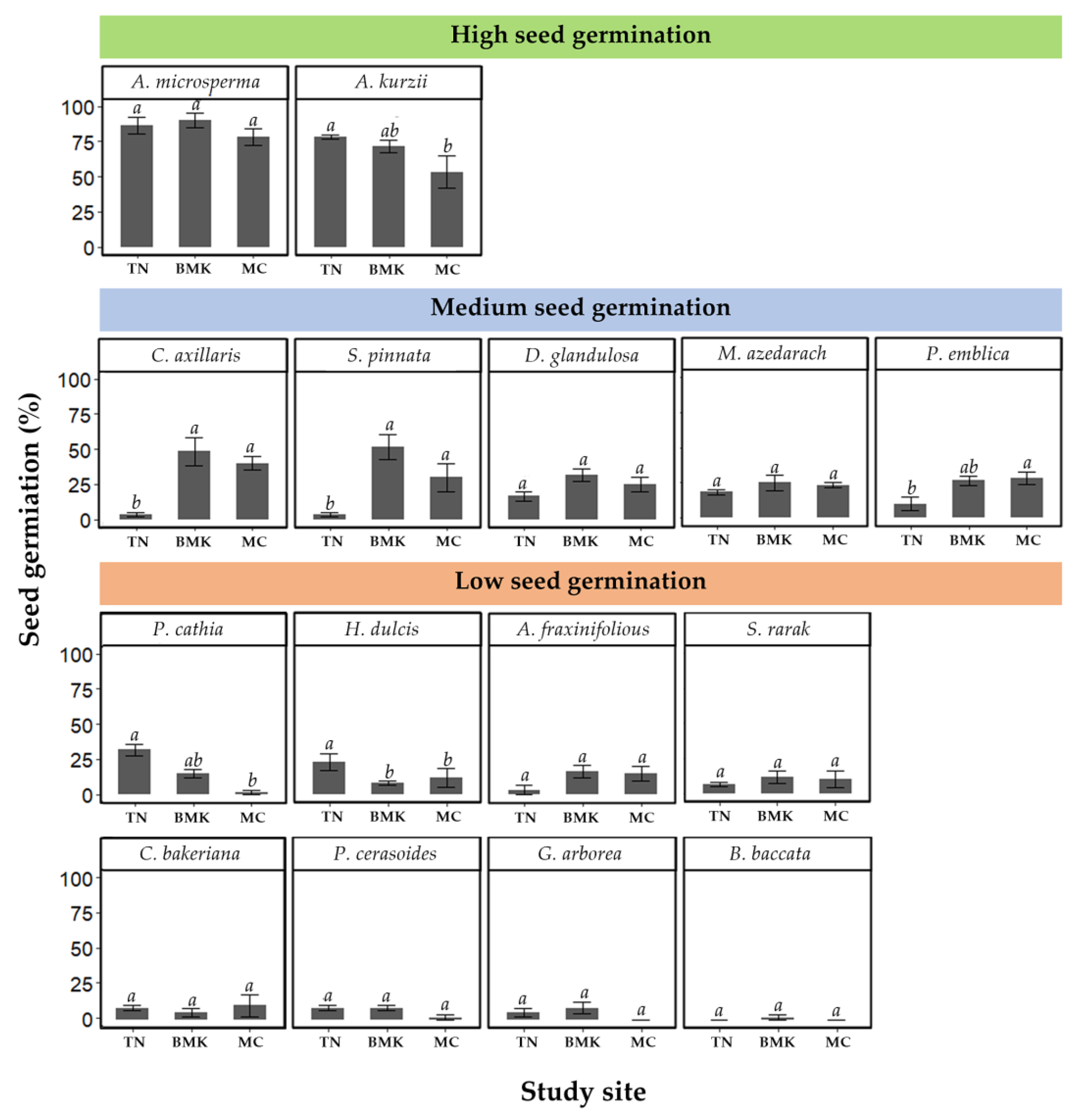
3.2. Seed Germination
3.3. Seedling Yield
| Species | n | %Yield | RCD | Height | CW | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | %RGR ± SE | Mean ± SE | %RGR ± SE | Mean ± SE | %RGR ± SE | ||
| A. microsperma | 72 | 66.67 ± 8.33 a | 2.2 ± 0.0 b | 57 ± 2.02 bc | 12.8 ± 0.3 cd | 83.64 ± 1.8 cd | 13.5 ± 0.5 cd | 74.0 ± 3.6 bc |
| S. pinnata | 32 | 33.33 ± 8.33 b | 2.8 ± 0.0 b | 25.19 ± 6.21 c | 11.2 ± 0.4 d | 50.9 ± 9.7 de | 9.6 ± 0.8 d | 44.2 ± 6.3 c |
| C. axillaris | 23 | 28.33 ± 1.67 b | 2.4 ± 0.1 b | 136.08 ± 18.75 ab | 22.4 ± 1.6 a | 133.99 ± 12.3 b | 19.0 ± 0.9 ab | 124.1 ± 5.5 ab |
| A. kurzii | 20 | 21.67 ± 1.68 c | 1.8 ± 0.1 b | 70.65 ± 13.64 bc | 9.6 ± 0.7 d | 113.52 ± 4.0 bc | 8.0 ± 0.4 d | 75.2 ± 20.4 bc |
| P. emblica | 17 | 17.50 ± 0.83 cd | 1.8 ± 0.1 b | 64.09 ± 7.43 bc | 18.4 ± 1.6 abc | 74.75 ± 12.2 cde | 15.4 ± 1.4 bcd | 75.3 ± 12.1 bc |
| M. azedarach | 11 | 15.00 ± 5.00 de | 2.6 ± 0.3 b | 47.37 ± 7.34 bc | 20.4 ± 2.6 ab | 101.68 ± 15.9 bcd | 22.6 ± 3.7 a | 146.4 ± 20.4 a |
| D. glandulosa | 8 | 15.00 ± 0.00 ef | 2.2 ± 0.1 b | 69.99 ± 6.35 bc | 11.6 ± 0.4 d | 84.56 ± 11.2 cd | 11.2 ± 1.1 cd | 33.0 ± 12.1 c |
| S. rarak | 8 | 11.25 ± 3.75 ef | 4.0 ± 0.4 a | 47.08 ± 20.70 bc | 17.0 ± 1.3 abcd | 65.75 ± 23.5 cde | 17.0 ± 2.0 abcd | 26.9 ± 6.6 c |
| H. dulcis | 2 | 10.00 ± 0.00 ef | 2.9 ± 0.5 ab | 160.84 ± 66.88 a | 26.2 ± 9.4 a | 236.16 ± 42.4 a | 11.6 ± 4.6 cd | 136.7 ± 68.4 ab |
| P. cerasoides | 2 | 10.00 ± 0.00 fg | 2.4 ± 0.3 b | 52.61 ± 37.65 bc | 25.5 ± 1.5 a | 65.96 ± 7.6 cde | 17.5 ± 0.1 abc | 25.5 ± 13.9 c |
| C. bakeriana | 8 | 10.00 ± 2.5 fg | 1.9 ± 0.1 b | 15.34 ± 5.21 c | 8.2 ± 1.1 d | 20.37 ± 7.7 e | 7.3 ± 1.0 d | 50.3 ± 29.2 bc |
| A. fraxinifolius | 4 | 6.25 ± 1.25 g | 1.8 ± 0.4 b | 157.41 ± 13.07 a | 10.1 ± 2.0 d | 107.58 ± 14.7 bcd | 8.9 ± 2.6 d | 79.8 ± 2.0 abc |
| G. arborea | 1 | 5.00 g | 2.9 ab | 139.2 ab | 14.0 bcd | 58.3 cde | 12.0 cd | 37.0 c |
