Abstract
In order to explore the effects of enhanced UV-B radiation on the decomposition and nutrient cycling characteristics of Cunninghamia lanceolata (Lamb.) Hook litter, we collected material from C. lanceolata in a middle-aged forest (16 years) and over-mature forest (49 years). Four different UV-B radiation enhancement gradient treatments of CK, CK + 30 uw/cm2 (T1), CK + 45 uw/cm2 (T2), and CK + 60 uw/cm2 (T3), with natural light (CK) as the baseline, were conducted to determine the impact of UV-B radiation indoor simulation enhancement on the litter decomposition and nutrient release of C. lanceolata at various developmental stages. The results indicate that UV-B radiation increases the dry weight decomposition rate and the nutrient decomposition rate of C. lanceolata litter, and the decomposition rate of C. lanceolata litter in an over-mature forest is always greater than that in a middle-aged forest litter, with observable influences on its chemical composition. Such changes significantly alter the nutrient release pattern of N, P, and K in litter in middle-aged forests and N in litter from over-mature forests, and promote the release of C, which may affect the nutrient cycle and carbon sink function of C. lanceolata plantations.
1. Introduction
In recent years, human activities have led to a large number of chlorofluorocarbons being discharged into the atmosphere, resulting in the thinning of the atmospheric ozone layer. In the past 30 years, the ultraviolet-B (UV-B) radiation in the middle latitudes of the northern hemisphere has increased by 5% [1], and this trend is expected to continue with increasing severity as we approach 2050 [2]. The average annual ozone consumption in subtropical China may be as high as 0.27% [3,4,5].
The litter decomposition process is the main method of energy conversion and material circulation in forest ecosystems, and plays an important role in ecosystem productivity [6]. UV-B radiation changes will directly or indirectly affect the decomposition rate of litter and the release of nutrients by acting on the photodegradation of lignin in litter and indirectly affecting the chemical components in the plant growth process and the microbial community structure, as well as the activity that takes place during the litter decomposition process [7,8]. Litter substrate quality [9] and litter chemical composition [10] are important contributors to the litter decomposition rate and nutrient return. Although the solar radiation entering the litter layer is weakened due to the coverage of the canopy in the forest, the death of trees caused by deforestation, fire, and pests and diseases increases the canopy gap, allowing a large amount of UV-B to reach the forest floor [11,12]. Therefore, it is crucial to explore the characteristics of litter decomposition and nutrient release under enhanced UV-B radiation [13,14,15].
Cunninghamia lanceolata (Lamb.) Hook is a fast-growing timber species that is highly prevalent in southern China, offering a substantial contribution to afforestation. In recent years, the declining productivity of C. lanceolata plantations as a result of multi-generation continuous loading is becoming increasingly serious, hindering the sustainable development of this plantation. Studies have shown that the low litter yield and slow decomposition rate of C. lanceolata plantations may facilitate this reduced productivity [16]. There are obvious differences in the physical structure and chemical composition of C. lanceolata litter at different developmental stages, which directly affect its decomposition rate and nutrient release [17]. Several researchers [18] have qualitatively revealed the response mechanism of C. lanceolata litter to UV-B radiation through UV-B filtration, but they have yet to explore the influence of the C. lanceolata litter decomposition process at different developmental stages in light of global warming through the quantitative application of UV-B radiation.
In view of this, in this study, we set different UV-B radiation intensities (CK, CK + 30 uw/cm2, CK + 45 uw/cm2, CK + 60 uw/cm2) for the collected middle-aged forests (16 years) and over-mature forests (49 years), using natural light (CK) as the baseline. The effects of C. lanceolata litter decomposition and nutrient release in response to UV-B radiation enhancement were studied in order to reveal the nutrient cycle characteristics of C. lanceolata litter under the background of UV-B radiation enhancement, providing a scientific basis for the scientific cultivation and rational management of C. lanceolata plantation considering the future of climate change.
2. Materials and Methods
2.1. Overview of the Test Site
The experimental site was situated in Xia’an Nursery Ground, Fuzhou City, Fujian Province, in a subtropical maritime monsoon climate characterized by warm and humid conditions, ample sunlight, and an annual average temperature of 20~25 °C. The coldest months are January and February, with an average temperature range of 6~10 °C. Conversely, the hottest months are July and August, with an average temperature range of 33~37 °C. The annual average sunshine duration ranges from 1700 to 1980 h and the average annual precipitation ranges from 900 to 2100 mm. The annual average relative humidity is 77%.
2.2. Experimental Design
Based on the cumulative annual observation data of meteorological stations (26°00′00″ N, 119°22′30″ E) in the experimental site (China Meteorological Science Data Center, http://data.cma.cn (accessed on 15 December 2023)), we determined the monthly variation in solar radiation intensity at the test site (Table 1). The total solar radiation and ultraviolet radiation tended to rise in summer and fall in winter. Based on the actual observation of the above solar radiation intensity, the total annual solar radiation was used as the standard, combined with the monthly average daily total radiation in Fujian Province (Table 2). Therefore, we referred to Smith’s method [19], using a random block design, with solar natural light (CK) as the reference value; according to the ozone consumption in the subtropical region in recent years, the trend of UV-B radiation enhancement in the next few decades was predicted. The four gradient treatments for daily UV-B radiation enhancement were set as CK, CK + 30 uw/cm2 (T1), CK + 45 uw/cm2 (T2), and CK + 60 uw/cm2 (T3), which was equivalent to the total annual UV-B radiation increasing by 0%, 8%, 12%, and 16%. The enhancement in UV-B radiation was simulated using a square wave method. Ultraviolet lamps with a peak wavelength of 313 nm served as the light source. These lamps were encased in a 125 μm thick cellulose acetate film, effectively filtering out UV-C radiation while allowing the transmission of UV-B and UV-A wavelengths. Firstly, twelve lampshades and twelve ultraviolet lamps were mounted on an 8 m long and 2 m wide frame. Next, aluminum foil was wrapped around the central portion of each lamp to ensure uniform ultraviolet radiation intensity. In the control group, only lampshades were installed. The radiation intensity under each experimental condition was measured and adjusted using a ultraviolet radiometer (UV-313, Beijing Normal University Photoelectric Instrument Factory, China). The experimental protocol involved 7 h of continuous irradiation, from 9:00 to 16:00 daily, with no exposure on rainy days.

Table 1.
The average monthly total radiation of the experimental site over the years (MJ/m).

