Integrated Metabolome and Transcriptome Analyses Provide New Insights into the Leaf Color Changes in Osmanthus fragrans cv. ‘Wucaigui’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chlorophyll Extraction and Determination
2.3. Carotenoid Extraction and Determination
2.4. Anthocyanin Extraction and Determination
2.5. Transcriptome Sequencing, Assembly, and Analysis
2.6. Screening of Hub Genes
2.7. Real-Time Fluorescence Quantitative PCR (qRT-PCR) Was Used to Verify Gene Expression
2.8. Data Analyses
3. Results
3.1. Metabolome Responses of Leaves at Different Developmental Stages
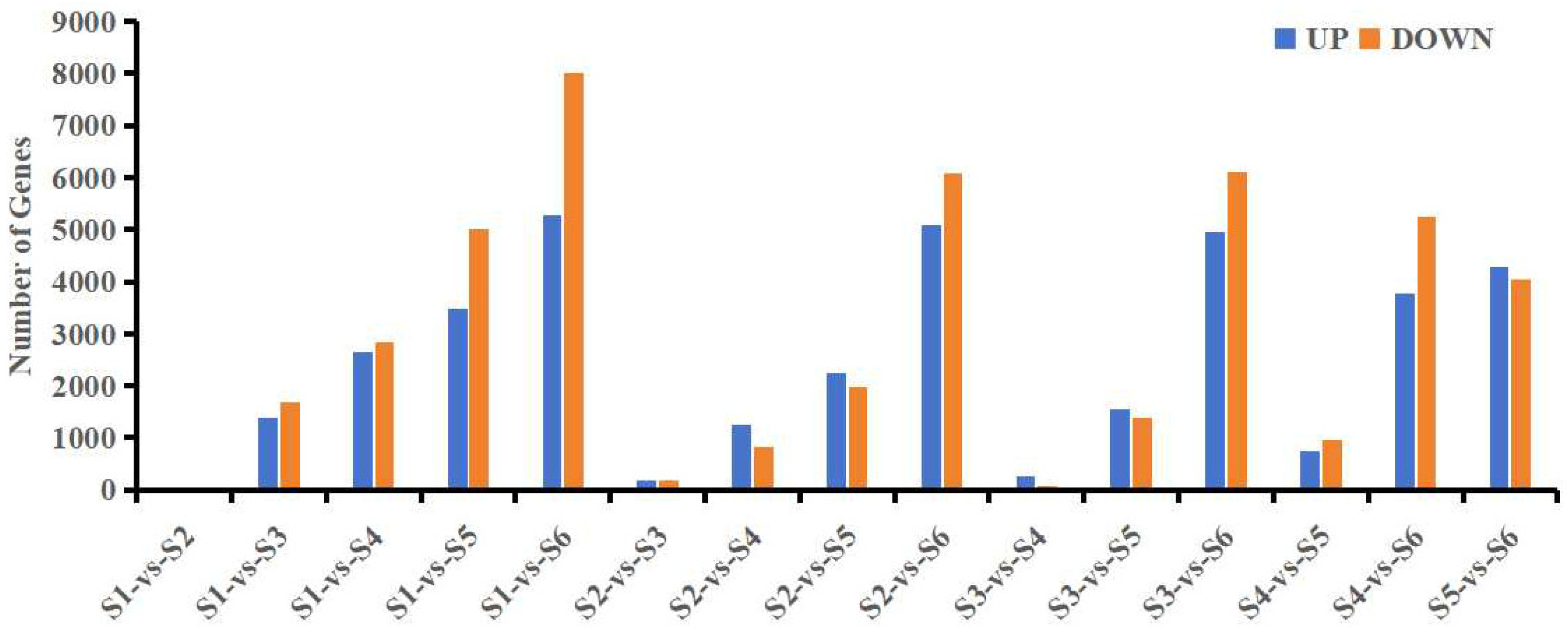
3.2. Transcriptome Analysis of Leaves at Different Developmental Stages
3.3. Key Metabolites in the Leaf Color-Change Process
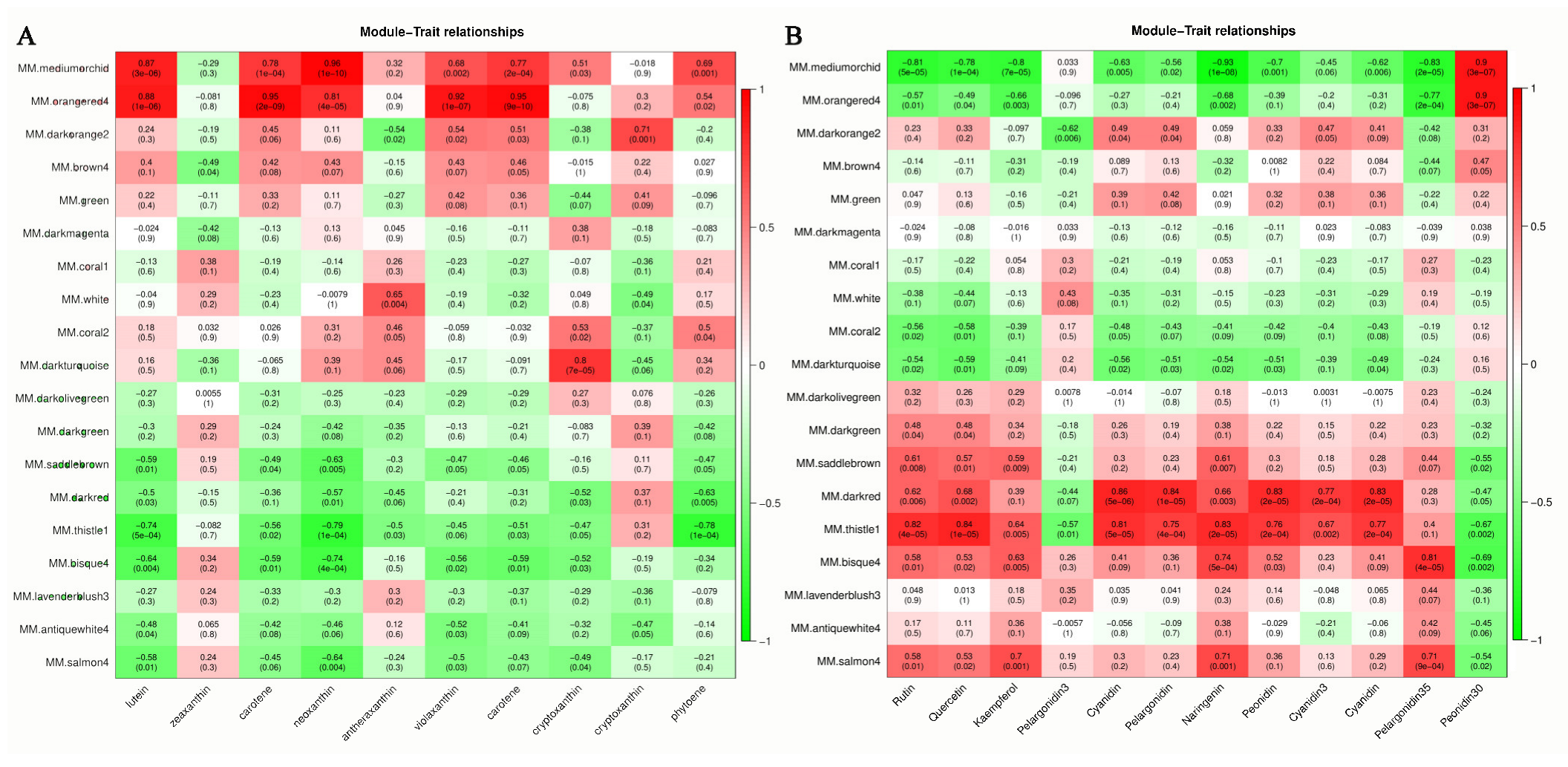
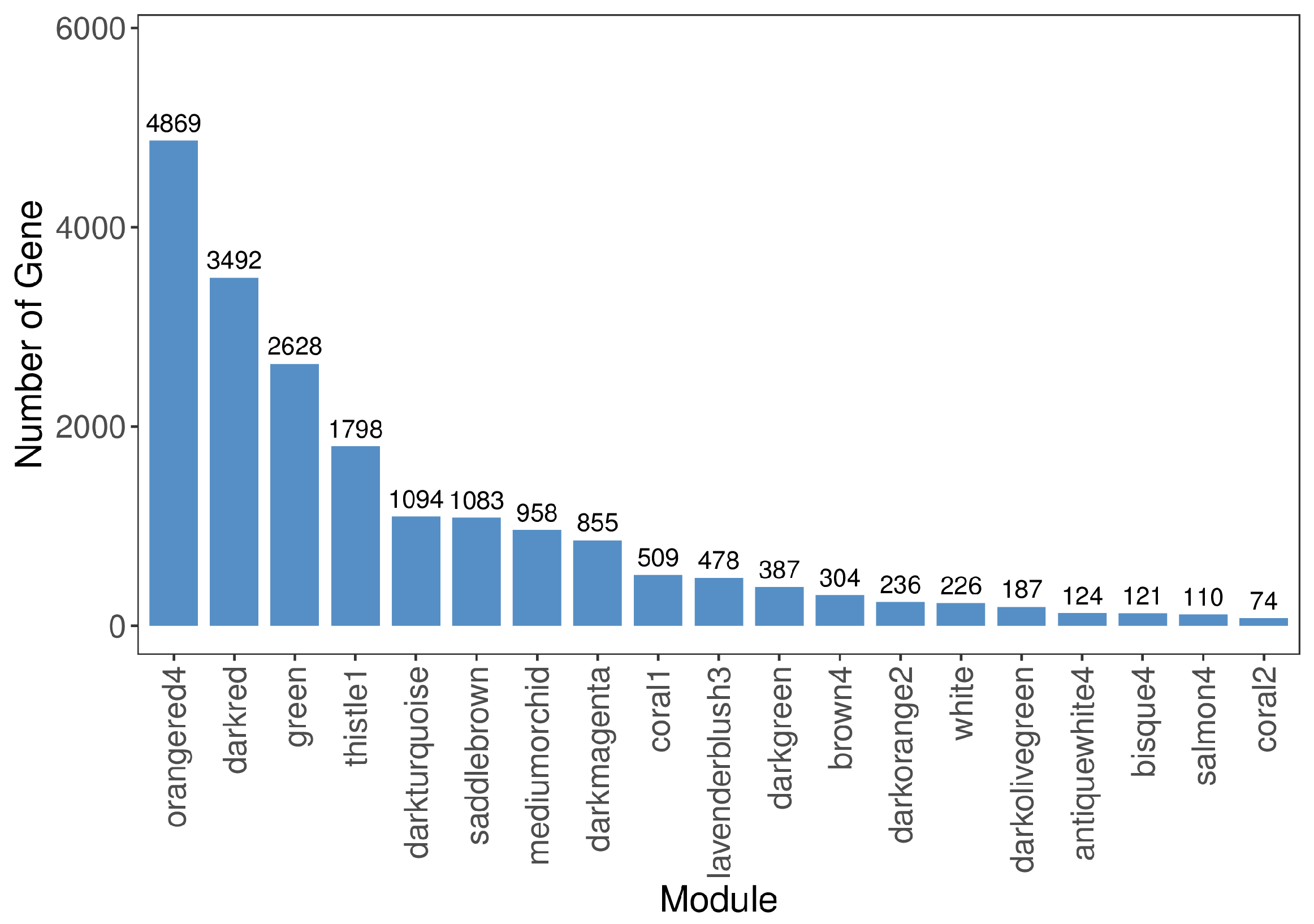
3.4. Hub Genes in the Leaf Color-Change Process
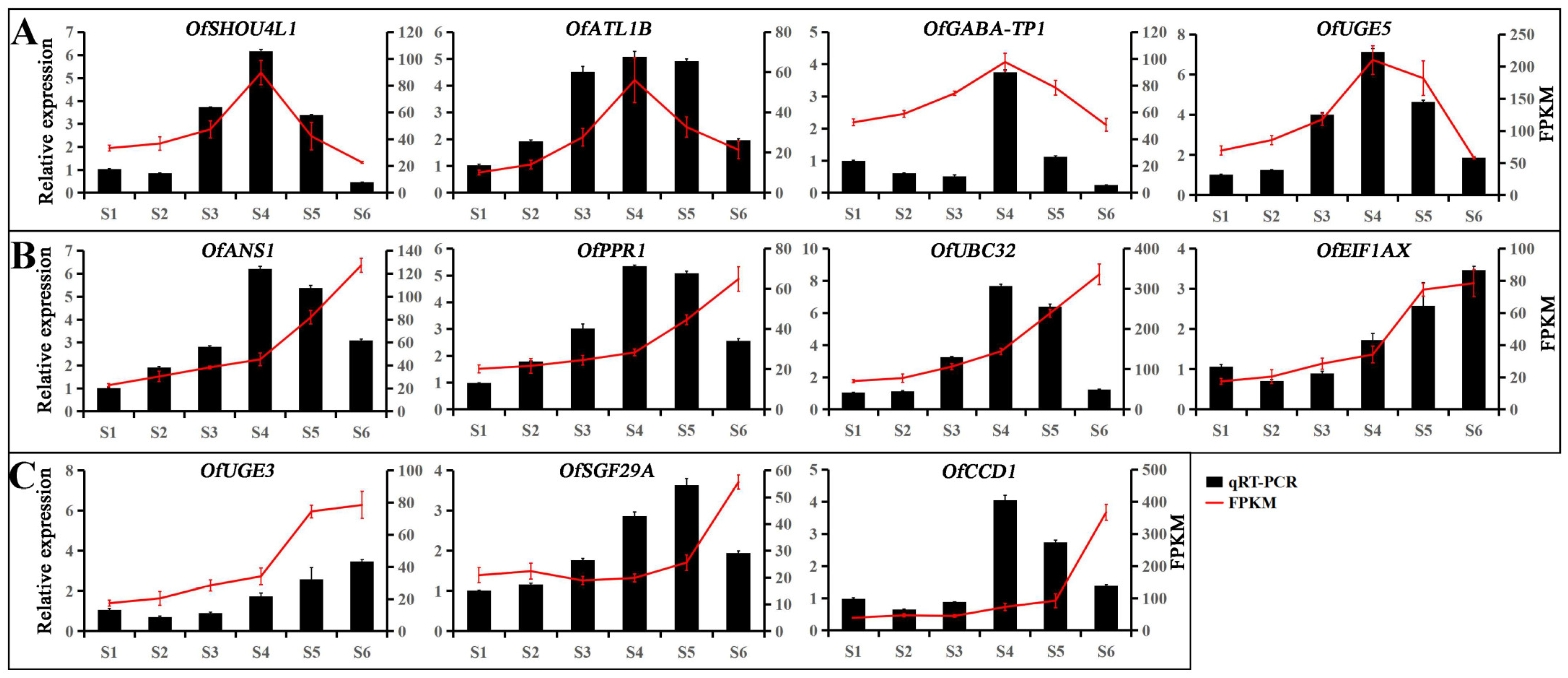
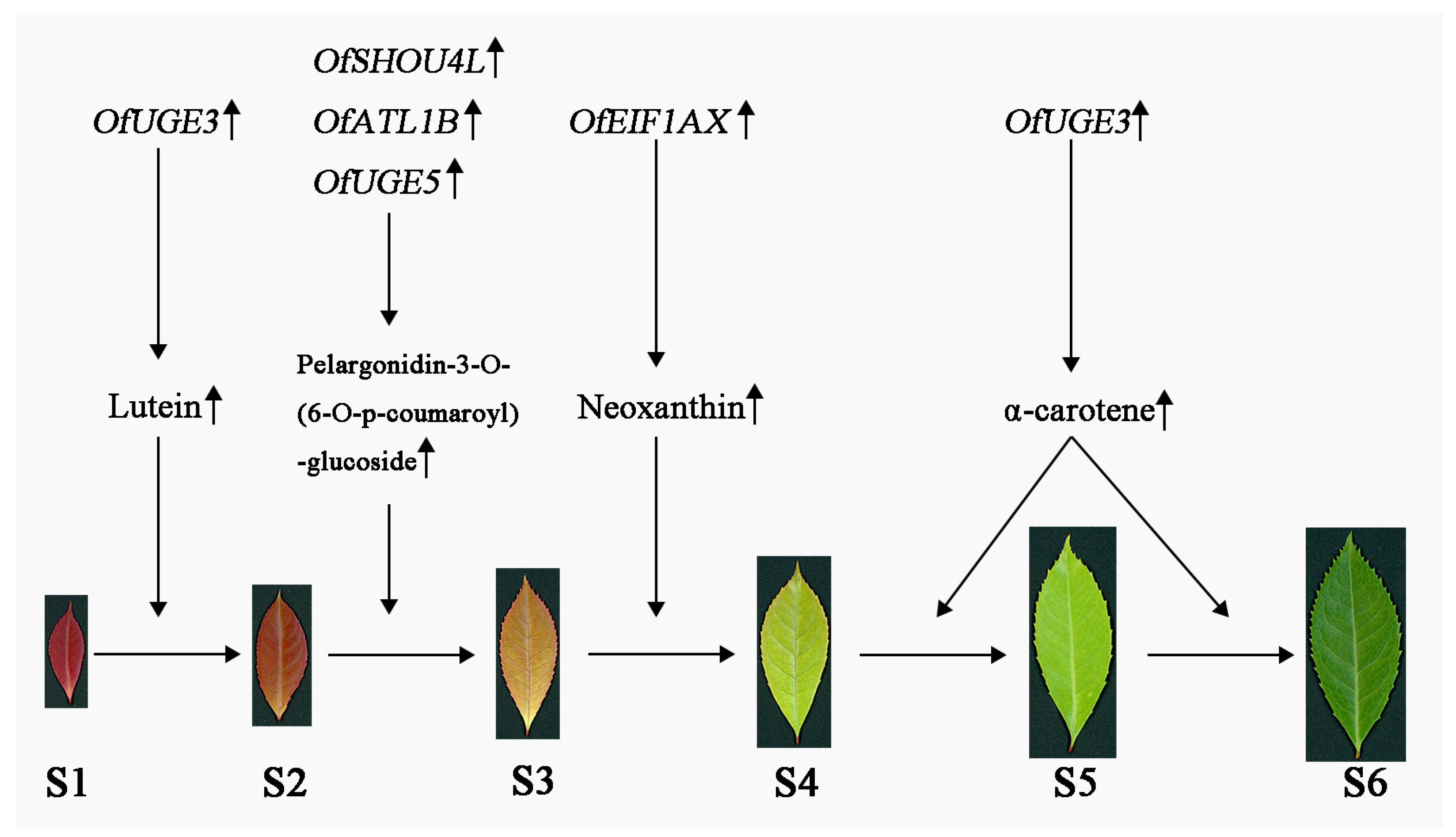
3.5. Hub Genes Regulate Leaf Color Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, T.; Wang, X.; Xu, Z.; Xu, J.; Li, R.; Liu, N.; Ding, G.C.; Sui, S.Z. Screening of key genes responsible for Pennisetum setaceum ‘Rubrum’ leaf color using transcriptome sequencing. PLoS ONE 2020, 15, e0242618. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Gu, H.; Cai, H.Y.; Guo, C.C.; Chen, Y.; Wang, L.G.; Chen, G.W. Integrated transcriptome and biochemical analysis provides new insights into the leaf color change in Acer fabri. Forests 2023, 14, 1638. [Google Scholar] [CrossRef]
- Luo, J.R.; Duan, J.J.; Huo, D.; Shi, Q.Q.; Niu, L.X.; Zhang, Y.L. Transcriptomic analysis reveals transcription factors related to leaf anthocyanin biosynthesis in Paeonia qiui. Molecules 2017, 22, 2186. [Google Scholar] [CrossRef]
- Chen, X.; Xie, J.; Yue, Y.Z.; Yang, X.L.; Wang, L.G. Advances in research on leaf coloration mechanism of colored leaf plants. J. Northwest Plant 2020, 40, 358–364. [Google Scholar]
- Zhang, Q.; Wang, L.L.; Liu, Z.G.; Zhao, Z.H.; Zhao, J.; Wang, Z.T.; Zhou, G.F.; Liu, P.; Liu, M.J. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba Mill. peel coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, M.H.; Hu, Y.B.; Meng, F.J.; Song, X.S.; Tigabu, M.; Chiang, V.L.; Sederoff, R.; Ma, W.J.; et al. Molecular and metabolic insights into anthocyanin biosynthesis for leaf color change in chokecherry (Padus virginiana). Int. J. Mol. Sci. 2021, 22, 10697. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.N.; Li, Y.J.; Zhu, Q.H.; Zhang, X.Y.; Liu, F.; Xue, F.; Sun, J. GhGSTF12, a glutathione S-transferase gene, is essential for anthocyanin accumulation in cotton (Gossypium hirsutum L.). Plant Sci. 2021, 305, 110827. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, C.P.; Zhang, Y.; Li, C.Z.Y.; Li, X.; Yu, Q.; Wang, S.; Wang, X.Y.; Chen, X.S.; Feng, S.Q. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear. BMC Plant Biol. 2020, 20, 129. [Google Scholar] [CrossRef]
- Yang, Y.X.; Chen, X.X.; Xu, B.; Li, Y.X.; Ma, Y.H.; Wang, G.D. Phenotype and transcriptome analysis reveals chloroplast development and pigment biosynthesis together influenced the leaf color formation in mutants of Anthurium andraeanum ‘Sonate’. PhytoKeys 2015, 6, 139. [Google Scholar] [CrossRef]