3.4. Seedling Growth
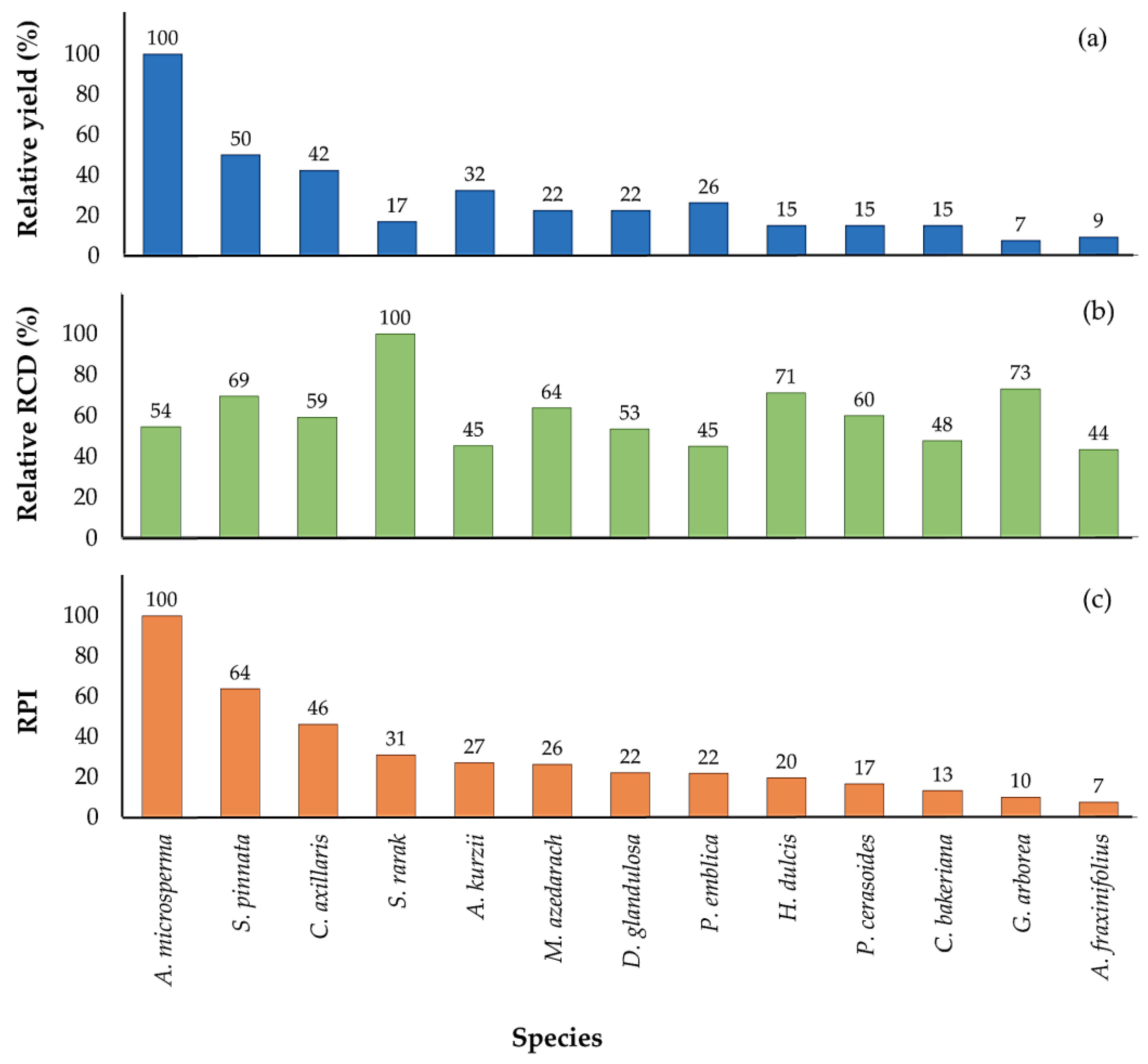
3.5. Relative Performance Indices
4. Discussion
4.1. Seed Removal
4.2. Seed Germination
4.3. Seedling Survival and Yield
4.4. Seedling Growth
4.5. Relative Performance Index (RPI)
4.6. Traits to Consider When Selecting Species for Direct Seeding
| Species | Collection Month | Sowing Time a | Storage Conditions b | Seed Pre-Treatments | Light Requirement for Germination | Usefulness c |
|---|---|---|---|---|---|---|
| A. fraxinifolius | Apr–Jun | RS | RT and RE 4 | Soaking in warm water for 24 h and scarification 2,5 | Full sunlight 2 | Timber |
| A. microsperma | Sep–Mar | RS | RT 3 | Without/with scarification 2,4 | Sunlight 4 | Timber, ornamental, dye |
| A. kurzii | Jun–Sep | RS | RE 3 | None | Sunlight 4 | Light timber |
| A. lacucha | Dec–Jun | IS | - | None 1 | Sunlight 1,4 | Timber, dye, medicinal, flower edible |
| B. baccata | Apr–Dec | IS | - | Soak in warm water for 2–3 days 1 | Full sunlight 2,4 | Dye, medicinal, oil, edible fruit |
| C. bakeriana | Sep–Jun | RS | RT and RE | Scarification 1 | Sunlight 4 | Timber, ornamental, medicinal |
| C. axillaris | Mar–Aug | RS | RE 3 | Soaking in water for 12 h 1 | Sunlight 4 | Edible fruit |
| D. glandulosa | May–Oct | RS | RT | Soaking in water for 24 h 1 | Partial shade 1 | Timber, edible fruit |
| G. cowa | Sep–Jun | IS | - | No 6 | Shade 4,6 | Medicinal, edible leaf and fruit, varnish |
| G. arborea | Mar–Jun | RS | RE 3 | Soaking in water for 12–24 h 1,3,4 | Sunlight 2 | Timber, medicinal |
| H. dulcis | Nov–Mar | RS | RE 3 | Soak in water for 1–2 days 1 | Shade 1,5 or 25% sunlight 2 | Medicinal |
| P. cathia | Jul–Sep | IS | - | None | - | - |
| M. azedarach | Apr–Aug | RS | RE 3 and RT 3,4 | Soak in water for 1–2 days 1 | Sunlight 1,4 | Timber, medicinal, insecticide, edible leaf and flower |
| M. baillonii | Aug–Mar | RS | RE 3 | None 1 | Sunlight 2 | Timber |
