Abundance and Species Richness of Lianas in a Karst Seasonal Rainforest: The Influence of Abiotic and Biotic Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plot Establishment and Data Collection
2.3. Data Analysis
3. Results
3.1. Diversity Estimates and Liana Species–Area Accumulation Curves
3.2. Liana Species–Area Patterns
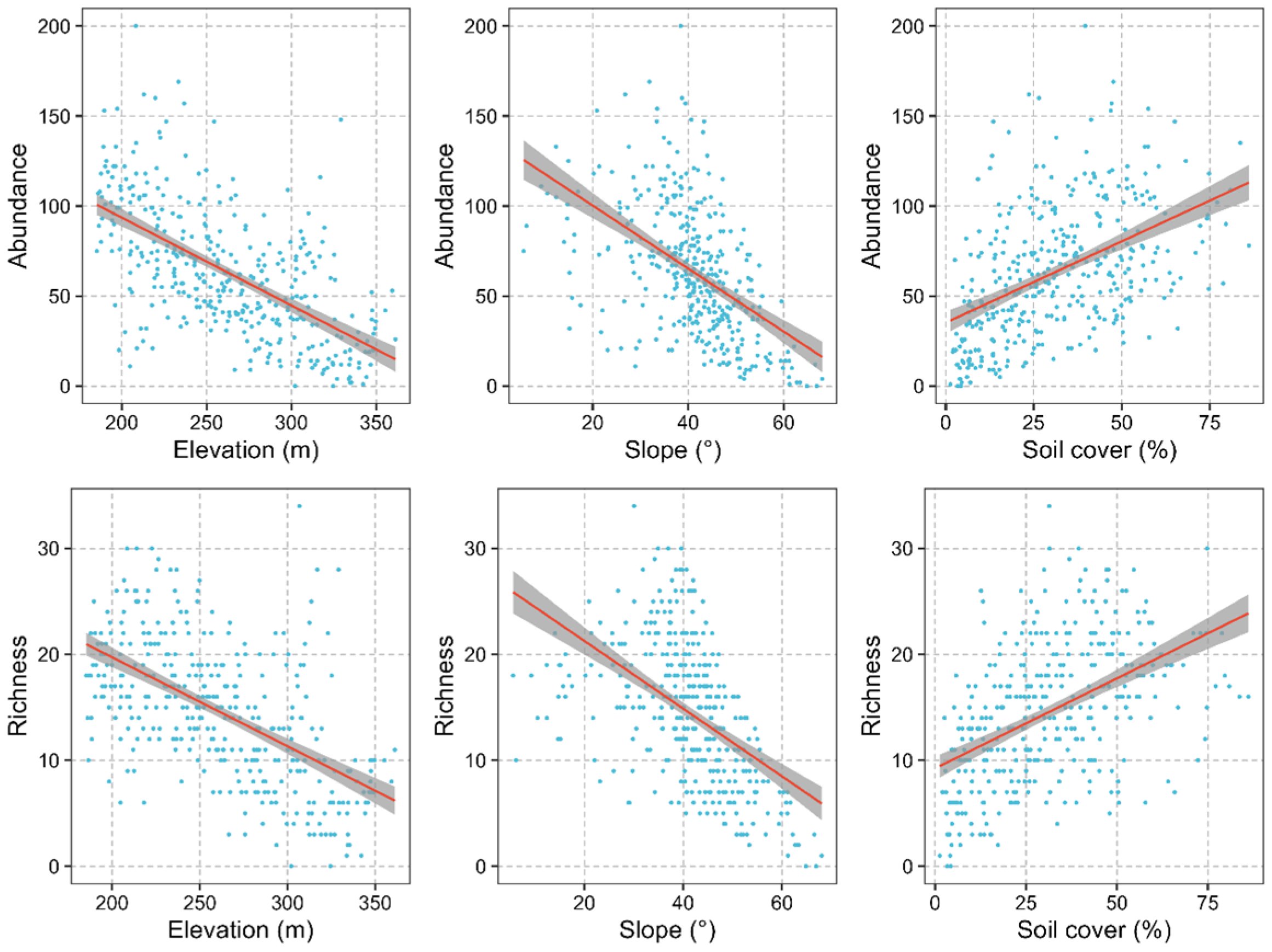
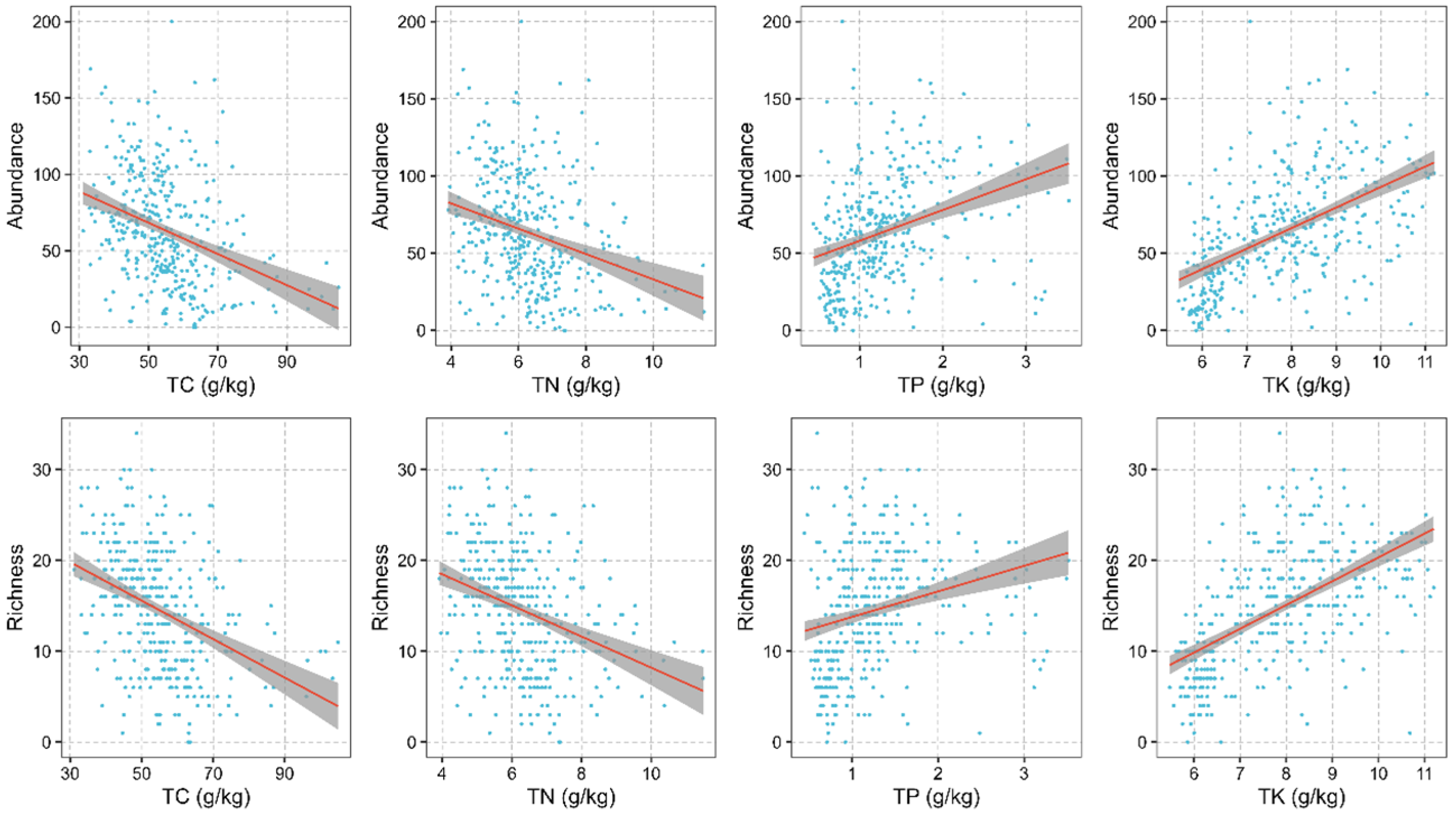
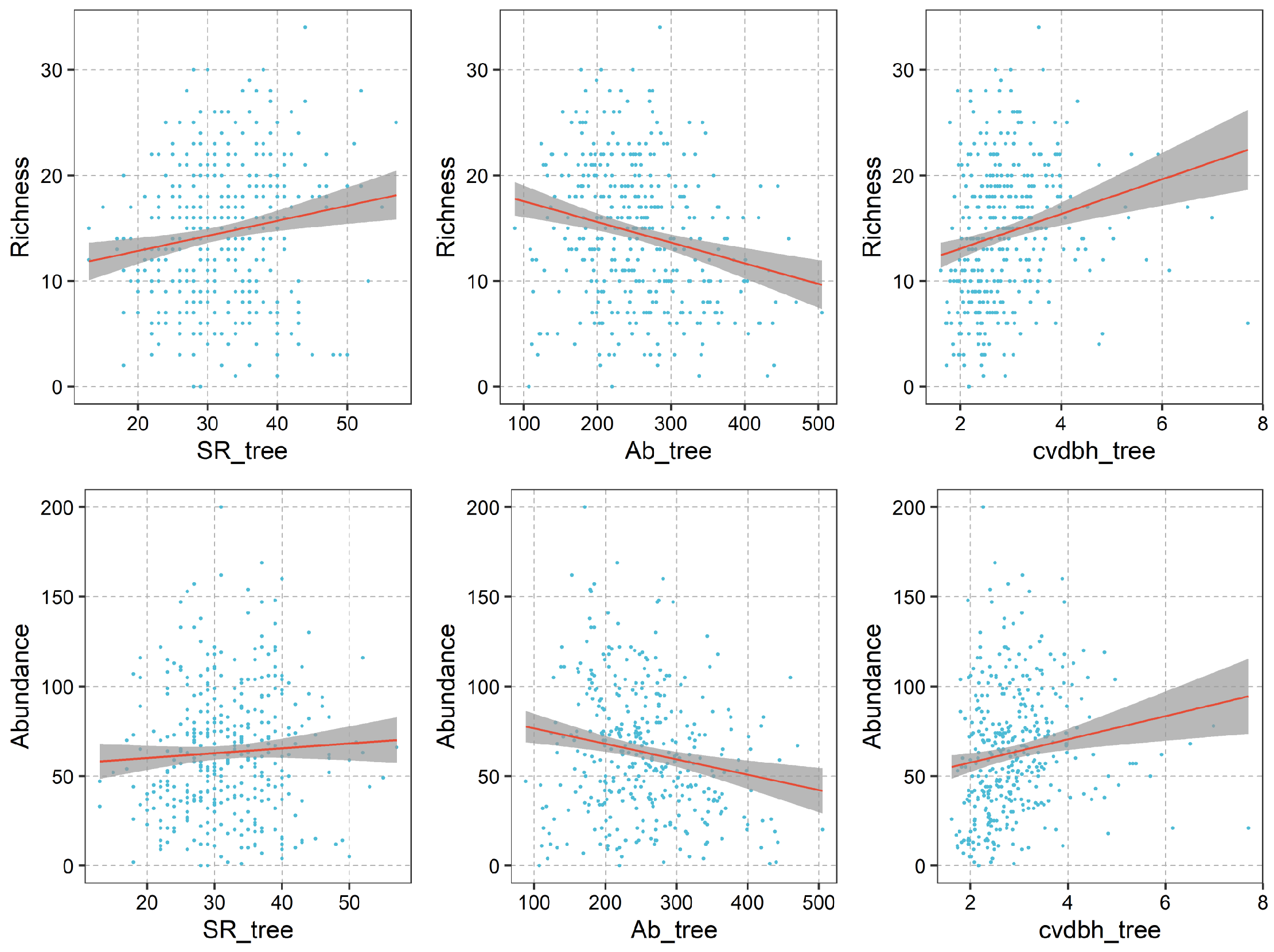
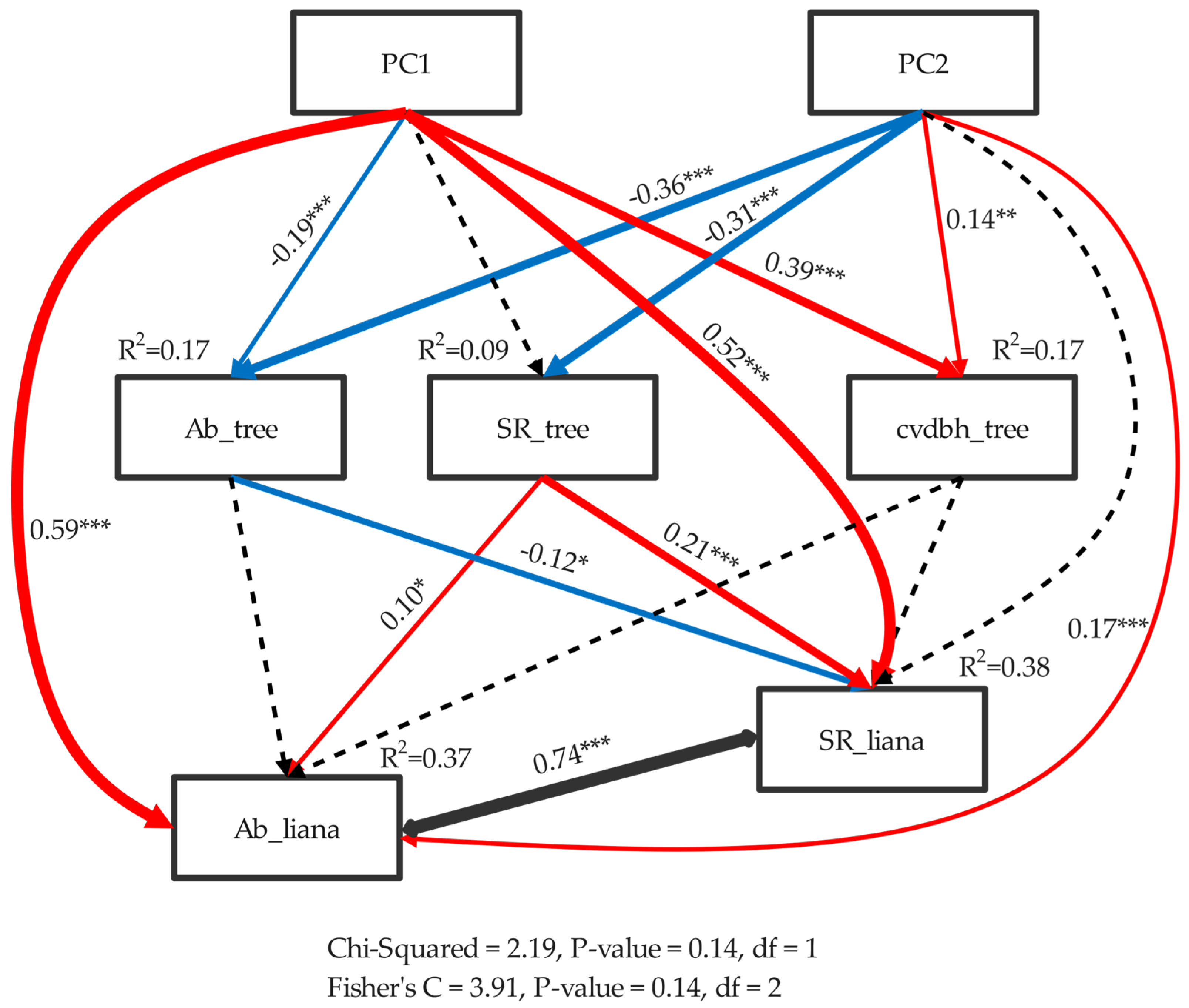
3.3. Abiotic and Biotic Drivers of Liana Diversity across the NG 15 ha Plot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
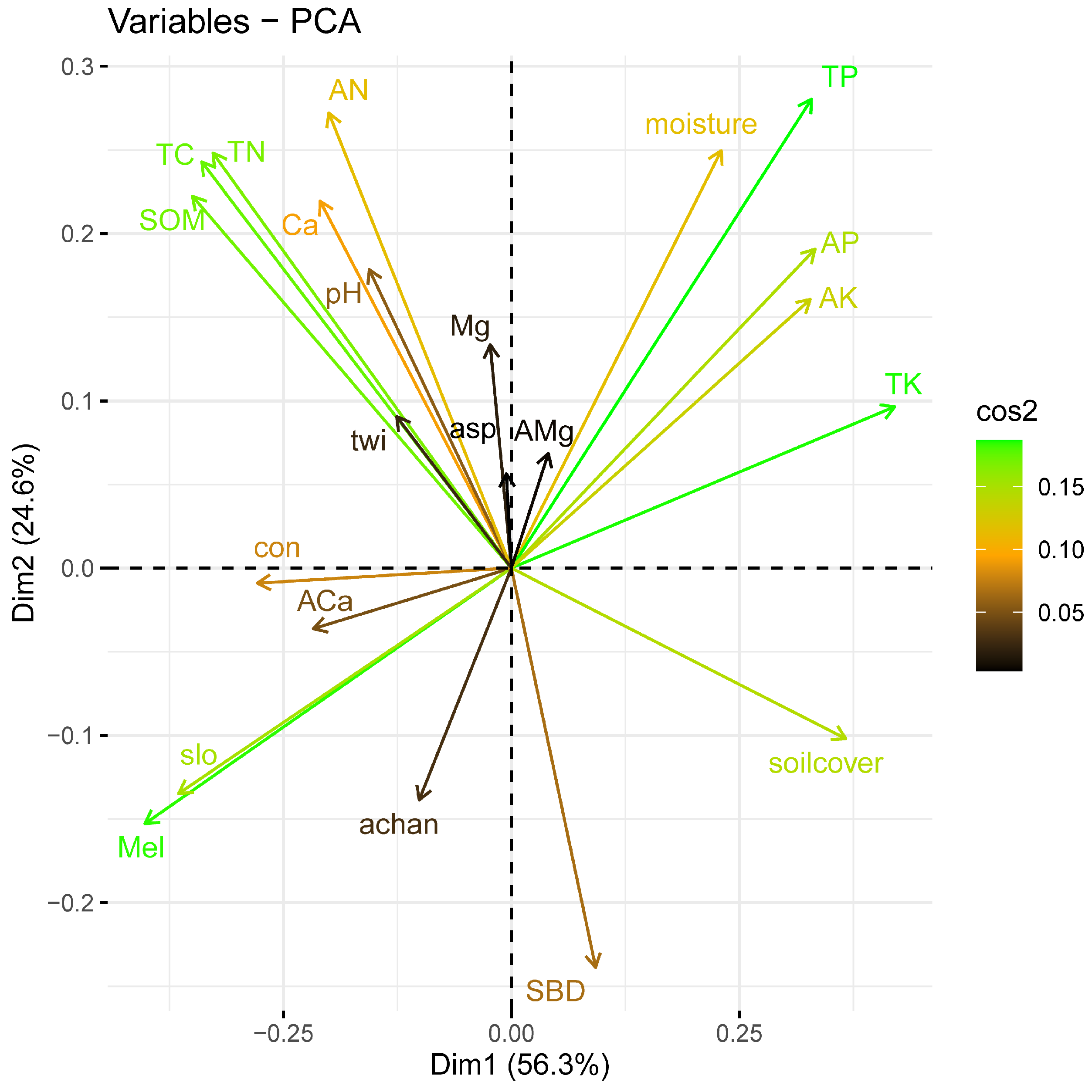
| Abiotic Factor | Comp. 1 | Comp. 2 |
|---|---|---|
| TK | 0.31 | 0.12 |
| TP | 0.26 | 0.34 |
| TN | −0.26 | 0.30 |
| TC | −0.27 | 0.29 |
| SOM | −0.28 | 0.27 |
| Mel | −0.32 | −0.18 |
| soilcover | 0.29 | −0.12 |
| AP | 0.26 | 0.23 |
| AK | 0.26 | 0.19 |
| moisture | 0.18 | 0.30 |
| SBD | 0.07 | −0.29 |
| AMg | 0.03 | 0.08 |
| asp | 0.00 | 0.07 |
| Mg | −0.02 | 0.16 |
| achan | −0.08 | −0.17 |
| twi | −0.10 | 0.11 |
| pH | −0.12 | 0.21 |
| AN | −0.16 | 0.33 |
| Ca | −0.17 | 0.26 |
| ACa | −0.17 | −0.04 |
| con | −0.22 | −0.01 |
| slo | −0.29 | −0.16 |
References
- Gentry, A. The distribution and evolution of climbing plants. In The Biology of Vines; Mooney, H.A., Putz, F.E., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 3–50. [Google Scholar]
- Schnitzer, S.; Bongers, F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef]
- Schnitzer, S.; Mangan, S.; Dalling, J.; Baldeck, C.; Hubbell, S.; Ledo, A.; Muller-Landau, H.; Tobin, M.; Aguilar, S.; Brassfield, D.; et al. Liana Abundance, Diversity, and Distribution on Barro Colorado Island, Panama. PLoS ONE 2012, 7, e52114. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S. The Contribution of Lianas to Forest Ecology, Diversity, and Dynamics. In Biodiversity of Lianas; Parthasarathy, N., Ed.; Springer: Cham, Switerland, 2015; Volume 5, pp. 149–160. [Google Scholar]
- Schnitzer, S. Testing ecological theory with lianas. New Phytol. 2018, 220, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cansino, L.; Schnitzer, S.A.; Reid, J.P.; Powers, J.S.J.E. Liana competition with tropical trees varies seasonally but not with tree species identity. Ecology 2015, 96, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- García León, M.M.; Martínez Izquierdo, L.; Mello, F.N.A.; Powers, J.S.; Schnitzer, S.A. Lianas reduce community-level canopy tree reproduction in a Panamanian forest. J. Ecol. 2018, 106, 737–745. [Google Scholar] [CrossRef]
- van der Heijden, G.M.F.; Schnitzer, S.A.; Meunier, F. Editorial: Lianas, ecosystems, and global change. Front. For. Glob. Chang. 2023, 6, 1079620. [Google Scholar] [CrossRef]