Table 2.
The monthly average daily total radiation in Fujian Province (MJ/m).
2.3. Research Method
The litter from C. lanceolata forests at various developmental stages, obtained from the Xinkou Teaching Forest of Fujian Agriculture and Forestry University, was selected for investigation. Three 400 m2 plots were established in middle-aged (16 years) and over-mature stands (49 years), each with several 1 × 1 m frames for litter collection. In December 2021, the litter was collected and returned to the laboratory for analysis, according to standard scientific procedures. In this study, the decomposition of C. lanceolata litter was investigated using the mesh bag method. Litter samples from both middle-aged and over-mature stands were dried at 70 °C to achieve a constant weight, followed by the determination of their initial nutrient content (as presented in Table 3); refer to the method of Song et al. [20] for further details. Nylon mesh bags measuring 20 cm × 20 cm with a pore diameter of 0.5 mm were used. In January 2022, these decomposition bags were placed within four distinct plots subjected to varying levels of UV-B radiation enhancement, each measuring 6 m in length and 4 m in width. Their perimeters were secured with stainless-steel needles to ensure close contact between the decomposition bags and the soil surface. Samples from the decomposition bags were collected at 90, 180, 270, and 360 days post-deployment. The bags were cleaned of surface soil and extraneous matter, then subsequently dried, and their weights were recorded. Twelve bags were collected each time. The sampling spanned from April 2022 to January 2023.

Table 3.
Initial nutrient content of C. lanceolata litter at different age stages.
2.4. Sample Tests
Different experimental treatments were employed to measure the total carbon (TC) and total nitrogen (TN) contents of litter using a carbon–nitrogen elemental analyzer (Elementar VarioMAX CN, Hanau, Germany). The total phosphorus (TP) and total potassium (TK) were determined via digestion with nitric–perchloric acid, followed by inductively coupled plasma optical emission spectrometry (ICP-OES, PekinElmer, USA).
2.5. Data Processing and Analysis
The formula for the dry weight residual rate of litter is expressed as follows:
where (Y) represents the percentage of residual litter weight at time (t), t represents the decomposition time, (M0) represents the initial weight of litter (g), and (Mt) represents the weight of litter at time (t).
Y(%) = Mt/M0 × 100
The Olson exponential decay model is expressed as follows [21]:
Y = (Mt/M0) = ae−kt
The half-life of decomposition (50% decay) is calculated as follows:
t0.5 = −ln0.5/k.
The decomposition turnover period (95% decay) is calculated as follows:
t0.95 = −ln0.05/k
In the formula, Y—residual rate (%); a—fitting parameter; and k—decomposition coefficient, quasi-decomposition rate (d−1), where a larger k value indicates a faster decomposition rate.
The litter nutrient residual rate formula is
where R represents the residual nutrient element rate (%), C0 represents the initial nutrient content (g/kg), and Ct represents the nutrient content (g/kg) of litter at time t.
R = [(Mt × Ct)/(M0 × C0)] × 100%
2.6. Statistical Analysis
We used SPSS (version 22.0) for Windows software package (SPSS Inc., Chicago, IL, USA) for one-way analysis of variance (ANOVA) and Duncan’s test for multiple comparisons. We also employed two-way analysis of variance to decompose the effects of time and UV-B radiation intensity as influencing factors. After that, we utilized simple linear regression to examine the relationship between the nutrient residual rate and dry weight residual rate during litter decomposition, which we assessed using Pearson correlation.
3. Results
3.1. Effects of Enhanced UV-B Radiation on Dry Weight Residual Rate of Litter from C. lanceolata at Different Ages
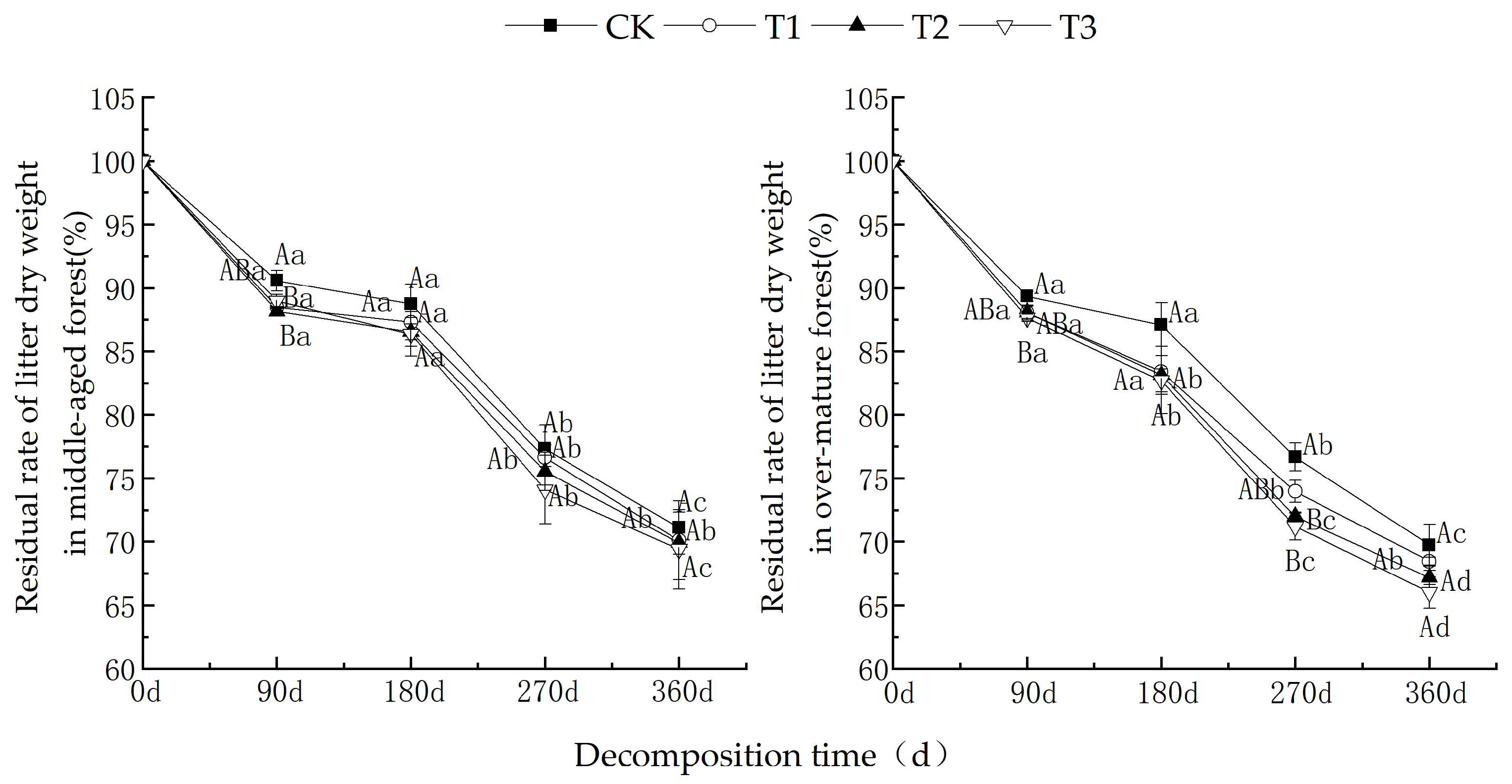
As shown in Figure 1, as the decomposition time increased, the dry weight residual rates of litter from middle-aged and over-mature forests exhibited a decreasing trend under various UV-B radiation intensities. The dry weight residual rates during the 180~270 d period were higher compared to those in the 0~180 d and 270~360 d periods. After one year of decomposition, under different UV-B radiation treatments, the dry weight residual rates of litter from middle-aged C. lanceolata forests were CK (71.1%) > T1 (70.1%) > T2 (69.9%) > T3 (69.4%). For C. lanceolata litter in over-mature forests, the dry weight residual rates were as follows: CK (69.7%) > T1 (68.5%) > T2 (67.2%) > T3 (66.0%).