| P. emblica | May–Mar | RS | RT 3 | Scarification 1 | Partial sunlight 1 | Timber, medicinal, edible fruit |
| P. viridis | Mar–May | IS | - | None | - | - |
| P. cerasoides | Feb–May | RS | RE 3 | None | Sunlight 1,4 | Ornamental, edible fruit, medicinal |
| Q. brandisiana | Feb–Jun | IS | - | None | Shade 4 | - |
| S. rarak | Jul–Jan | RS | RT and RE | Scarification 1 | Partial sunlight 1,5 or full sunlight 2 | Soap (from fruit) |
| S. arboreum | Apr-Jul | IS | - | No 4 | Shade 5 | - |
| S. pentandrum | Aug–Oct | RS | - | Scarification 4 | - | |
| S. pinnata | Sep–Mar | RS | RT 3 | None | Sunlight 4 | Medicinal, edible flower, fruit, and young leaf |
| S. fruticosum | Mar–Aug | IS | - | None 2 | Full sunlight 2 | - |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasi, R. The Glasgow Leaders’ declaration on forests and land use: Significance toward “Net Zero”. Glob. Chang. Biol. 2022, 28, 1951–1952. [Google Scholar] [CrossRef] [PubMed]
- Uhlich, T.; Burck, J. Glasgow Leader’s Declaration on Forests and Land Use. Federal Ministry for Economic Cooperation and Development. 2022. Available online: https://www.germanwatch.org/sites/default/files/germanwatch_gdflu_factsheet_g7-g20_track-2.pdf (accessed on 31 August 2022).
- IUCN (International Union for Conservation of Nature). Restore Our Future Bonn Challenge. 2020. Available online: https://www.bonnchallenge.org/about (accessed on 31 August 2022).
- World Economic Forum. A Platform for the Trillion Trees Community. 2022. Available online: https://www.1t.org/ (accessed on 31 August 2022).
- Besseau, P.; Graham, S.; Christophersen, T. Restoring Forests and Landscapes: The Key to a Sustainable Future; Global Partnership on Forest and Landscape Restoration: Vienna, Austria, 2018. [Google Scholar]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Evol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Keene, C.L.; Zahawi, R.A. Direct seeding of late-successional trees to restore tropical montane forest. For. Ecol. Manag. 2011, 261, 1590–1597. [Google Scholar] [CrossRef]