- Estrada-Villegas, S.; Narvaez, S.; Sanchez, A.; Schnitzer, S. Lianas Significantly Reduce Tree Performance and Biomass Accumulation across Tropical Forests: A Global Meta-Analysis. Front. For. Glob. Chang. 2022, 4, 812066. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; van der Heijden, G.; Mascaro, J.; Carson, W.P. Lianas in gaps reduce carbon accumulation in a tropical forest. Ecology 2014, 95, 3008–3017. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Visser, M.D. How do lianas and vines influence competitive differences and niche differences among tree species? Concepts and a case study in a tropical forest. J. Ecol. 2019, 107, 1469–1481. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L. Lianas and self-supporting plants during tropical forest succession. For. Ecol. Manag. 2009, 257, 2150–2156. [Google Scholar] [CrossRef]
- Kusumoto, B.; Enoki, T.; Watanabe, Y. Community structure and topographic distribution of lianas in a watershed on Okinawa, south-western Japan. J. Trop. Ecol. 2008, 24, 675–683. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Zakaria, R. Environmental factors associated with liana community assemblages in a tropical forest reserve, Ghana. J. Trop. Ecol. 2015, 31, 69–79. [Google Scholar] [CrossRef]
- Rahmad, Z.; Johari, M.; Addo-Fordjour, P. Local environmental factors shape liana community structure along an elevation gradient in a tropical rainforest. Écoscience 2021, 29, 1–13. [Google Scholar] [CrossRef]
- Fadrique, B.; Homeier, J. Elevation and topography influence community structure, biomass and host tree interactions of lianas in tropical montane forests of southern Ecuador. J. Veg. Sci. 2016, 27, 958–968. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Zakaria, R.; Anuar, S. Environmental factors influencing liana community diversity, structure and habitat associations in a tropical hill forest, Malaysia. Plant Ecol. Divers. 2014, 7, 485–496. [Google Scholar] [CrossRef]
- Bruy, D.; Ibanez, T.; Munzinger, J.; Isnard, S. Abundance, richness and composition of lianas in forest communities along an elevation gradient in New Caledonia. Plant Ecol. Divers. 2018, 10, 469–481. [Google Scholar] [CrossRef]
- Gerolamo, C.S.; Nogueira, A.; Capellotto Costa, F.R.; de Castilho, C.V.; Angyalossy, V. Local dynamic variation of lianas along topography maintains unchanging abundance at the landscape scale in central Amazonia. J. Veg. Sci. 2018, 29, 651–661. [Google Scholar] [CrossRef]
- Dalling, J.; Schnitzer, S.; Baldeck, C.; Harms, K.; John Chandran, R.; Mangan, S.; Lobo, E.; Yavitt, J.; Hubbell, S. Resource-based habitat associations in a neotropical liana community. J. Ecol. 2012, 100, 1174–1182. [Google Scholar] [CrossRef]
- Malizia, A.; Grau, H.R.; Lichstein, J.W. Soil phosphorus and disturbance influence liana communities in a subtropical montane forest. J. Veg. Sci. 2010, 21, 551–560. [Google Scholar] [CrossRef]
- Liu, Q.; Sterck, F.J.; Medina-Vega, J.A.; Sha, L.-Q.; Cao, M.; Bongers, F.; Zhang, J.-L.; Poorter, L. Soil nutrients, canopy gaps and topography affect liana distribution in a tropical seasonal rain forest in southwestern China. J. Veg. Sci. 2021, 32, e12951. [Google Scholar] [CrossRef]