Figure 1.
Comparison of the remaining weight ratio of cedar litter under various UV-B radiation treatments. (Different capital letters indicate significant differences in different UV-B radiation periods, while different lowercase letters indicate significant differences in the same UV-B radiation periods (p < 0.05)).
3.2. Effects of Enhanced UV-B Radiation on Litter Decomposition Dynamics of C. lanceolata at Different Ages
Regression equations were fitted to model the residual rates and decomposition times of middle-aged and over-mature forest litterfall, estimating their half-lives and turnover periods. The Olson exponential decay model demonstrated an R2 > 0.9 for the fitted equations obtained in all treatments, reaching a statistically significant level (Table 4). The decomposition rates of middle-aged and over-mature forest litterfall under different UV-B radiation intensities exhibited the following trend: T3 > T2 > T1 > CK. The half-lives of middle-aged forest litterfall in CK, T1, T2, and T3 were 2.1 a, 2.045 a, 1.998 a, and 1.904 a, respectively, with turnover periods of 9.078 a, 8.837 a, 8.633 a, and 8.230 a, while the half-lives of over-mature forest litterfall were 2.009 a, 1.843 a, 1.729 a, and 1.662 a, respectively, with turnover periods of 8.683 a, 7.967 a, 7.471 a, and 7.184 a. The two-way analysis of variance (Table 5) demonstrated that the decomposition time significantly influenced the dry weight residual rate of litter from both middle-aged and over-mature forests (p < 0.001). UV-B radiation intensity significantly affected the dry weight residual rate of over-mature forest litter (p < 0.01), but the same was not true for middle-aged forest litter (p > 0.05). The interaction between decomposition time and UV-B radiation intensity had very little impact on the dry weight residual rate of litter from either middle-aged or over-mature forests (p > 0.05).

Table 4.
Decomposition model of C. lanceolata litter under different UV-B radiation treatments.

Table 5.
Two-way ANOVA of decomposition time and UV-B radiation intensity on litter dry weight residual rate.
3.3. Effects of Enhanced UV-B Radiation on Nutrient Release Dynamics of C. lanceolata Litter at Different Ages
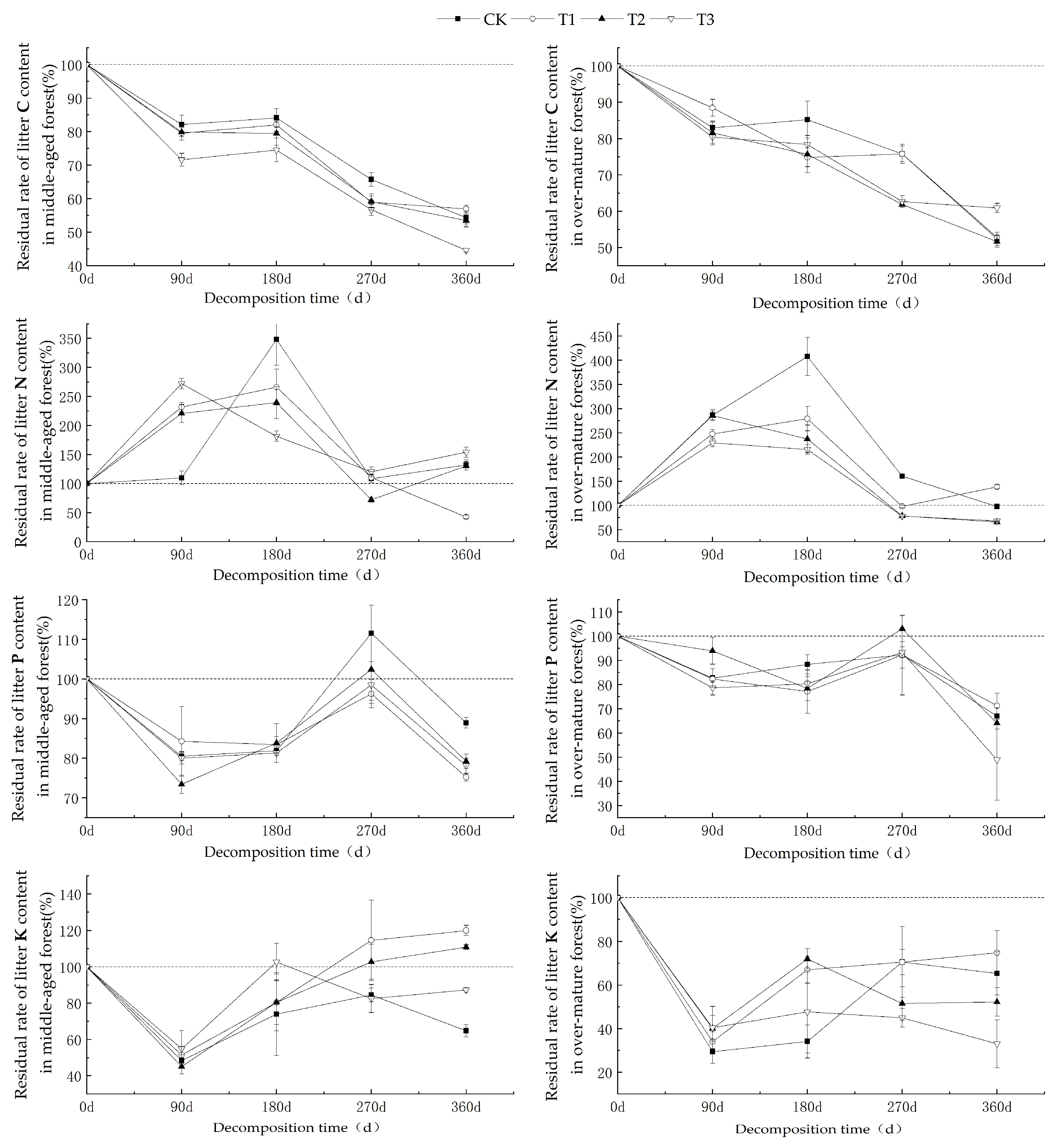
As shown in Figure 2, the carbon (C) residual rates of litter from middle-aged and over-mature C. lanceolata forests exhibited a decreasing trend with prolonged decomposition time. Over the 360-day decomposition period, the C residual rates under various UV-B radiation intensities consistently remained below 100%, indicating a distinct carbon net release phenomenon. After 360 days of decomposition, the C residual rates for middle-aged forest litter ranged from 44.58% to 56.94%, while those of over-mature forest litter ranged from 51.63% to 60.92%. The nitrogen (N) residual rate initially increased, followed by a decrease during the decomposition process. Within the 0–270-day decomposition period, the N content residual rates for middle-aged and over-mature forest litters both exceeded 100%, signifying a state of nitrogen