- Chang, C.C.; Turner, B.L. Ecological succession in a changing world. J. Ecol. 2018, 107, 503–509. [Google Scholar] [CrossRef]
- Egerer, M.H.; Fricke, E.C.; Rogers, H.S. Seed dispersal as an ecosystem service: Frugivore loss leads to decline of a socially valued plant, Capsicum frutescens. Ecol. Appl. 2018, 28, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ranjitkar, S.; Harrison, R.D.; Xu, J.; Ou, X.; Ma, X.; He, J. Selection of native tree species for subtropical forest restoration in Southwest China. PLoS ONE 2017, 12, e0170418. [Google Scholar] [CrossRef] [PubMed]
- Grossnickle, S.C.; Ivetic, V. Direct seeding in reforestation—A field performance review. Reforesta 2017, 4, 94–142. [Google Scholar] [CrossRef]
- Woods, K.; Elliott, S. Direct seeding for forest restoration on abandoned agricultural land in northern Thailand. J. Trop. For. Sci. 2004, 16, 248–259. [Google Scholar]
- Willoughby, I.; Richard, L.; Jinks, R.; Kerr, G.; Gosling, P. Factors affecting the success of direct seeding for lowland afforestation in the UK. Forestry 2004, 77, 467–482. [Google Scholar] [CrossRef]
- Naruangsri, K.; Tiansawat, P.; Elliott, S. Differential seed removal, germination and seedling growth as determinants of species suitability for forest restoration by direct seeding—A case study from northern Thailand. For. Ecosyst. 2023, 10, 100133. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Silva, R.R.P.; Oliveira, D.R.; da Rocha, G.P.E.; Vieira, D.L.M. Direct seeding of Brazilian savanna trees: Effects of plant cover and fertilization on seedling establishment and growth. Restor. Ecol. 2015, 23, 393–401. [Google Scholar] [CrossRef]
- Tunjai, P. Direct Seeding for Restoring Tropical Lowland Forest Ecosystems in Southern Thailand. Ph.D. Thesis, The Graduate School, Walailak University, Nakhon Si Thammarat, Thailand, 2011. [Google Scholar]