- Leicht-Young, S.A.; Pavlovic, N.B.; Frohnapple, K.J.; Grundel, R. Liana habitat and host preferences in northern temperate forests. For. Ecol. Manag. 2010, 260, 1467–1477. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Luan, F.; Yuan, Z.; Ali, A.; Chu, C.; Bin, Y. Habitat Conditions and Tree Species Shape Liana Distribution in a Subtropical Forest. Forests 2022, 13, 1358. [Google Scholar] [CrossRef]
- Bai, X.L.; Liu, Q.; Mohandass, D.; Cao, M.; Wen, H.D.; Chen, Y.J.; Gupta, S.K.; Lin, L.X.; Zhang, J.L. Spatial autocorrelation shapes liana distribution better than topography and host tree properties in a subtropical evergreen broadleaved forest in SW China. Biotropica 2022, 54, 301–308. [Google Scholar] [CrossRef]
- Nakada, I.; Uehara, I.; Mori, H. More lianas on larger host trees on steep slopes in a secondary temperate forest, Japan. Plant Ecol. 2024, 19, 1–15. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.M.; Li, X.K. The types of natural vegetation in karst region of Guangxi and its classified system. Guihaia 2003, 23, 289–293. [Google Scholar]
- Guo, Y.; Wang, B.; Li, D.; Mallik, A.; Xiang, W.; Ding, T.; Wen, S.; Lu, S.; Huang, F.; He, Y.; et al. Effects of topography and spatial processes on structuring tree species composition in a diverse heterogeneous tropical karst seasonal rainforest. Flora 2017, 231, 21–28. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Frank, E., Zachos, J.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Wang, B.; Ding, T.; Liu, S.; Peng, D.; Li, D.; Lu, M.; Nong, Z.; Nong, Z.; Nong, S.; Guo, Y.; et al. Layer structure and species composition of primeval habitat forest communities that include the tallest tree in karst areas of China. Chin. Sci. Bull. 2024, 69, 912–924. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Xiang, W.; Ding, T.; Lu, S.; Huang, F.; Li, D.; Wen, S.; He, Y.; Li, X. Density-dependent effects of tree species in a 15 ha seasonal rain forest plot in northern tropical karst in Nonggang, Guangxi, southern China. Chin. Sci. Bull. 2015, 60, 1602–1611. [Google Scholar]
- Su, Z.M.; Zhao, T.L.; Huang, Q.C. Vegetation survey report of Nonggang Nature Reserve. Guihaia S1 1988, 12, 185–214. (In Chinese) [Google Scholar]
- Su, Z.M.; Li, X.K.; Ding, T.; Ning, S.J.; Chen, W.L.; Mo, X.L. The Vegetation of Guangxi; China Forestry Publishing House: Beiing, China, 2014. (In Chinese) [Google Scholar]
- Condit, R.S. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Schnitzer, S.A.; Rutishauser, S.; Aguilar, S. Supplemental protocol for liana censuses. For. Ecol. Manag. 2008, 255, 1044–1049. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Carson, W.P. Lianas suppress tree regeneration and diversity in treefall gaps. Ecol. Lett. 2010, 13, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Gerwing, J.J.; Schnitzer, S.A.; Burnham, R.J.; Bongers, F.; Chave, J.; DeWalt, S.J.; Ewango, C.E.N.; Foster, R.; Kenfack, D.; Martínez-Ramos, M.; et al. A Standard Protocol for Liana Censuses. Biotropica 2006, 38, 256–261. [Google Scholar] [CrossRef]