enrichment. However, from 270 to 360 days, nitrogen enrichment in middle-aged forest litter was observed only under CK and T3 conditions, while over-mature forest litter exhibited nitrogen enrichment under CK and T1 conditions. After 360 days of decomposition, the N residual rates for middle-aged forest litter ranged from 42.49% to 153.90%, and those of over-mature forest litter ranged from 65.50% to 138.37%. The phosphorus (P) residual rates of litter from middle-aged and over-mature C. lanceolata forests demonstrated a general decrease–increase–decrease under various UV-B radiation intensities. Notably, only around the 270-day mark did middle-aged forest litter under CK conditions exhibit significant enrichment, while during other decomposition periods, it predominantly signified a state of P release. After 360 days of decomposition, the P residual rates for middle-aged forest litter ranged from 75.19% to 88.92%, whereas those of over-mature forest litter ranged from 48.81% to 71.28%. The potassium (K) residual rate during both middle-aged and over-mature forest litter decomposition generally showcased an initial decrease followed by an increase before stabilizing. During the 270–360-day decomposition period, middle-aged forest litter displayed K enrichment under T1 and T2 conditions, while it was mostly indicative of a release under other UV-B radiation treatments. In contrast, over the entire 360-day decomposition period, the over-mature forest litter consistently exhibited a net release of K. After 360 days of decomposition, the residual K content for middle-aged forest litter ranged from 64.77% to 119.95%, and from 33.01% to 74.78% for over-mature forest litter.
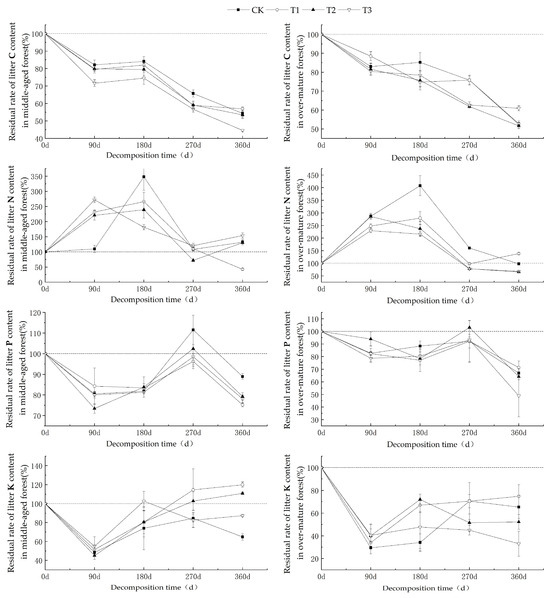
Figure 2.
The nutrient residual rates of C, N, P, and K during the decomposition of C. lanceolata litter under different UV-B radiation treatments.
3.4. The Interaction Effects of UV-B Radiation Enhancement and Decomposition Time on C, N, P, and K Residual Rates of C. lanceolata Litter
Table 6 indicates that decomposition time had a highly significant impact (p < 0.01) on the residual rates of carbon (C), nitrogen (N), phosphorus (P), and potassium (K) in litter from both middle-aged and over-mature C. lanceolata forests. UV-B radiation intensity had a significant impact (p < 0.01) on the C residual rate in middle-aged forest litter and the N residual rate in over-mature forest litter. Additionally, it influenced (p < 0.05) the K residual rate in middle-aged forest litter and the C residual rate in over-mature forest litter, although it failed to reach extreme significance. The interaction between decomposition time and UV-B radiation intensity had a highly significant impact (p < 0.01) on the N residual rate in middle-aged forest litter and the C and N residual rates in over-mature forest litter, as well as (p < 0.05) the K residual rate in over-mature forest litter, although again, it failed to reach extreme significance.

Table 6.
The effects (F-value) of decomposition time, UV-B radiation intensity, and their interaction on litter nutrient residual rate.