- Hossain, F.; Elliott, S.; Chairuangsri, S. Effectiveness of direct seeding for forest restoration on severely degraded land in Lampang Province, Thailand. Open J. For. 2014, 4, 512–519. [Google Scholar] [CrossRef][Green Version]
- Waiboonya, P. Developing New Techniques of Seed Storage and Direct Seeding of Native Tree Species for Tropical Forest Restoration. Ph.D. Thesis, Chiang Mai University, Chiang Mai, Thailand, 2017. [Google Scholar]
- Ceccon, E.; González, E.J.; Martorell, C. Is direct seeding a biologically viable strategy for restoring forest ecosystems? evidence from a meta-analysis. Land. Degrad. Dev. 2016, 27, 511–520. [Google Scholar] [CrossRef]
- Souza, D.C. Forest restoration by direct seeding: A global bibliometric analysis. Restor. Ecol. 2022, 30, e13631. [Google Scholar] [CrossRef]
- Meli, P.; Martınez-Ramos, M.; Rey-Benayas, J.M.; Carabias, J. Combining ecological, social and technical criteria to select species for forest restoration. Appl. Veg. Sci. 2014, 7, 744–753. [Google Scholar] [CrossRef]
- Meteorological Department of Thailand. Climatic Data of Ban Nong Hoi, Mae Rim, Chiang Mai; Meteorological Department of Thailand: Bangkok, Thailand, 2022. [Google Scholar]
- Gardner, S.; Sidisunthorn, P.; Anusarnsunthorn, V. A Field Guide to Forest Trees of Northern Thailand; Kobfai Publishing Project: Bangkok, Thailand, 2000. [Google Scholar]
- FORRU (Forest Restoration Research Unit). How to Plant a Forest: The Principles and Practice of Restoring Tropical Forests; Department of Biology, Faculty of Science, Chiang Mai University: Chiang Mai, Thailand, 2005. [Google Scholar]
- FORRU (Forest Restoration Research Unit). Tree Seeds and Seedlings for Restoring Forests in Northern Thailand; Biology Department, Chiang Mai University: Chiang Mai, Thailand, 2000. [Google Scholar]
- SER; INSR; RBGK. Seed Information Database (SID). 2023. Available online: https://ser-sid.org/about (accessed on 1 August 2023).