- Harms, K.; Condit, R.; Hubbell, S.; Foster, R. Habitat associations of trees and shrubs in a 50-ha Neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.; Gorky, V.; Condit, R.; Svenning, J.-C.; Hernandez, C.; Romoleroux, K.; Losos, E.; Magard, E.; Balslev, H. Tree Species Distributions and Local Habitat Variation in the Amazon: Large Forest Plot in Eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Parthasarathy, N.; Sankaralingam, M.; Reddy, M. Patterns of liana diversity in tropical evergreen forests of peninsular India. For. Ecol. Manag. 2004, 190, 15–31. [Google Scholar] [CrossRef]
- Ewango, C.; Bongers, F.; Makana, J.-R.; Poorter, L.; Sosef, M. Structure and composition of the liana assemblage of a mixed rain forest in the Congo Basin. Plant Ecol. Evol. 2015, 148, 29–42. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Li, X.; Xiang, W.; Ding, T.; Huang, F.; Lu, S.; Han, W.; Wen, S.; He, L. Species composition and spatial distribution of a 15 ha northern tropical karst seasonal rain forest dynamics study plot in Nonggang, Guangxi, southern China. Biodivers. Sci. 2014, 22, 141–156. [Google Scholar]
- Schnitzer, S. A Mechanistic Explanation for Global Patterns of Liana Abundance and Distribution. Am. Nat. 2005, 166, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, B. Liana diversity and abundance of a tropical montane rainforest in Mengsong, Southern Yunnan, China. Guihaia 2008, 28, 67–72. [Google Scholar]
- Liu, Q.; Huaidong, W.; Yunhong, T.; Jiaolin, Z. Liana Diversity and its Climbing Situation on Trees in Xishuangbanna Tropical Seasonal Rainforest. Sci. Silvae Sin. 2017, 53, 1–8. [Google Scholar]
- Guo, Y.; Wang, B.; Xiang, W.; Ding, T.; Lu, S.; Huang, F.; Wen, S.; Li, D.; He, Y.; Li, X. Responses of spatial pattern of woody plants’ basal area to topographic factors in a tropical karst seasonal rainforest in Nonggang, Guangxi, southern China. Biodivers. Sci. 2016, 24, 30–39. [Google Scholar] [CrossRef][Green Version]
- Schnitzer, S.; Bongers, F.; Burnham, R.J.; Putz, F.E. Ecology of Lianas; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]

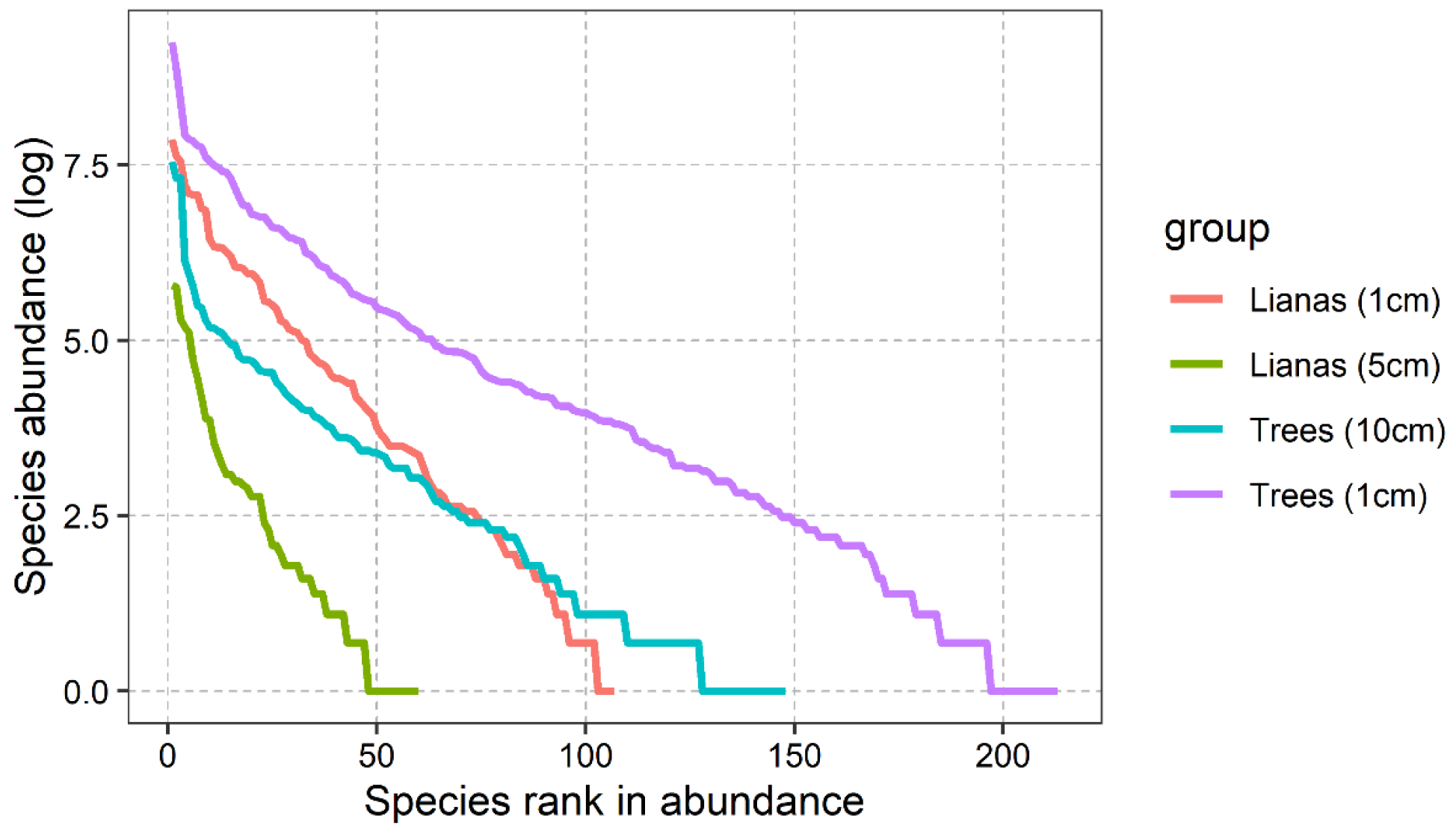

| Diameter Class (cm) | Lianas | Trees | ||||
|---|---|---|---|---|---|---|
| Size Class. Freq | Mean dbh | Total Basal Area (m2) | Size Class. Freq | Mean dbh | Total Basal Area (m2) | |
| 1 | 23,819 | 2.55 | 16.48 | 74,758 | 5.37 | 365.11 |
| 2 | 14,165 | 3.36 | 15.01 | 55,800 | 6.67 | 361.6 |
| 5 | 1950 | 6.27 | 6.42 | 24,557 | 11.24 | 336.54 |
| 10 | 88 | 11.54 | 0.95 | 10,679 | 16.79 | 281.34 |
| 20 | 0 | 0.00 | 0.00 | 2435 | 27.48 | 156.33 |
| No. | Family | Genus | Species | Stem Density | Total BA (cm2 15 ha−1) | Mean dbh (cm) | rel.den (%) | rel.dom (%) | rel.fre (%) | IV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Annonaceae | Artabotrys | hongkongensis | 2069 | 22,090.43 | 3.33 | 8.69 | 13.41 | 4.66 | 8.92 |