3.5. Correlation Analysis between Dry Weight Residual Rate and Nutrient Residual Rate of Litter from C. lanceolata at Different Ages
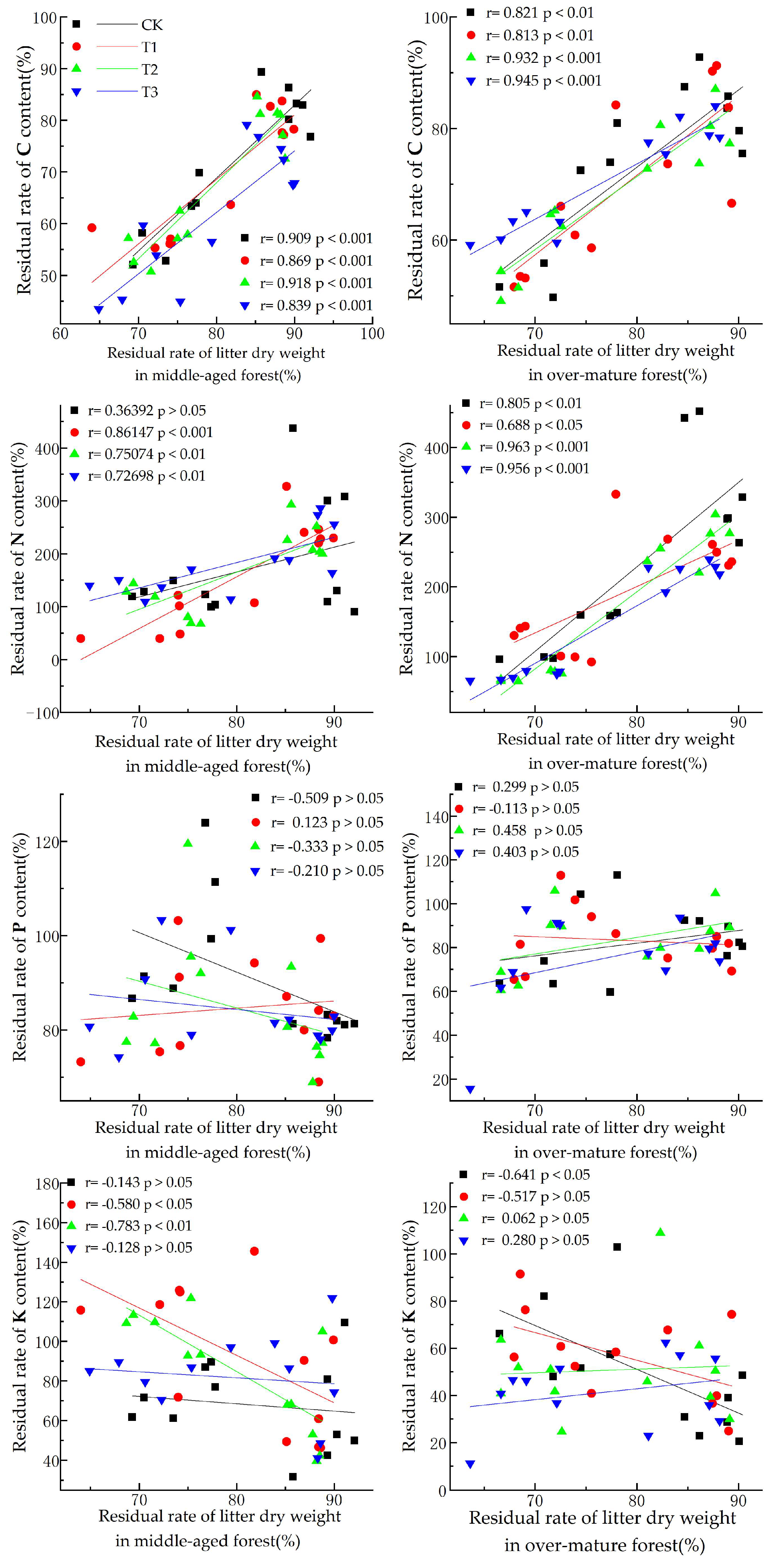
As shown in Figure 3, the dry weight residual rate of litter from middle-aged forests exhibited a highly significant positive correlation (p < 0.001) with the C residual rate under different UV-B radiation conditions. It also showed a highly significant positive correlation with the N content residual rates under T1, T2, and T3 conditions, while the correlation was not significant under natural light conditions. However, no significant correlation was observed between the residual rate of phosphorus (P) and the different UV-B radiation intensity treatments. Under the T1 conditions, we observed a significant negative correlation (p < 0.05) with the K residual rate, while the correlation is extremely significant under the T2 condition (p < 0.01). Nevertheless, no significant correlation was observed under the CK and T3 conditions.
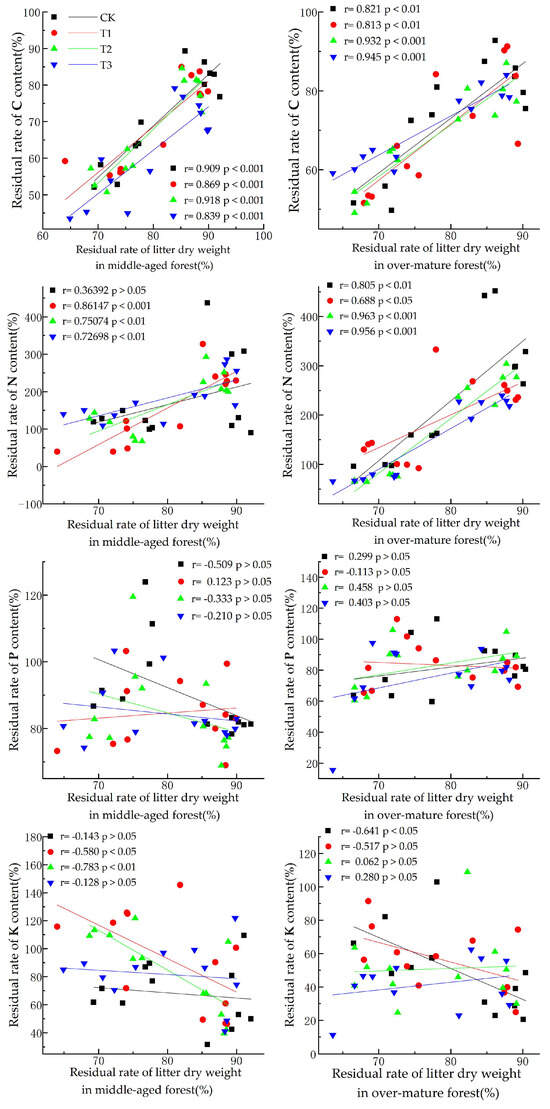
Figure 3.
Correlation between litter dry weight residual rate and C, N, P, and K nutrient residual rate.