- Nagata, N.; Tanaka, R.; Satoh, S.; Tanaka, A. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell 2005, 17, 233–240. [Google Scholar] [CrossRef]
- Celebioglu, B.; Hart, J.P.; Porch, T.; Griffiths, P.; Myers, J.R. Genome-wide association study to identify possible candidate genes of snap bean leaf and pod color. Genes 2023, 14, 2234. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Liu, Q.; Yan, K.; Wang, X.Y.; Xu, P. Transcriptomic and metabolomic analyses reveal how girdling promotes leaf color expression in Acer rubrum L. BMC Plant Biol. 2022, 22, 498. [Google Scholar]
- Li, W.X.; Yang, S.B.; Lu, Z.G.; He, Z.C.; Ye, Y.L.; Zhao, B.B.; Wang, L.; Jin, B. Cytological, physiological, and transcriptomic analyses of golden leaf coloration in Ginkgo biloba L. Hortic. Res. 2018, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.K.; Zhan, W.; Sun, A.R.; Xie, Y.; Han, Z.M.; Qu, X.B.; Wang, J.Y.; Zhang, L.F.; Tian, M.S.; Pang, X.H.; et al. Combined transcriptome and metabolome integrated analysis of Acer mandshuricum to reveal candidate genes involved in anthocyanin accumulation. Sci. Rep. 2021, 11, 23148. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, X.L.; Jie, J.; Ding, W.J.; Li, Y.L.; Yue, Y.Z.; Wang, L.G. Biochemical and comparative transcriptome analyses reveal key genes involved in major metabolic regulation related to colored leaf formation in Osmanthus fragrans ‘Yinbi Shuanghui’ during development. Biomolecules 2020, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhen, J.P.; Che, X.Y.; Zhang, K.; Zhang, G.W.; Yang, H.J.; Wen, J.; Wang, J.X.; Wang, J.M.; He, B.; et al. Transcriptomic and metabolomic analysis of autumn leaf color change in Fraxinus angustifolia. PeerJ 2023, 11, e15319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Yu, X.R.; Chen, M.J.; Chang, C.F.; Zhu, J.L.; Zhao, H. Comparative transcriptome and metabolome profiling reveal mechanisms of red leaf color fading in Populus × euramericana cv. ‘Zhonghuahongye’. Plants 2023, 12, 3511. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Yue, Y.Z.; Li, H.Y.; Ding, W.J.; Chen, G.W.; Shi, T.T.; Chen, J.H.; Park, M.S.; Chen, F.; Wang, L.G. The chromosome-level quality genome provides insights into the evolution of the biosynthesis genes for aroma compounds of Osmanthus fragrans. Hortic. Res. 2018, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ding, W.J.; Shi, T.T.; Ouyang, Q.X.; Yang, X.L.; Yue, Y.Z.; Wang, L.G. Integrated transcriptome and endogenous hormone analysis provides new insights into callus proliferation in Osmanthus fragrans. Sci. Rep. 2022, 12, 7609. [Google Scholar] [CrossRef]
- Guo, P.; Huang, Z.Q.; Zhao, W.; Lin, N.; Wang, Y.H.; Shang, F.D. Mechanisms for leaf color changes in Osmanthus fragrans ‘Ziyan Gongzhu’ using physiology, transcriptomics and metabolomics. BMC Plant Biol. 2023, 23, 453. [Google Scholar] [CrossRef]