| 2 | Annonaceae | Uvaria | tonkinensis | 2612 | 11,780.14 | 2.16 | 10.97 | 7.15 | 4.94 | 7.69 |
| 3 | Fabaceae | Bauhinia | championii | 1190 | 18,925.14 | 3.89 | 5.00 | 11.49 | 4.85 | 7.11 |
| 4 | Vitaceae | Tetrastigma | kwangsiense | 1366 | 13,108.88 | 3.04 | 5.74 | 7.96 | 3.18 | 5.62 |
| 5 | Apocynaceae | Urceola | rosea | 1183 | 11,936.32 | 3.17 | 4.97 | 7.25 | 3.21 | 5.14 |
| 6 | Araceae | Rhaphidophora | hongkongensis | 1897 | 3212.40 | 1.41 | 7.96 | 1.95 | 4.42 | 4.78 |
| 7 | Vitaceae | Tetrastigma | planicaule | 977 | 11,223.13 | 3.30 | 4.10 | 6.81 | 2.29 | 4.40 |
| 8 | Apocynaceae | Trachelospermum | jasminoides | 1218 | 3715.14 | 1.79 | 5.11 | 2.26 | 4.78 | 4.05 |
| 9 | Erythropalaceae | Erythropalum | scandens | 955 | 8295.60 | 3.01 | 4.01 | 5.04 | 2.53 | 3.86 |
| 10 | Moraceae | Malaisia | scandens | 629 | 1407.89 | 1.40 | 2.64 | 0.86 | 3.45 | 2.31 |
| 11 | Rutaceae | Zanthoxylum | dissitum | 561 | 2386.07 | 2.14 | 2.36 | 1.45 | 3.05 | 2.28 |
| 12 | Apocynaceae | Secamone | elliptica | 422 | 4159.16 | 3.10 | 1.77 | 2.52 | 2.38 | 2.23 |
| 13 | Lamiaceae | Premna | fulva | 368 | 3658.29 | 3.35 | 1.55 | 2.22 | 2.87 | 2.21 |
| 14 | Rubiaceae | Uncaria | hirsuta | 556 | 4425.77 | 2.97 | 2.33 | 2.69 | 1.34 | 2.12 |
| 15 | Gnetaceae | Gnetum | montanum | 571 | 3103.59 | 2.41 | 2.40 | 1.88 | 1.91 | 2.07 |
| 16 | Rubiaceae | Uncaria | rhynchophylla | 527 | 3478.81 | 2.73 | 2.21 | 2.11 | 1.71 | 2.01 |
| 17 | Icacinaceae | Iodes | vitiginea | 393 | 1964.34 | 2.32 | 1.65 | 1.19 | 2.67 | 1.84 |
| 18 | Malpighiaceae | Aspidopterys | concava | 417 | 2029.58 | 2.30 | 1.75 | 1.23 | 2.15 | 1.71 |
| 19 | Primulaceae | Embelia | undulata | 423 | 2443.29 | 2.48 | 1.78 | 1.48 | 1.73 | 1.66 |
| 20 | Convolvulaceae | Argyreia | capitiformis | 494 | 1307.12 | 1.71 | 2.07 | 0.79 | 1.91 | 1.59 |
| 21 | Apocynaceae | Marsdenia | tinctoria | 346 | 1401.44 | 2.06 | 1.45 | 0.85 | 2.17 | 1.49 |
| 22 | Fabaceae | Caesalpinia | sinensis | 259 | 837.27 | 1.87 | 1.09 | 0.51 | 2.06 | 1.22 |
| 23 | Fabaceae | Bowringia | callicarpa | 247 | 1610.40 | 2.56 | 1.04 | 0.98 | 1.50 | 1.17 |
| 24 | Menispermaceae | Diploclisia | glaucescens | 198 | 1712.73 | 3.01 | 0.83 | 1.04 | 1.53 | 1.14 |
| 25 | Vitaceae | Cissus | subtetragona | 236 | 1160.52 | 2.32 | 0.99 | 0.70 | 1.66 | 1.12 |
| 26 | Fabaceae | Dalbergia | pinnata | 148 | 1823.28 | 3.52 | 0.62 | 1.11 | 1.43 | 1.05 |
| 27 | Fabaceae | Senegalia | rugata | 177 | 1856.58 | 3.20 | 0.74 | 1.13 | 1.17 | 1.01 |
| 28 | Opiliaceae | Cansjera | rheedei | 155 | 1458.18 | 3.05 | 0.65 | 0.89 | 1.41 | 0.98 |
| 29 | Vitaceae | Tetrastigma | erubescens | 259 | 1908.00 | 2.72 | 1.09 | 1.16 | 0.63 | 0.96 |
| 30 | Malpighiaceae | Aspidopterys | nutans | 192 | 803.25 | 2.13 | 0.81 | 0.49 | 1.28 | 0.86 |
| 31 | Hernandiaceae | Illigera | rhodantha | 169 | 535.57 | 1.82 | 0.71 | 0.33 | 1.17 | 0.74 |