The dry weight residual rate of litter from over-mature forests demonstrated a highly significant positive correlation with the C residual rate under different UV-B radiation conditions (p < 0.01). Furthermore, a significant negative correlation with the residual rate of N was observed under each UV-B radiation treatment (p < 0.05), reaching extreme significance under the CK, T2, and T3 conditions (p < 0.01). No significant correlation was found between the dry weight residual rate and the residual rate of P under all UV-B radiation treatments. Under CK conditions, a significant negative correlation with the K residual rate was observed (p < 0.05), whereas this correlation was not significant under other UV-B amplification conditions.
4. Discussion
The decomposition of litter is pivotal in the nutrient cycling of forest ecosystems. UV-B radiation intensity is recognized as a primary environmental factor influencing litter decomposition [22], and empirical evidence suggests that heightening its intensity augments the rate of litter decomposition [23,24]. In this investigation, the litter decomposition rate of C. lanceolata increased with the UVB radiation intensity. Both middle-aged and over-mature forests exhibited decomposition rates in the order of T3 > T2 > T1 > CK, in accordance with the findings of Song et al. [23]. This means that the decomposition rate of C. lanceolata litter will gradually accelerate in the next few decades. Enhancing UV-B radiation facilitates the photodegradation of lignin within litter. This enhancement is primarily attributed to photochemical reactions that modify the composition of lignin and recalcitrant compounds, thereby expediting the material cycling rate of plant litter [25]. Additionally, in our study, over-mature forests consistently displayed higher decomposition rates than middle-aged forests under varying UV-B radiation intensities, in agreement with the results presented by Wang [26]. The disparities in litter decomposition rates between these forest types across different UV-B radiation treatments may arise from differential microbial community structures and quantities, as well as variations in initial substrate quality. Middle-aged forests, undergoing rapid growth, manifest elevated demands for N and P, leading to enhanced initial nutrient concentrations of N and P in the litter relative to over-mature forests. Conversely, over-mature forests possess a stabilized stand structure, bolstering resistance against pests and adverse conditions. Consequently, investments in protective compound synthesis, such as tannins, are diminished [27], further amplifying litter degradability. In our study, the peak periods for the accelerated decomposition of cedar litter in both middle-aged and over-mature forests spanned from 180 to 270 days (July to September). This timeframe corresponds to elevated temperatures and concentrated precipitation during the summer months, fostering heightened microbial activity and thereby accelerating litter decomposition [26].
During litter decomposition, nutrient enrichment and release are closely tied to the chemical composition of the litter itself, exhibiting distinct stage-related characteristics, including three primary release modes: direct release, enrichment–release, and leaching–enrichment–release [28]. Li et al. [29] investigated the litter decomposition and carbon emissions from Pinus massoniana, Pinus elliottii, Cunninghamia lanceolata, and a mixed Schima superba and Pinus massoniana forest, and discovered that carbon consistently exhibited a net release throughout litter decomposition, which increased progressively over time. In the context of this research, both middle-aged and over-mature cedar forest litter showed a net release of carbon content during decomposition in natural and UV-B radiation-enhanced environments, which supported previous findings in this regard. After decomposition, under the influence of T3 UV-B radiation treatment, there was a notable reduction in carbon content. Moreover, the T3 treatment exhibited the fastest release rate, indicating that UV-B radiation can stimulate carbon release to a certain extent, thereby accelerating nutrient cycling. These observations are similar to the findings of Guo et al. [30]. This phenomenon is attributed to the capability of UV-B radiation to expedite decomposition by facilitating the photodegradation of lignin, a non-structural carbon compound present in litter [25]. This change may reduce the carbon storage of the litter layer of C. lanceolata plantation, increase its carbon flux, and subsequently affect the carbon sink function of C. lanceolata plantation against the background of global change. However, post-decomposition, the carbon content in middle-aged forest litter under natural conditions was lower than that observed under T1 and T2 UV-B treatments. Similarly, for over-mature forests, the carbon content was lower than that under the T2 treatment. This divergence can be attributed to UV-B radiation altering the structure and quantity of microbial communities, and thus reducing decomposition rates [14]. For T1 and T2 UV-B radiation treatments implemented in middle-aged forests, and following T2 UV-B radiation treatment in over-mature forests, microbial activity plays a predominant role.
The release of nutrients from litter is significantly influenced by the litter quality. High-quality litter, characterized by higher nitrogen (N) and phosphorus (P) contents, tends to release nutrients more rapidly. Conversely, litter with diminished N and P contents may sequester nutrients from the environment before gradually releasing them during the initial stages of decomposition [31,32]. Studies by Parton et al. [33], conducted over a decade-long observation of litter decomposition across seven distinct biomes, emphasize that nitrogen release is contingent upon both the initial nitrogen content of the litter and the residual litter mass. High-nitrogen litter swiftly meets microbial demands, facilitating rapid nitrogen release. However, low-nitrogen litter relies on external nitrogen sources to satisfy microbial demands, slowing the release of nitrogen. In this study, litter from both middle-aged and over-mature cedar forests exhibited a pattern of nutrient enrichment and release for total nitrogen (N) under both natural light conditions and UV-B enhancement. Compared to natural light conditions, the UV-B enhancement treatments accelerated the initial enrichment of nitrogen during the early stages of litter decomposition. This acceleration can be attributed to the enhanced decomposition of litter induced by UV-B radiation, resulting in a reduction in initial residual mass and an increase in nitrogen content. Moreover, the enrichment and release of litter nutrients are influenced by the ratios of initial carbon (C) to other nutrients. Microorganisms absorb nutrient elements from organic matter and exchange inorganic nutrients with the environment to maintain their chemical stoichiometric balance. However, before achieving this balance, they must undergo continuous nutrient enrichment, followed by release [34].
The C:P ratio in litter is a crucial factor that determines the enrichment and release of phosphorus (P), typically falling within a threshold of 200 to 480 [35,36]. In this study, the C:P ratio in litter from both middle-aged and over-mature forests surpassed 480, exceeding this threshold. Middle-aged forest litter exhibited a release–enrichment–release pattern for phosphorus in its natural state, while it generally remained in a net release state under other treatments (T1, T2, and T3). This indicates that UV-B radiation enhancement altered the phosphorus release pattern in middle-aged forest litter. At around 270 days of decomposition, the CK condition for middle-aged forest litter showed clear signs of phosphorus enrichment. This may be attributed to the high temperature and rainy season leading to changes in microbial community structure and increased diversity, and thus increased activity. The phosphorus released during decomposition failed to meet the turnover demands of the microbial community, leading to significant phosphorus accumulation [37]. Additionally, varying intensities of UV-B radiation altered microbial community structure and quantity [15], influencing the release of phosphorus. Over-mature forest litter, under four different UV-B radiation conditions, consistently exhibited a net release pattern, similar to that described by Song [38]. Potassium (K), which is among the most mobile elements in plant nutrients, is primarily present in litter in the form of soluble salts. Consequently, litter from over-mature cedar forests showed a direct release pattern for potassium [39]. However, in the later stages of decomposition, specifically during the 270–360-day period under T1 and T2 treatments for middle-aged forest litter, the intensity of potassium exhibited signs of enrichment, suggesting that UV-B radiation enhancement transiently altered its release pattern. The underlying mechanisms, potentially influenced by other environmental factors (such as temperature, humidity, and microorganisms), remain unclear and in need of further investigation.