- Zhang, X.Z. Comparative study on determination methods of plant chlorophyll content. J. Shenyang Agr. Univ. 1985, 4, 84–87. [Google Scholar]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Buchfnk, B.; Xie, C.; Huson, H.D. Fast and sensitive protein alignment using diamond. Nat. Methods 2014, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, 121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Guo, J.; Zhou, Q.; Xin, Y.; Wang, G.B.; Xu, L.A. De novo transcriptome analysis revealed genes involved in flavonoid biosynthesis, transport and regulation in Ginkgo biloba. Ind. Crop. Prod. 2018, 124, 226–235. [Google Scholar] [CrossRef]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.W.; Zhang, X.G. DEGseq: An R package for identifying diferentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.X.; Wang, Y.G.; Bao, Z.Y.; Zhao, H.B. Identifcation of suitable reference genes for gene expression normalization in the quantitative real-time PCR analysis of Sweet Osmanthus (Osmanthus fragrans Lour.). PLoS ONE 2015, 10, 1–17. [Google Scholar]
- Cai, W.Q.; Zhang, D.M.; Zhang, X.; Chen, Q.R.; Liu, Y.; Lin, L.; Xiang, L.L.; Yang, Y.J.; Xu, L.; Yu, X.Y.; et al. Leaf color change and photosystem function evaluation under heat treatment revealed the stress resistance variation between Loropetalum chinense and L. chinense var. Rubrum. PeerJ 2023, 11, e14834. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T. A hidden link between leaf development and senescence. Plant Sci. 2018, 276, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Wang, X.X.; Li, J.Y.; Zhu, W.P.; Cui, J.T.; Zhang, K.Z. Regulatory mechanisms of leaf color change in Acer pictum subsp. Mono. Genome 2019, 62, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, L.; Liang, Z.J.; He, X.M.; Liu, W.R.; Jiang, B.; Yan, J.Q.; Sun, P.Y.; Cao, Z.Q.; Peng, Q.W.; et al. Metabolome and transcriptome analyses reveal chlorophyll and anthocyanin metabolism pathway associated with cucumber fruit skin color. BMC Plant Biol. 2020, 20, 386. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhou, Y.; Chen, H.; Chen, C.; Liu, Z.M.; Han, C.; Wu, Q.K.; Yu, F.Y. Transcriptomic analyses reveal key genes involved in pigment biosynthesis related to leaf color change of Liquidambar formosana Hance. Molecules 2022, 27, 5433. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies-challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.x.; Chang, C.; Jiang, P.; Wei, K.; Zhang, R.R.; Jin, Y.H.; Zhao, J.N.; Xu, L.S.; Shi, Y.M.; Guo, S.C.; et al. Metabolomics in rheumatoid arthritis: Advances and review. Front Immunol. 2022, 11, 13:961708. [Google Scholar] [CrossRef]
- Li, Y.K.; Fang, J.B.; Qi, X.J.; Lin, M.M.; Zhong, Y.P.; Sun, L.M.; Cui, W. Combined analysis of the fruit metabolome and transcriptome reveals candidate genes involved in flavonoid biosynthesis in Actinidia arguta. Int. J. Mol. Sci. 2018, 19, 1471. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Zhang, L.T.; Wu, Y.; Zheng, Y.S.; Nie, L.B.; Zhang, S.N.; Lan, T.; Zhao, Y.; Zhu, S.D.; Hou, J.F.; et al. Comparative transcriptome analysis reveals that chlorophyll metabolism contributes to leaf color changes in wucai (Brassica campestris L.) in response to cold. BMC Plant Biol. 2021, 21, 438. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Q.; Shen, Q.Q.; Zhuo, B.P.; He, J.R. Transcriptome analysis reveals genes associated with leaf color mutants in Cymbidium longibracteatum. Tree Genet. Genomes 2020, 16, 44. [Google Scholar]