| 32 | Urticaceae | Boehmeria | nivea var. tenacissima | 108 | 691.04 | 2.64 | 0.45 | 0.42 | 1.23 | 0.70 |
| 33 | Menispermaceae | Tinomiscium | petiolare | 173 | 813.14 | 2.25 | 0.73 | 0.49 | 0.76 | 0.66 |
| 34 | Apocynaceae | Beaumontia | grandiflora | 123 | 999.28 | 2.92 | 0.52 | 0.61 | 0.81 | 0.65 |
| 35 | Dioscoreaceae | Dioscorea | polystachya | 115 | 135.04 | 1.12 | 0.48 | 0.08 | 1.16 | 0.57 |
| 36 | Euphorbiaceae | Mallotus | repandus | 101 | 802.14 | 2.91 | 0.42 | 0.49 | 0.78 | 0.56 |
| 37 | Oleaceae | Jasminum | albicalyx | 96 | 237.28 | 1.60 | 0.40 | 0.14 | 1.12 | 0.56 |
| 38 | Fabaceae | Dalbergia | dyeriana | 86 | 716.50 | 3.01 | 0.36 | 0.44 | 0.83 | 0.54 |
| 39 | Rhamnaceae | Gouania | javanica | 87 | 567.44 | 2.63 | 0.37 | 0.34 | 0.90 | 0.54 |
| 40 | Apocynaceae | Cryptolepis | buchananii | 107 | 647.88 | 2.55 | 0.45 | 0.39 | 0.76 | 0.53 |
| 41 | Araceae | Spirodela | polyrhiza | 67 | 715.63 | 3.19 | 0.28 | 0.43 | 0.83 | 0.52 |
| 42 | Fabaceae | Afgekia | filipes | 72 | 1061.61 | 3.84 | 0.30 | 0.64 | 0.49 | 0.48 |
| 43 | Fabaceae | Cheniella | tenuiflora | 60 | 1354.76 | 4.62 | 0.25 | 0.82 | 0.24 | 0.44 |
| 44 | Menispermaceae | Tinospora | sinensis | 88 | 208.35 | 1.57 | 0.37 | 0.13 | 0.78 | 0.42 |
| 45 | Fabaceae | Pterolobium | punctatum | 82 | 483.45 | 2.53 | 0.34 | 0.29 | 0.63 | 0.42 |
| 46 | Convolvulaceae | Argyreia | wallichii | 81 | 255.32 | 1.84 | 0.34 | 0.16 | 0.76 | 0.42 |
| 47 | Vitaceae | Vitis | sp | 87 | 287.49 | 1.89 | 0.37 | 0.17 | 0.60 | 0.38 |
| 48 | Apocynaceae | Ichnocarpus | frutescens | 66 | 235.15 | 1.96 | 0.28 | 0.14 | 0.61 | 0.34 |
| 49 | Vitaceae | Vitis | heyneana | 40 | 388.14 | 3.01 | 0.17 | 0.24 | 0.45 | 0.29 |
| 50 | Vitaceae | Ampelopsis | glandulosa var. hancei | 33 | 242.67 | 2.15 | 0.14 | 0.15 | 0.47 | 0.25 |
| 51 | Apocynaceae | Marsdenia | koi | 62 | 138.68 | 1.53 | 0.26 | 0.08 | 0.40 | 0.25 |
| 52 | Fabaceae | Callerya | cinerea | 33 | 178.36 | 2.43 | 0.14 | 0.11 | 0.45 | 0.23 |
| 53 | Vitaceae | Tetrastigma | retinervium var. pubescens | 57 | 193.48 | 1.94 | 0.24 | 0.12 | 0.33 | 0.23 |
| 54 | Rosaceae | Rosa | henryi | 36 | 364.83 | 2.33 | 0.15 | 0.22 | 0.31 | 0.23 |
| 55 | Fabaceae | Derris | fordii var. lucida | 51 | 164.04 | 1.82 | 0.21 | 0.10 | 0.36 | 0.23 |
| 56 | Vitaceae | Tetrastigma | ceratopetalum | 33 | 173.57 | 2.15 | 0.14 | 0.11 | 0.38 | 0.21 |
| 57 | Ranunculaceae | Clematis | meyeniana | 44 | 87.22 | 1.50 | 0.19 | 0.05 | 0.38 | 0.21 |
| 58 | Asclepiadaceae | sp | sp | 37 | 81.93 | 1.60 | 0.16 | 0.05 | 0.40 | 0.20 |
| 59 | Apocynaceae | Telosma | procumbens | 25 | 173.82 | 2.71 | 0.11 | 0.11 | 0.38 | 0.20 |
| 60 | Smilacaceae | Smilax | astrosperma | 32 | 33.99 | 1.15 | 0.13 | 0.02 | 0.42 | 0.19 |
| 61 | Vitaceae | Tetrastigma | serrulatum | 39 | 109.12 | 1.62 | 0.16 | 0.07 | 0.27 | 0.17 |
| 62 | Ranunculaceae | Clematis | finetiana | 33 | 79.57 | 1.68 | 0.14 | 0.05 | 0.31 | 0.17 |
| 63 | Capparaceae | Capparis | acutifolia | 24 | 109.51 | 2.21 | 0.10 | 0.07 | 0.29 | 0.15 |