5. Conclusions
UV-B radiation of varying intensities significantly impacts the residual dry weight accumulation rates and nutrient release patterns of C. lanceolata litter. Our study findings reveal that an elevation in UV-B radiation levels expedites the decomposition process of C. lanceolata litter. This suggests that, over the forthcoming decades, there will be a progressive increment in the decomposition rate of this litter, particularly in over-mature forest stands where the effect is more pronounced. The intensification of UV-B radiation stimulates the release of C and K from the litter in middle-aged forests, and C and N in over-mature forests. This acceleration in nutrient release not only hastens the nutrient cycling process but also modifies the patterns of N, P, and K release in middle-aged forests, and N in over-mature forests. Such alterations carry significant implications for the nutrient cycling dynamics and the carbon sequestration capabilities of C. lanceolata plantations. Over the next few decades, it is essential to consider appropriate planting densities for C. lanceolata and to enhance light penetration within the plantations through artificial pruning, which can play a significant role in improving the fertility of the soil in these artificial forests. Taken together, this study is crucial for a comprehensive understanding of the effects of climate change on the decomposition of C. lanceolata litter.
Author Contributions
Conceptualization, Z.L. and A.L.; methodology, Z.L.; software, L.C.; validation, L.C., Y.D. and S.C.; formal analysis, Z.L.; investigation, Z.L., S.C., C.L. and T.Y.; resources, L.C.; data curation, Y.D.; writing—original draft preparation, Z.L.; writing—review and editing, A.L. and X.M.; visualization, A.L.; supervision, A.L.; project administration, X.M.; funding acquisition, X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2021YFD2201302, 2016YFD0600301).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns but may be provided by the corresponding author on request.
Acknowledgments
We wish to express our gratitude for the experimental materials provided by Xinkou Forest Farm and for the unwavering support of the National Fund Committee.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Herman, J.R. Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data. J. Geophys. Res. 2010, 115, D04203. [Google Scholar] [CrossRef]
- Wu, R.H.; Zheng, Y.F.; Wang, C.H.; Hu, Z.H. Comparison of the effects of enhanced ultraviolet radiation on the growth of aboveground and root of maize. Ecol. Environ. Sci. 2007, 16, 323–326. [Google Scholar]
- Zhou, P.; Chen, Z.Y. Analysis of the Spatio-temporal Characteristics of UV-B Strength Change over the Yunnan Plateau. J. Nat. Resour. 2008, 23, 487–493. [Google Scholar]
- Liao, Y.F.; Wang, W.Y.; Zhang, L.; Yang, L.S. Distribution of biologically effective solar ultraviolet radiation intensity on theground in China. Geogr. Res. 2007, 26, 821–827+860. [Google Scholar]
- Zhou, X.J.; Luo, C.; Li, W.L.; Shi, J.E. The variation of total ozone in China and the low value center of the Qinghai-Tibet Plateau. Chin. Sci. Bull. 1995, 40, 1396–1398. (In Chinese) [Google Scholar]
- Dias AT, C.; Cornelissen, J.H.; Berg, M.P. Litter for life: Assessing the multifunctional legacy of plant traits. J. Ecol. 2017, 105, 1163–1168. [Google Scholar] [CrossRef]
- Austin, A.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef]
- Baker, N.R.; Allison, S.D. Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate. Ecology 2016, 96, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Uselman, S.M.; Snyder, K.A.; Blank, R.R.; Jones, T.J. UVB exposure doesnot accelerate rates of litter decomposition in a semi-arid riparianecosystem. Soil Biol. Biochem. 2011, 43, 1254–1265. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Milchunas, D.G. Effects of ultravioletradiation on liter decomposition depend on precipitation and iterchemistry in a shortgrass steppe ecosystem. Glob. Change Biol. 2007, 13, 2193–2205. [Google Scholar] [CrossRef]
- WU, Z.M.; Huang, C.L.; Wei, C.L. Gap light energy effect of Pinus taiwanensis community and regeneration of Pinus taiwanensis. Chin. J. Appl. Ecol. 2000, 11, 14–19. [Google Scholar]
- Yan, S.J.; Hong, W.; Wu, C.Z.; Bi, X.L.; Wang, X.G.; Feng, L. Gaps and their natural disturbance characteristics in mid-subtropical evergreenbroad-leaved forest in Wanmulin. Chin. J. Appl. Ecol. 2004, 15, 1126–1130. [Google Scholar]
- Rozema, J.; Tosserams, M.; Nelissen, H.J.M.; Heerwaarden, L.; Broekman, R.A.; Flierman, N. UV-B and Aquatic Ecosystems || Stratospheric Ozone Reduction and Ecosystem Processes: Enhanced UV-B Radiation Affects Chemical Quality and Decomposition of Leaves of the Dune Grassland Species Calamagrostis epigeios. Plant Ecol. 1997, 128, 284–294. [Google Scholar]
- Moody, S.A.; Paul, N.D.; Bjorn, L.O.; Callaghan, T.V.; Lee, J.A.; Manetas, Y.; Rozema, J.; Gwynn-Jones, D.; Johanson, U.; Kyparissis, A. The direct effects of UV-B radiation on Betula pubescens litter decomposing at four European field sites. Plant Ecol. 2001, 154, 27–36. [Google Scholar] [CrossRef]