- Gao, Y.F.; Zhao, D.H.; Zhang, J.Q.; Chen, J.S.; Li, J.L.; Weng, Z.; Rong, L.P. De novo transcriptome sequencing and anthocyanin metabolite analysis reveals leaf color of Acer pseudosieboldianum in autumn. BMC Genom. 2021, 22, 383. [Google Scholar] [CrossRef]
- Wu, Q.B.; Pan, Y.B.; Su, Y.C.; Zou, W.H.; Xu, F.; Sun, T.T.; Grisham, M.P.; Yang, S.L.; Xu, L.P.; Que, Y.X. WGCNA identifies a comprehensive and dynamic gene co-expression network that associates with smut resistance in sugarcane. Int. J. Mol. Sci. 2022, 23, 10770. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Liu, H.L.; Zhang, D.; Zou, D.T.; Wang, J.G.; Zheng, H.L.; Jia, Y.; Qu, Z.J.; Sun, B.; Zhao, H.W. Photosynthetic carbon fixation and sucrose metabolism supplemented by weighted gene co-expression network analysis in response to water stress in rice with overlapping growth stages. Front Plant Sci. 2022, 13, 864605. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yang, H.H.; Li, M.M.; Bai, Y.; Chen, C.; Guo, D.L.; Guo, C.H.; Shu, Y.J. A pan-transcriptome analysis indicates efficient downregulation of the FIB genes plays a critical role in the response of alfalfa to cold stress. Plants 2022, 11, 3148. [Google Scholar] [CrossRef]
- Zhu, L.; Wen, J.; Ma, Q.Y.; Yan, K.Y.; Du, Y.M.; Chen, Z.; Lu, X.Y.; Ren, J.; Wang, Y.L.; Li, S.S.; et al. Transcriptome profiling provides insights into leaf color changes in two Acer palmatum genotypes. BMC Plant Biol. 2022, 22, 589. [Google Scholar] [CrossRef]
- Zhong, S.W.; Dong, B.; Zhou, J.; Miao, Y.F.; Yang, L.Y.; Wang, Y.G.; Xiao, Z.; Fang, Q.; Wan, Q.Q.; Zhao, H.B. Highly efficient transient gene expression of three tissues in Osmanthus fragrans mediated by Agrobacterium tumefaciens. Sci. Hortic. 2023, 310, 111725. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.M.; Qin, P.; Prasad, K.; Hu, Y.X.; Xu, L. The WOX11-LBD16 pathway promotes pluripotency acquisition in callus cells during de novo shoot regeneration in tissue culture. Plant Cell Physiol. 2018, 59, 734–748. [Google Scholar] [CrossRef]
- Xu, C.Y.; Cao, H.F.; Zhang, Q.Q.; Wang, H.Z.; Xin, W.; Xu, E.J.; Zhang, S.Q.; Yu, R.X.; Yu, D.X.; Hu, Y.X. Control of auxin-induced callus formation by bZIP59-LBD complex in Arabidopsis regeneration. Nat. Plants 2018, 4, 108–115. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, J.; Yang, Z.H.; Matsui, A.; Seki, M.; Li, S.B.; Yan, X.Y.; Kohnen, M.V.; Gu, L.F.; Prasad, K.; et al. PtWOX11 acts as master regulator conducting the expression of key transcription factors to induce de novo shoot organogenesis in poplar. Plant Mol. Biol. 2018, 98, 389–406. [Google Scholar] [CrossRef]
- Dai, X.H.; Liu, N.; Wang, L.J.; Li, J.; Zheng, X.J.; Xiang, F.N.; Liu, Z.H. MYB94 and MYB96 additively inhibit callus formation via directly repressing LBD29 expression in Arabidopsis thaliana. Plant Sci. 2020, 293, 110323. [Google Scholar] [CrossRef] [PubMed]


| Stages | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| chlorophyll a | 216.1 ± 13.95 c | 232.8 ± 35.54 c | 306.1 ± 24.8 c | 357.4 ± 52.07 c | 810 ± 55.43 b | 1691.6 ± 198.03 a |
| chlorophyll b | 104.4 ± 7.59 c | 104.4 ± 39.28 c | 121.6 ± 17.96 c | 189.5 ± 48.98 bc | 352.3 ± 21.59 b | 642.5 ± 79.73 a |
| Stages | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| α-carotene | 2.0202 ± 0.22477 b | 2.1009 ± 0.38257 b | 1.8701 ± 0.27284 b | 2.8983 ± 0.45855 b | 8.8319 ± 0.46579 b | 118.6902 ± 4.30442 a |