| 64 | Vitaceae | Tetrastigma | caudatum | 29 | 44.54 | 1.33 | 0.12 | 0.03 | 0.31 | 0.15 |
| 65 | Primulaceae | Maesa | balansae | 18 | 206.78 | 3.31 | 0.08 | 0.13 | 0.25 | 0.15 |
| 66 | Ranunculaceae | Naravelia | pilulifera | 33 | 58.89 | 1.42 | 0.14 | 0.04 | 0.27 | 0.15 |
| 67 | Euphorbiaceae | Mallotus | yunnanensis | 10 | 349.06 | 4.20 | 0.04 | 0.21 | 0.13 | 0.13 |
| 68 | Celastraceae | Salacia | sessiliflora | 19 | 108.39 | 2.54 | 0.08 | 0.07 | 0.22 | 0.12 |
| 69 | Vitaceae | Ampelopsis | cantoniensis | 16 | 67.49 | 2.09 | 0.07 | 0.04 | 0.25 | 0.12 |
| 70 | Fabaceae | Bauhinia | corymbosa | 17 | 294.75 | 4.31 | 0.07 | 0.18 | 0.11 | 0.12 |
| 71 | Celastraceae | Pristimera | setulosa | 14 | 151.68 | 3.10 | 0.06 | 0.09 | 0.20 | 0.12 |
| 72 | Fabaceae | Derris | tonkinensis var. compacta | 16 | 91.32 | 2.44 | 0.07 | 0.06 | 0.22 | 0.11 |
| 73 | Piperaceae | Piper | semiimmersum | 25 | 26.04 | 1.12 | 0.11 | 0.02 | 0.22 | 0.11 |
| 74 | Rhamnaceae | Ventilago | leiocarpa | 13 | 46.55 | 2.04 | 0.06 | 0.03 | 0.22 | 0.10 |
| 75 | Dioscoreaceae | Dioscorea | esquirolii | 19 | 17.84 | 1.09 | 0.08 | 0.01 | 0.20 | 0.10 |
| 76 | Oleaceae | Jasminum | elongatum | 14 | 30.63 | 1.60 | 0.06 | 0.02 | 0.18 | 0.09 |
| 77 | Rhamnaceae | Ziziphus | oenopolia | 11 | 51.12 | 2.30 | 0.05 | 0.03 | 0.18 | 0.09 |
| 78 | Vitaceae | Cissus | assamica | 10 | 11.56 | 1.20 | 0.04 | 0.01 | 0.18 | 0.08 |
| 79 | Fabaceae | Sophora | prazeri | 14 | 45.03 | 1.86 | 0.06 | 0.03 | 0.13 | 0.07 |
| 80 | Rosaceae | Rubus | pirifolius | 12 | 55.35 | 2.33 | 0.05 | 0.03 | 0.13 | 0.07 |
| 81 | Araceae | Rhaphidophora | megaphylla | 14 | 25.13 | 1.45 | 0.06 | 0.02 | 0.13 | 0.07 |
| 82 | Aristolochiaceae | Aristolochia | kwangsiensis | 11 | 51.83 | 2.17 | 0.05 | 0.03 | 0.11 | 0.06 |
| 83 | Primulaceae | Embelia | laeta | 6 | 61.56 | 3.23 | 0.03 | 0.04 | 0.11 | 0.06 |
| 84 | Rosaceae | Rubus | alceifolius | 8 | 16.27 | 1.54 | 0.03 | 0.01 | 0.13 | 0.06 |
| 85 | Vitaceae | Tetrastigma | sp | 5 | 95.36 | 4.82 | 0.02 | 0.06 | 0.07 | 0.05 |
| 86 | Oleaceae | Jasminum | prainii | 6 | 16.53 | 1.72 | 0.03 | 0.01 | 0.11 | 0.05 |
| 87 | Araceae | Pothos | chinensis | 11 | 9.53 | 1.05 | 0.05 | 0.01 | 0.09 | 0.05 |
| 88 | Phyllanthaceae | Phyllanthus | reticulatus | 7 | 61.46 | 3.10 | 0.03 | 0.04 | 0.07 | 0.05 |
| 89 | Euphorbiaceae | Mallotus | millietii | 6 | 19.43 | 1.93 | 0.03 | 0.01 | 0.09 | 0.04 |
| 90 | Fabaceae | Dalbergia | hancei | 4 | 35.46 | 3.03 | 0.02 | 0.02 | 0.07 | 0.04 |
| 91 | Rosaceae | Rubus | feddei | 9 | 25.01 | 1.82 | 0.04 | 0.02 | 0.05 | 0.04 |
| 92 | Vitaceae | Vitis | retordii | 5 | 44.49 | 2.98 | 0.02 | 0.03 | 0.05 | 0.03 |
| 93 | Annonaceae | Desmos | chinensis | 5 | 13.60 | 1.78 | 0.02 | 0.01 | 0.07 | 0.03 |
| 94 | Primulaceae | Embelia | sp | 4 | 22.27 | 2.45 | 0.02 | 0.01 | 0.05 | 0.03 |
| 95 | Fabaceae | Millettia | pachyloba | 3 | 20.28 | 2.60 | 0.01 | 0.01 | 0.05 | 0.03 |
| 96 | Caprifoliaceae | Lonicera | confusa | 4 | 7.56 | 1.50 | 0.02 | 0.01 | 0.05 | 0.03 |