- Manohan, B.; Shannon, D.P.; Tiansawat, P.; Chairuangsri, S.; Jainuan, J.; Elliott, S. Use of functional traits to distinguish successional guilds of tree species for restoring forest ecosystems. Forests 2023, 14, 1075. [Google Scholar] [CrossRef]
- Vander Wall, S.B.; Kuhn, K.M.; Beck, M.J. Seed removal, seed predation, and secondary dispersal. Ecology 2005, 86, 801–806. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Dylewski, L.; Ortega, Y.K.; Bogdziewicz, M.; Pearson, D.E. Seed size predicts global effects of small mammal seed predation on plant recruitment. Ecol. Lett. 2020, 23, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.H. Tree seed predation on degraded hillsides in Hong Kong. For. Ecol. Manag. 1997, 99, 215–221. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Schaefer, H.M. The conservation physiology of seed dispersal. Phil. Trans. R. Soc. 2012, 367, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Fricke, E.C.; Tewksbury, J.J.; Rogers, H.S. Multiple natural enemies cause distance-dependent mortality at the seed-to-seedling transition. Ecol. Lett. 2014, 17, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Doust, S.J.; Erskine, P.D.; Lamb, D. Restoring rainforest species by direct seeding: Tree seedling establishment and growth performance on degraded land in the wet tropics of Australia. For. Ecol. Manag. 2008, 256, 1178–1188. [Google Scholar] [CrossRef]
- Souza, M.L.; Silva, D.R.P.; Fantecelle, L.B.; Lemos Filho, J.P.D. Key factors affecting seed germination of Copaifera langsdorffii, a Neotropical tree. Acta Bot. Bras. 2015, 29, 473–477. [Google Scholar] [CrossRef]
- Silva, R.R.P.; Vieira, D.L.M. Direct seeding of 16 Brazilian savanna trees: Responses to seed burial, mulching and an invasive grass. Appl. Veg. Sci. 2017, 20, 410–421. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Vaughton, G.; Ramsey, M. Relationships between seed mass, seed nutrients, and seedling growth in Banksia cunninghamii (Proteaceae). Int. J. Plant Sci. 2001, 162, 599–606. [Google Scholar] [CrossRef]
- Cordazzo, C.V. Effect of seed mass on germination and growth in three dominant species in southern Brazilian coastal dunes. Braz. J. Biol. 2002, 62, 427–435. [Google Scholar] [CrossRef]
- Flores, J.; González-Salvatierra, C.; Jurado, E. Effect of light on seed germination and seedling shape of succulent species from Mexico. J. Plant Ecol. 2016, 9, 174–179. [Google Scholar] [CrossRef]
- Aud, F.F.; Ferraz, I.D.K. Seed size influence on germination responses to light and temperature of seven pioneer tree species from the Central Amazon. An. Acad. Bras. Cienc. 2012, 84, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.C.; Laurance, S.G.W. A review of the use of direct seeding and seedling plantings in restoration: What do we know and where should we go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Saverimuttu, T.; Westoby, M. Components of variation in seedling potential relative growth rate: Phylogenetically independent contrasts. Oecologia 1996, 105, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Coomes, D.A.; Grubb, P.J. Colonization, tolerance, competition and seed-size variation within functional groups. Trends Ecol. Evol. 2003, 18, 283–291. [Google Scholar] [CrossRef]
- Kidson, R.; Westoby, M. Seed mass and seedling dimensions in relation to seedling establishment. Oecologia 2000, 125, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Yang, Y.; Curtis, R.; Bartlow, A.W.; Agosta, S.J.; Steele, M.A. Alternative strategies of seed predator escape by early-germinating oaks in Asia and North America. Ecol. Evol. 2012, 2, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Plant Flower Seeds. Tree. 2023. Available online: https://plantflowerseeds.com/collections/trees (accessed on 15 September 2023).
- NParks Flora and Fauna Web. Database for Plants and Animals Found in Singapore. 2023. Available online: https://www.nparks.gov.sg/florafaunaweb (accessed on 15 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naruangsri, K.; Pathom-aree, W.; Elliott, S.; Tiansawat, P. Selecting Suitable Tree Species for Direct Seeding to Restore Forest Ecosystems in Northern Thailand. Forests 2024, 15, 674. https://doi.org/10.3390/f15040674
Naruangsri K, Pathom-aree W, Elliott S, Tiansawat P. Selecting Suitable Tree Species for Direct Seeding to Restore Forest Ecosystems in Northern Thailand. Forests. 2024; 15(4):674. https://doi.org/10.3390/f15040674
Chicago/Turabian StyleNaruangsri, Khuanphirom, Wasu Pathom-aree, Stephen Elliott, and Pimonrat Tiansawat. 2024. "Selecting Suitable Tree Species for Direct Seeding to Restore Forest Ecosystems in Northern Thailand" Forests 15, no. 4: 674. https://doi.org/10.3390/f15040674
APA StyleNaruangsri, K., Pathom-aree, W., Elliott, S., & Tiansawat, P. (2024). Selecting Suitable Tree Species for Direct Seeding to Restore Forest Ecosystems in Northern Thailand. Forests, 15(4), 674. https://doi.org/10.3390/f15040674