| ε-carotene | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.3718 ± 0.00919 a |
| phytofluene | 0.8395 ± 0.10317 ab | 0.8021 ± 0.08205 ab | 1.3913 ± 0.02097 a | 0.3415 ± 0.34152 bc | 0.7856 ± 0.12336 ab | 0 ± 0 c |
| β-carotene | 19.2999 ± 0.53642 c | 25.2141 ± 1.53285 bc | 20.7461 ± 2.59892 bc | 35.3598 ± 2.65966 b | 31.4712 ± 2.27726 bc | 144.4781 ± 6.79325 a |
| (E/Z)-phytoene | 0.6935 ± 0.0633 b | 0.7769 ± 0.037 b | 1.7952 ± 0.17079 ab | 1.9186 ± 0.45996 ab | 1.9327 ± 0.28795 ab | 2.5695 ± 0.37205 a |
| β-cryptoxanthin palmitate | 0.074 ± 0.074 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| zeaxanthin | 78.648 ± 13.89694 ab | 136.2735 ± 29.5574 ab | 145.9202 ± 17.13689 a | 144.2718 ± 15.2421 ab | 66.0319 ± 8.80972 b | 112.9632 ± 4.70251 ab |
| violaxanthin | 3.5234 ± 0.34962 bc | 3.5538 ± 0.40363 bc | 2.0529 ± 0.19068 c | 4.0291 ± 0.42941 b | 3.0504 ± 0.37767 bc | 8.796 ± 0.56817 a |
| neoxanthin | 9.0619 ± 0.68292 d | 11.0697 ± 1.9487 d | 11.8445 ± 0.77803 d | 23.8524 ± 0.56168 c | 35.9063 ± 2.81685 b | 57.4593 ± 2.49658 a |
| lutein | 252.6791 ± 12.5571 c | 327.7297 ± 5.14785 c | 303.3848 ± 16.24441 c | 455.4933 ± 14.83089 b | 458.7924 ± 34.21441 b | 871.3145 ± 24.57139 a |
| β-cryptoxanthin | 2.6305 ± 0.69132 b | 3.261 ± 0.65776 b | 3.1777 ± 0.67226 b | 2.9472 ± 0.29273 b | 6.5685 ± 0.43987 a | 3.5697 ± 0.15147 b |
| 8’-apo-beta-carotenal | 0.0208 ± 0.00159 c | 0.0246 ± 0.00227 c | 0.027 ± 0.00337 c | 0.0348 ± 0.00432 bc | 0.0895 ± 0.0053 a | 0.0465 ± 0.00313 b |
| α-cryptoxanthin | 1.8543 ± 0.60653 | 1.382 ± 0.36117 | 0.7002 ± 0.08712 | 0.6937 ± 0.04528 | 0.7997 ± 0.07594 | 1.6454 ± 0.15539 |
| echinenone | 0.0058 ± 0.0007 c | 0.0038 ± 0.00029 c | 0.0079 ± 0.00219 c | 0.009 ± 0.0022 bc | 0.028 ± 0.00183 a | 0.0163 ± 0.00119 b |
| β-citraurin | 0.0153 ± 0.00253 | 0.0196 ± 0.00226 | 0.0205 ± 0.00428 | 0.021 ± 0.0011 | 0.0171 ± 0.0011 | 0.0124 ± 0.00076 |
| antheraxanthin dipalmitate | 0.0124 ± 0.00623 b | 0.0198 ± 0.00159 b | 0.0154 ± 0.00094 b | 0.0153 ± 0.00259 b | 0.0101 ± 0.00124 b | 0.1106 ± 0.01585 a |
| antheraxanthin | 2.4446 ± 0.66069 | 3.4623 ± 0.7224 | 5.7288 ± 1.61515 | 8.1202 ± 3.32925 | 6.6285 ± 0.70874 | 5.1785 ± 0.95917 |
| lutein palmitate | 0.131 ± 0.02524 a | 0.1058 ± 0.02103 a | 0.0661 ± 0.00944 ab | 0.0649 ± 0.02596 ab | 0.0063 ± 0.00631 b | 0.0119 ± 0.01194 b |
| capsorubin | 0.0365 ± 0.03652 b | 0.1045 ± 0.03652 ab | 0.0703 ± 0.03556 ab | 0.1482 ± 0.04598 ab | 0.0719 ± 0.00352 ab | 0.2089 ± 0.01193 a |
| lutein myristate | 0.0483 ± 0.01155 a | 0.0413 ± 0.00481 ab | 0.0204 ± 0.00472 bc | 0.0093 ± 0.00542 c | 0 ± 0 c | 0 ± 0 c |
| β-cryptoxanthin myristate | 0.0036 ± 0.00362 | 0.0035 ± 0.00355 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.0033 ± 0.00326 |
| β-cryptoxanthin oleate | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.0043 ± 0.0043 | 0.0034 ± 0.0034 |
| rubixanthin palmitate | 0.0094 ± 0.00944 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| violaxanthin dilaurate | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.014 ± 0.01403 | 0 ± 0 |
| zeaxanthin dipalmitate | 0.026 ± 0.026 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| violaxanthin dibutyrate | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0039 ± 0.00392 b | 0.0329 ± 0.00403 a |