| 97 | Sabiaceae | Sabia | swinhoei | 3 | 6.68 | 1.67 | 0.01 | 0.00 | 0.04 | 0.02 |
| 98 | Fabaceae | Derris | sp | 2 | 11.98 | 2.75 | 0.01 | 0.01 | 0.04 | 0.02 |
| 99 | Vitaceae | Vitis | flexuosa | 2 | 6.07 | 1.95 | 0.01 | 0.00 | 0.04 | 0.02 |
| 100 | Ranunculaceae | Clematis | florida | 2 | 3.60 | 1.50 | 0.01 | 0.00 | 0.04 | 0.02 |
| 101 | Primulaceae | Embelia | scandens | 2 | 3.31 | 1.45 | 0.01 | 0.00 | 0.04 | 0.02 |
| 102 | Piperaceae | Piper | kadsura | 2 | 2.11 | 1.15 | 0.01 | 0.00 | 0.04 | 0.02 |
| 103 | Vitaceae | Tetrastigma | obtectum | 2 | 2.08 | 1.15 | 0.01 | 0.00 | 0.04 | 0.02 |
| 104 | Vitaceae | Tetrastigma | sp | 2 | 22.56 | 3.75 | 0.01 | 0.01 | 0.02 | 0.01 |
| 105 | Apocynaceae | Hoya | carnosa | 3 | 2.52 | 1.03 | 0.01 | 0.00 | 0.02 | 0.01 |
| 106 | Rutaceae | Zanthoxylum | calcicola | 2 | 2.67 | 1.30 | 0.01 | 0.00 | 0.02 | 0.01 |
| 107 | Rutaceae | Citrus | trifoliata | 1 | 6.16 | 2.80 | 0.00 | 0.00 | 0.02 | 0.01 |
| 108 | Rhamnaceae | Sageretia | thea | 1 | 4.91 | 2.50 | 0.00 | 0.00 | 0.02 | 0.01 |
| 109 | Oleaceae | Jasminum | sp | 1 | 3.46 | 2.10 | 0.00 | 0.00 | 0.02 | 0.01 |
| 110 | Rhamnaceae | sp | sp | 1 | 3.14 | 2.00 | 0.00 | 0.00 | 0.02 | 0.01 |
| 111 | Rosaceae | Rubus | sp | 1 | 3.14 | 2.00 | 0.00 | 0.00 | 0.02 | 0.01 |
| 112 | Vitaceae | Ampelopsis | chaffanjonii | 1 | 1.77 | 1.50 | 0.00 | 0.00 | 0.02 | 0.01 |
| 113 | Oleaceae | Jasminum | microcalyx | 1 | 1.33 | 1.30 | 0.00 | 0.00 | 0.02 | 0.01 |
| Diversity Index | Lianas | Trees |
|---|---|---|
| Stem density | 23,819 (24.16%) | 74,758 |
| Total basal area (m2) | 16.48 (4.32%) | 365.11 |
| Species richness | 113 (34.66%) | 213 |
| Shannon diversity | 3.51 | 3.82 |
| Simpson dominance | 0.95 | 0.96 |
| Pielou evenness | 0.75 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, F.; Li, D.; Wang, B.; Guo, Y.; Wen, S.; Huang, F.; Tao, W.; Tang, N.; Li, X.; et al. Abundance and Species Richness of Lianas in a Karst Seasonal Rainforest: The Influence of Abiotic and Biotic Factors. Forests 2024, 15, 1011. https://doi.org/10.3390/f15061011
Li J, Lu F, Li D, Wang B, Guo Y, Wen S, Huang F, Tao W, Tang N, Li X, et al. Abundance and Species Richness of Lianas in a Karst Seasonal Rainforest: The Influence of Abiotic and Biotic Factors. Forests. 2024; 15(6):1011. https://doi.org/10.3390/f15061011
Chicago/Turabian StyleLi, Jianxing, Fang Lu, Dongxing Li, Bin Wang, Yili Guo, Shujun Wen, Fuzhao Huang, Wanglan Tao, Nianwu Tang, Xiankun Li, and et al. 2024. "Abundance and Species Richness of Lianas in a Karst Seasonal Rainforest: The Influence of Abiotic and Biotic Factors" Forests 15, no. 6: 1011. https://doi.org/10.3390/f15061011
APA StyleLi, J., Lu, F., Li, D., Wang, B., Guo, Y., Wen, S., Huang, F., Tao, W., Tang, N., Li, X., & Xiang, W. (2024). Abundance and Species Richness of Lianas in a Karst Seasonal Rainforest: The Influence of Abiotic and Biotic Factors. Forests, 15(6), 1011. https://doi.org/10.3390/f15061011