- Pancotto, V.A.; Sala, O.E.; Cabello, M.; López, N.I.; Robson, T.M.; Ballaré, C.L.; Caldwell, M.M.; Scopel, A.L. Solar UV-B decreases decomposition in herbaceous plant litter in Tierra del Fuego, Argentina: Potential role of an altered decomposer community. Blackwell Sci. Ltd. 2003, 9, 1465–1474. [Google Scholar] [CrossRef]
- Xia, L.D.; Yu, J.D.; Deng, L.L.; Li, X.Y.; Zhou, C.F.; Xu, Y.X. Research Progress on Soil Fertility Decline of C. lanceolata Plantation. World For. Res. 2018, 31, 37–42. [Google Scholar]
- Yang, Q.P.; LI, R.S.; Zhang, W.D.; Zheng, W.H.; Wang, Q.K.; Chen, L.C.; Guan, X.; Xu, M.; Wang, S.L. Decomposition of harvest residue needles of different needle ages in a C.lanceolata (Cunninghamia lanceolata) plantation. Plant Soil 2018, 423, 273–284. [Google Scholar] [CrossRef]
- Zhang, H.L.; Song, X.Z.; Zhang, Z.T.; Jiang, H.; Wang, Y.X.; Bai, S.B. Effects of UV-B radiation on the decomposition of Cunninghamia lanceolata leaf litter. Chin. J. Appl. Ecol. 2011, 22, 845–850. [Google Scholar]
- Smith, W.K.; Gao, W.; Steltzer, H.; Wallenstein, M.D.; Tree, R. Moisture availability influences the effect of ultraviolet-B radiation on leaf litter decomposition. Glob. Change Biol. 2010, 16, 484–495. [Google Scholar] [CrossRef]
- Song, X.Z.; Jiang, H.; Zhang, H.L.; Peng, C.H.; Yu, S.Q. Elevated UV-B radiation did not affect decomposition rates of needles of two coniferous species in subtropical China. Eur. J. Soil Biol. 2011, 47, 343–348. [Google Scholar] [CrossRef]
- Liu, Z.W.; Pan, W.K. Problems and corrections of Olson litter decomposition model. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2005, 33, 69–70. [Google Scholar]
- Zhang, Y.; Zhang, Y.f.; Ma, Y.B.; Liu, C.E.; Duan, C.Q.; Zi, Y.F.; Tang, B.C.; Zhang, W.L. Research progress on the influencing factors of litter decomposition in forest ecosystem. Environ. Ecol. 2023, 5, 45–56. [Google Scholar]
- Song, X.Z.; Bu, T.; Zhang, S.K.; Jiang, H.; Wang, Z.K.; Zhao, M.S.; Liu, Y.J. Effects of UV-B radiation on the chemical composition and decomposition of Cyclobalanopsis glauca leaf litter. Environ. Ecol. 2013, 34, 2355–2360. [Google Scholar]
- Mi, X.; Xie, T.T.; Wu, Z.X. Effects of UV-B radiation on litter decomposition of Reaumuria soongorica and Pearl Shrub in arid desert area. J. Gansu Agric. Univ. 2023, 58, 163–171. [Google Scholar]
- Zhang, H.L.; Song, X.Z.; Ai, J.G.; Jiang, H.; Yu, S.Q. Review on the effects of enhanced ultraviolet-B radiation on litter decomposition. J. Zhejiang A&F Univ. 2010, 27, 134–142. [Google Scholar]
- Wang, X.; Gao, M.D.; Yang, F.; Guo, Y.P.; Ma, C.M. Comparison of leaf litter decomposition and nutrient dynamics in different forest ages of Larix principis-rupprechtii plantation. J. Northeast For. Univ. 2012, 40, 56–60+66. [Google Scholar]
- Ye, G.F.; Zhang, S.J.; Zhang, L.H.; Lu, C.Y.; Lin, Y.M. Tannin content and nutrient reabsorption dynamics of Casuarina equisetifolia twigs at different forest ages. Acta Ecol. Sin. 2013, 33, 6107–6113. [Google Scholar]
- Guo, J.F.; Yang, Y.S.; Chen, G.S.; Lin, P.; Xie, J.S. Research Progress on Forest Litter Decomposition. Sci. Silvae Sin. 2006, 42, 93–100. [Google Scholar]
- Li, H.T.; Yu, G.R.; Li, J.Y.; Chen, Y.R.; Liang, T. Litter decomposition dynamics and nutrient release of four plantations in subtropical red soil hilly region. Acta Ecol. Sin. 2007, 27, 898–908. [Google Scholar]
- Guo, B.H.; Bu, T.; Wang, Z.K.; Song, X.Z.; Liu, G.L.; Fan, S.H. Effects of UV-B radiation on chemical composition and decomposition of Elaeocarpus decipiens litter. Chin. J. Ecol. 2013, 32, 2314–2319. [Google Scholar]
- Gallardo, A.; Merino, J. Nitrogen immobilization in leaf litter at two Mediterranean ecosystems of SW Spain. Biogeochemistry 1992, 15, 213–228. [Google Scholar] [CrossRef]
- McClaugherty, C.A.; Pastor, J.; Aber, J.D.; Melillo, J.M. Forest Litter Decomposition in Relation to Soil Nitrogen Dynamics and Litter Quality. Ecology 1985, 66, 266–275. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-Scale Similarities in Nitrogen Release Patterns During Long-Term Decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Gosz, J.R.; Likens, G.E.; Herbert, B.F. Nutrient Release From Decomposing Leaf and Branch Litter in the Hubbard Brook Forest, New Hampshire. Ecol. Monogr. 1973, 43, 173–191. [Google Scholar] [CrossRef]
- Dziadowiec, H. The decomposition of plant litterfall in a oak-linden-hornbeam forest and an oak-pine mixedforest of the Bialoweza national Park. Acta Soc. Bot. Pol. 1987, 56, 169–185. [Google Scholar] [CrossRef]
- Li, N.; Zhao, C.Y.; Hao, H.; Zang, F.; Chang, Y.P.; Wang, H.; Yang, J.H. Effects of altitude and canopy density on leaf litter decomposition of Qinghai spruce forest in Qilian Mountains. Acta Ecol. Sin. 2021, 41, 4493–4502. [Google Scholar]
- Song, X.Z.; Zhang, H.L.; Jiang, H.; Yu, S.Q. Effects of UV-B radiation on the release of nitrogen and phosphorus from leaf litter in subtropical forests. Environ. Sci. 2012, 33, 545–550. [Google Scholar]
- Bruder, A.; Schindler, M.H.; Moretti, M.S.; Gessner, M.O. Litter decomposition in a temperate and a tropical stream: The effects of species mixing, litter quality and shredders. Freshw. Biol. 2014, 59, 438–449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).