| Stages | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Cyanidin-3,5-O-diglucoside | 0.4353 ± 0.05688 a | 0.1801 ± 0.0158 b | 0.0968 ± 0.00174 bc | 0.0717 ± 0.00985 bc | 0.0954 ± 0.02975 bc | 0.0325 ± 0.00368 c |
| Cyanidin-3-O-arabinoside | 0.0855 ± 0.0309 a | 0.013 ± 0.00199 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Cyanidin-3-O-rutinoside-5-O-glucoside | 1.445 ± 0.40599 a | 0.5831 ± 0.06172 b | 0.4126 ± 0.02887 b | 0.2424 ± 0.06033 b | 0.1741 ± 0.03911 b | 0 ± 0 b |
| Cyanidin-3-(6″-caffeylsophoroside)-5-glucoside | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0011 ± 0.00056 b | 0.0054 ± 0.00109 a |
| Cyanidin-3-(6-O-p-caffeoyl)-glucoside | 0 ± 0 c | 0.0649 ± 0.00775 b | 0.0887 ± 0.0103 b | 0.1063 ± 0.01142 b | 0.2158 ± 0.01788 a | 0.1945 ± 0.01243 a |
| Cyanidin-3-O-(6-O-malonyl-beta-D-glucoside) | 0.0038 ± 0.00167 | 0 ± 0 | 0 ± 0 | 0.0014 ± 0.00167 | 0 ± 0 | 0 ± 0 |
| Cyanidin-3-O-sophoroside | 0.0319 ± 0.016 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Cyanidin-3-O-xyloside | 0.0098 ± 00491 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Cyanidin-3,5,3′-O-triglucoside | 0.0222 ± 0.00307 bc | 0.048 ± 0.00995 ab | 0.0879 ± 0.00865 a | 0.0485 ± 0.01621 ab | 0 ± 0 c | 0 ± 0 c |
| Cyanidin-3-O-sambubioside | 0.0385 ± 0.01258 a | 0.0122 ± 0.0016 b | 0.005 ± 0.00065 b | 0.0034 ± 0.00088 b | 0.0018 ± 0.00022 b | 0.0018 ± 0.00017 b |
| Cyanidin-3-O-rutinoside | 20.2637 ± 5.28889 a | 9.0883 ± 0.79503 b | 5.0819 ± 0.14054 b | 2.2312 ± 0.72849 b | 0.958 ± 0.36382 b | 0.2357 ± 0.04616 b |
| Cyanidin-3-O-glucoside | 2.8567 ± 0.89241 a | 0.954 ± 0.10062 b | 0.4329 ± 0.0179 5 b | 0.2998 ± 0.04351 b | 0.5354 ± 0.1856 b | 0.2095 ± 0.02935 b |
| Delphinidin-3-O-5-O-(6-O-coumaroyl)-diglucoside | 0.0142 ± 0.00454 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Delphinidin-3-O-(6-O-acetyl)-glucoside | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0367 ± 0.00694 a |
| Delphinidin-3-O-(6-O-malonyl-beta-D-glucoside) | 0 ± 0 b | 0.0038 ± 0.00193 b | 0.0029 ± 0.0029 b | 0.0017 ± 0.00167 b | 0.0064 ± 0.0032 b | 0.024 ± 0.00289 a |
| Delphinidin-3,5-O-diglucoside | 0.0001 ± 0.00008 c | 0.0018 ± 0.00015 a | 0.0013 ± 0.00018 a | 0.002 ± 0.00022 ab | 0 ± 0 c | 0.0007 ± 0.00044 bc |
| Delphinidin-3-O-sambubioside | 0.0113 ± 0.00136 bc | 0.0175 ± 0.00123 a | 0.0189 ± 0.0007 a | 0.0161 ± 0.002 ab | 0.0092 ± 0.00116 cd | 0.0034 ± 0.0004 d |
| Delphinidin-3-O-rutinoside | 0.0046 ± 0.00459 b | 0.0212 ± 0.00155 a | 0.0076 ± 0.0038 ab | 0.0057 ± 0.00291 b | 0.0052 ± 0.0026 b | 0.0082 ± 0.0008 ab |
| Delphinidin-3-O-arabinoside | 0.0068 ± 0.00161 d | 0.0081 ± 0.00044 d | 0.0099 ± 0.00123 cd | 0.0147 ± 0.00068 c | 0.0217 ± 0.00095 b | 0.0318 ± 0.00196 a |
| Delphinidin-3-O-galactoside | 0.0148 ± 0.00054 b | 0.0117 ± 0.00091 b | 0.0133 ± 0.00027 b | 0.0117 ± 0.00207 b | 0.0164 ± 0.00101 b | 0.0249 ± 0.002 a |
| Delphinidin-3-O-rutinoside-5-O-glucoside | 0.027 ± 0.00197 ab | 0.0343 ± 0.00111 a | 0.0294 ± 0.00128 ab | 0.0232 ± 0.00281 b | 0.021 ± 0.00139 b | 0.0245 ± 0.00217 b |
| Malvidin-3,5-O-diglucoside | 0.0145 ± 0.00034 a | 0.0136 ± 0.00047 a | 0.0134 ± 0.00043 a | 0 ± 0 b | 0.0085 ± 0.00428 ab | 0.008 ± 0.00398 ab |
| Malvidin-3-O-glucoside | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0118 ± 0.00146 a |
| Malvidin-3-O-galactoside | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0036 ± 0.00032 a |
| Pelargonidin-3-O-(6-O-malonyl-beta-D-glucoside) | 0.0065 ± 0.0064 7 b | 0.0333 ± 0.00293 a | 0.0213 ± 0.01075 ab | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Pelargonidin-3-O-glucoside | 0.0175 ± 0.00877 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Pelargonidin-3-O-arabinoside | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0133 ± 0.00094 a |
| Pelargonidin-3,5-O-diglucoside | 0.6045 ± 0.08294 bc | 0.8783 ± 0.00316 a | 1.0345 ± 0.02331 a | 0.7947 ± 0.08205 ab | 0.4928 ± 0.06224 c | 0.1638 ± 0.01162 d |
| Pelargonidin-3-O-(6-O-p-coumaroyl)-glucoside | 127.1063 ± 6.39837 b | 153.5045 ± 0.44048 a | 166.2193 ± 1.41302 a | 163.8305 ± 2.41484 a | 150.9447 ± 2.57229 a | 151.7783 ± 3.73283 a |
| Pelargonidin-3-O-rutinoside | 13.4871 ± 3.76768 a | 4.0461 ± 0.51719 b | 2.4218 ± 0.22746 b | 1.2958 ± 0.44617 b | 0.3847 ± 0.07238 b | 0.084 ± 0.00697 b |
| Peonidin-3-O-(6-O-p-coumaroyl)-glucoside | 0.3092 ± 0.04391 d | 0.699 ± 0.05671 cd | 2.4569 ± 0.33936 cd | 4.0014 ± 0.52503 c | 9.7389 ± 0.44988 b | 29.25 ± 1.6666 a |
| Peonidin-3-O-5-O-(6-O-coumaroyl)-diglucoside | 0 ± 0 c | 0 ± 0 c | 0.003 ± 0.00088 c | 0.0019 ± 0.00028 c | 0.0241 ± 0.00447 b | 0.1458 ± 0.00333 a |
| Peonidin-3,5-O-diglucoside | 0 ± 0 c | 0 ± 0 c | 0.0163 ± 0.00815 bc | 0.0376 ± 0.00406 a | 0.0315 ± 0.00334 ab | 0 ± 0 c |
| Peonidin-3-O-sophoroside | 0.0073 ± 0.00438 ab | 0.0154 ± 0.00061 a | 0.0043 ± 0.00425 ab | 0.0026 ± 0.00259 ab | 0 ± 0 b | 0 ± 0 b |
| Peonidin-3-O-glucoside | 0.0063 ± 0.00252 bc | 0.0016 ± 0.00077 c | 0.0099 ± 0.00298 abc | 0.04 ± 0.01459 ab | 0.0449 ± 0.0108 a | 0 ± 0 c |
| Peonidin | 0 ± 0 | 0.0037 ± 0.00189 | 0.0033 ± 0.00335 | 0.0046 ± 0.0025 | 0.0026 ± 0.00262 | 0 ± 0 |
| Peonidin-3-O-rutinoside | 6.5679 ± 1.95878 a | 3.5425 ± 0.49228 ab | 2.8087 ± 0.1773 ab | 1.9558 ± 0.48003 b | 1.0596 ± 0.17776 b | 0.1688 ± 0.0143 b |
| Peonidin-3-O-galactoside | 0.0297 ± 0.0037 8 b | 0.0489 ± 0.00458 b | 0.0558 ± 0.00145 b | 0.0383 ± 0.0055 b | 0.0251 ± 0.0055 b | 0.1084 ± 0.01434 a |
| Petunidin-3,5-O-diglucoside | 0.1003 ± 0.01161 a | 0.0629 ± 0.00357 b | 0.0305 ± 0.00901 c | 0.0106 ± 0.00901 cd | 0.0059 ± 0.00901 cd | 0 ± 0 d |
| Petunidin-3-O-rutinoside | 0.0256 ± 0.00328 ab | 0.033 ± 0.00328 a | 0.0167 ± 0.00322 b | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c |
| Petunidin-3-O-(6-O-malonyl-beta-D-glucoside) | 0.0188 ± 0.0014 cd | 0.0255 ± 0.00087 bc | 0.0329 ± 0.00145 ab | 0.0362 ± 0.00109 a | 0.0338 ± 0.00376 ab | 0.0099 ± 0.00187 d |
| Petunidin-3-O-arabinoside | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0.0195 ± 0.00366 c | 0.0417 ± 0.00258 b | 0.1008 ± 0.00655 a |
| Procyanidin A1 | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.0118 ± 0.00066 a |
| Procyanidin B3 | 0.0294 ± 0.00109 bc | 0.0269 ± 0.00241 c | 0.0329 ± 0.00168 abc | 0.0352 ± 0.0018 abc | 0.0368 ± 0.00254 ab | 0.0403 ± 0.00251 a |
| Procyanidin B2 | 0 ± 0 | 0.0097 ± 0.00966 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Rutin | 756.2563 ± 64.21963 a | 882.8901 ± 34.55686 a | 697.5998 ± 27.95971 ab | 465.7452 ± 48.99165 bc | 434.002 ± 81.47855 c | 343.7775 ± 35.91914 c |
| Kaempferol-3-O-rutinoside | 223.141 ± 9.15061 bc | 334.9178 ± 24.16805 a | 298.4348 ± 19.96219 ab | 199.6224 ± 19.96219 c | 154.9266 ± 33.73202 cd | 104.0808 ± 6.73921 d |
| Naringenin-7-O-glucoside | 3.86060 ± 0.51811 a | 4.1340 ± 0.29852 a | 3.60640 ± 0.33668 ab | 2.28420 ± 0.23061 bc | 0.96270 ± 0.18936 cd | 0 ± 0 d |
| Dihydrokaempferol | 0.0833 ± 0.00766 a | 0.0496 ± 0.00684 b | 0.0303 ± 0.00976 bc | 0.0153 ± 0.00144 cd | 0.0059 ± 0.00591 cd | 0 ± 0 d |
| Quercetin-3-O-glucoside | 381.4945 ± 42.41605 a | 425.5015 ± 17.93373 a | 320.1362 ± 7.69342 ab | 211.1826 ± 22.08354 bc | 198.8025 ± 31.03374 c | 174.3214 ± 12.57217 c |
| Naringenin | 0.3095 ± 0.00357 a | 0.3234 ± 0.01599 a | 0.296 ± 0.00785 ab | 0.2459 ± 0.01469 b | 0.1831 ± 0.01575 c | 0.0987 ± 0.00329 d |
| Sample | Raw Reads | Clean Reads | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|
| S1-1 | 8,696,665,200 | 8,299,815,303 | 44.45 | 96.96 | 91.76 |
| S1-2 | 7,528,551,300 | 7,280,491,759 | 44.90 | 96.41 | 90.60 |
| S1-3 | 6,741,765,000 | 6,594,652,421 | 43.23 | 96.18 | 90.09 |
| S2-1 | 6,712,422,900 | 6,565,193,547 | 44.28 | 97.82 | 93.61 |
| S2-2 | 6,504,189,900 | 6,384,983,903 | 43.51 | 97.76 | 93.06 |
| S2-3 | 12,265,493,400 | 10,910,017,551 | 47.33 | 97.55 | 93.45 |
| S3-1 | 10,777,563,900 | 10,047,579,955 | 45.47 | 97.34 | 92.81 |
| S3-2 | 8,863,207,500 | 8,408,586,068 | 44.67 | 97.29 | 92.65 |
| S3-3 | 7,874,278,800 | 7,724,751,444 | 43.48 | 97.50 | 92.66 |
| S4-1 | 9,042,206,100 | 8,493,640,356 | 44.89 | 97.48 | 93.05 |
| S4-2 | 14,078,341,800 | 13,777,599,646 | 43.69 | 97.44 | 92.70 |
| S4-3 | 11,819,290,500 | 11,327,978,014 | 43.93 | 97.16 | 92.12 |
| S5-1 | 10,417,861,800 | 9,506,739,383 | 47.13 | 97.78 | 93.79 |
| S5-2 | 7,561,083,900 | 7,326,157,819 | 44.75 | 97.68 | 93.29 |
| S5-3 | 8,346,830,700 | 7,997,110,370 | 44.37 | 97.48 | 92.87 |
| S6-1 | 7,765,364,700 | 7,681,276,006 | 44.24 | 97.54 | 92.78 |
| S6-2 | 7,433,418,600 | 7,202,084,857 | 43.16 | 96.62 | 91.02 |
| S6-3 | 10,267,528,200 | 9,962,439,176 | 43.35 | 97.79 | 93.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Qiu, H.; Wang, R.; Wang, L.; Yang, X. Integrated Metabolome and Transcriptome Analyses Provide New Insights into the Leaf Color Changes in Osmanthus fragrans cv. ‘Wucaigui’. Forests 2024, 15, 709. https://doi.org/10.3390/f15040709
Zhang S, Qiu H, Wang R, Wang L, Yang X. Integrated Metabolome and Transcriptome Analyses Provide New Insights into the Leaf Color Changes in Osmanthus fragrans cv. ‘Wucaigui’. Forests. 2024; 15(4):709. https://doi.org/10.3390/f15040709
Chicago/Turabian StyleZhang, Songyue, Hanruo Qiu, Rui Wang, Lianggui Wang, and Xiulian Yang. 2024. "Integrated Metabolome and Transcriptome Analyses Provide New Insights into the Leaf Color Changes in Osmanthus fragrans cv. ‘Wucaigui’" Forests 15, no. 4: 709. https://doi.org/10.3390/f15040709
APA StyleZhang, S., Qiu, H., Wang, R., Wang, L., & Yang, X. (2024). Integrated Metabolome and Transcriptome Analyses Provide New Insights into the Leaf Color Changes in Osmanthus fragrans cv. ‘Wucaigui’. Forests, 15(4), 709. https://doi.org/10.3390/f15040709





