Neighborhood Competition and Understory-Associated Vegetation Are Important Factors Influencing the Natural Regeneration of Subtropical Mountain Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plot Selection and Vegetation Investigation Methodology
2.3. Selection and Interpretation of Explanatory and Response Variables
2.4. Data Analysis
3. Results
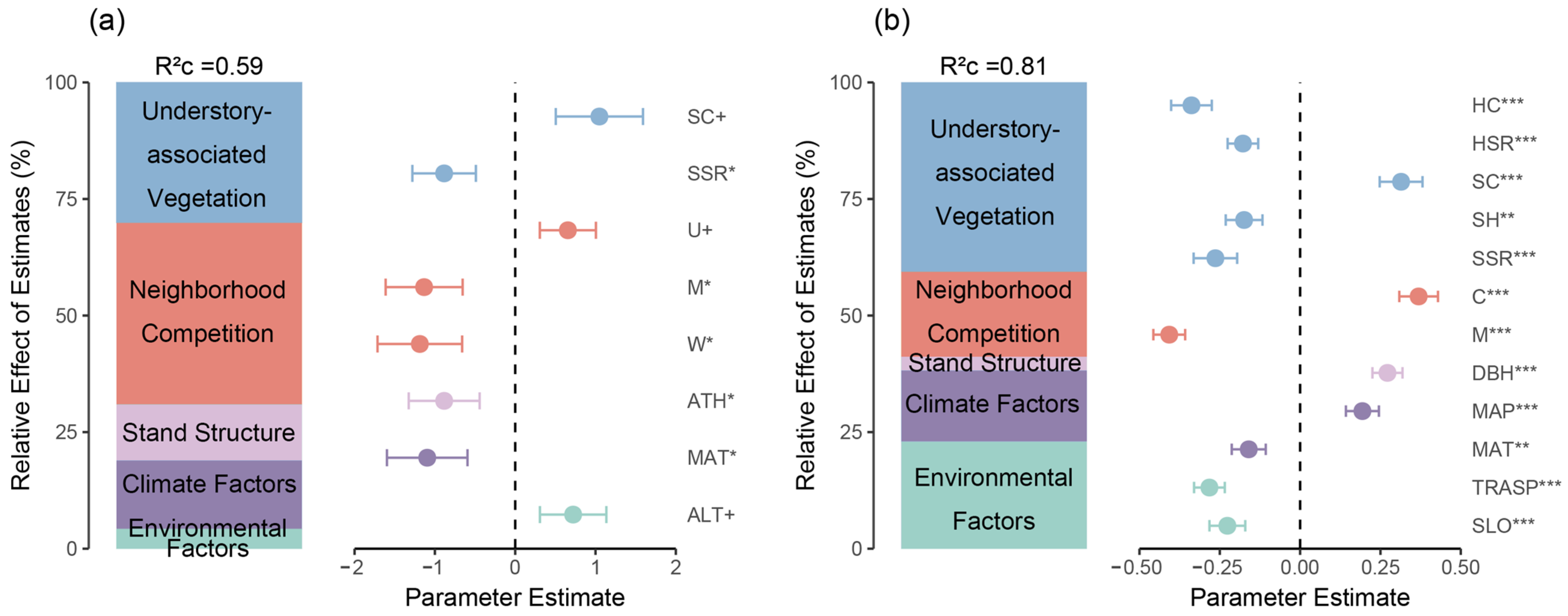
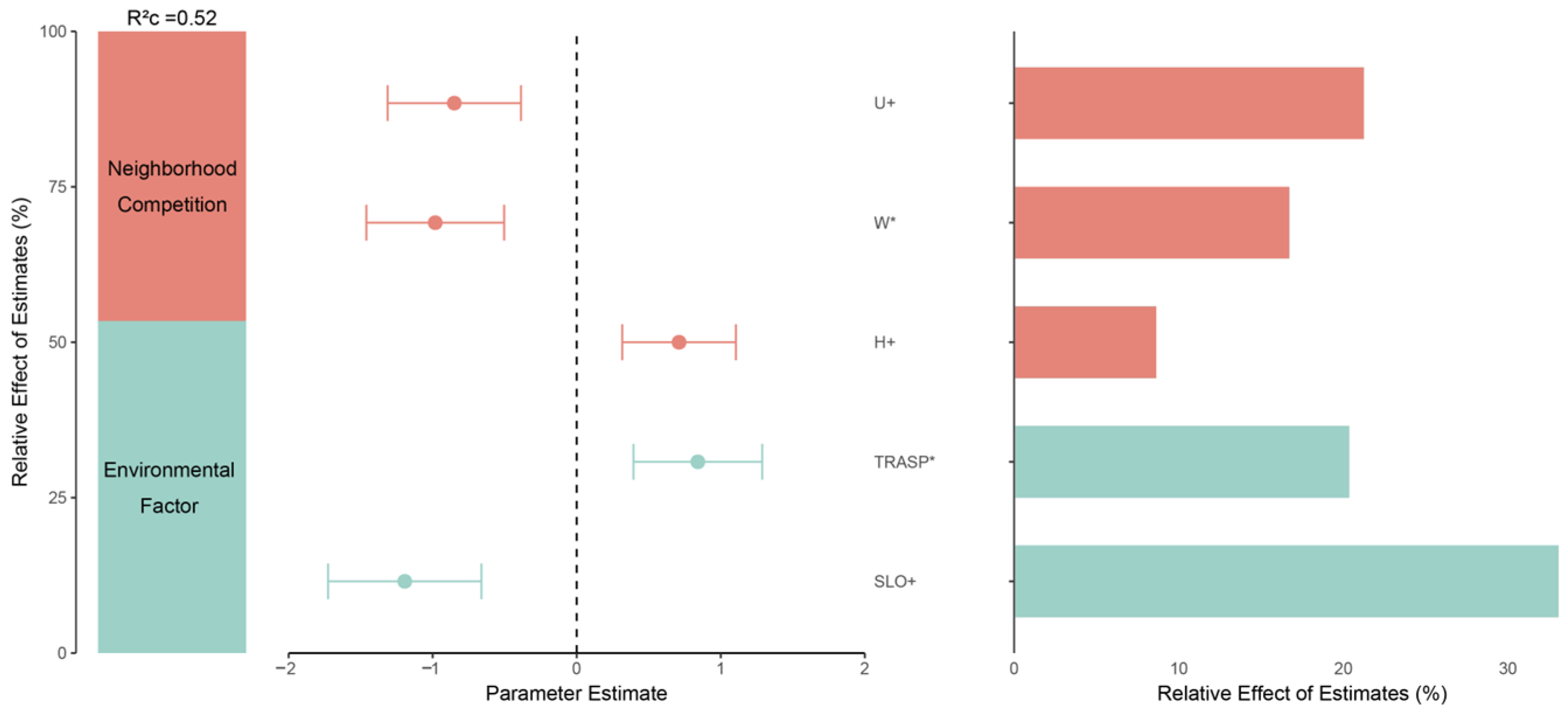
3.1. Natural Regeneration Basics and Important Factors Affecting Regeneration
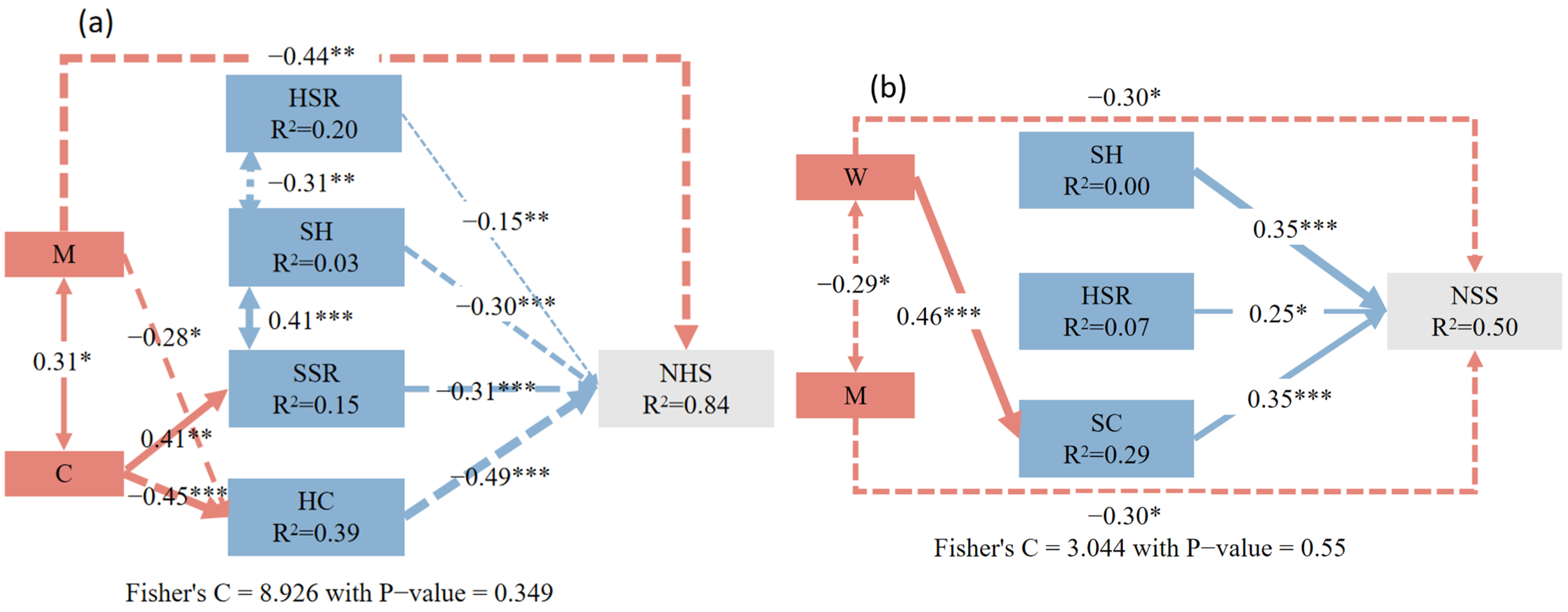
3.2. The Influence of Neighborhood Competition and Understory-Associated Vegetation on Natural Regeneration in the Shrub and Herb Layers
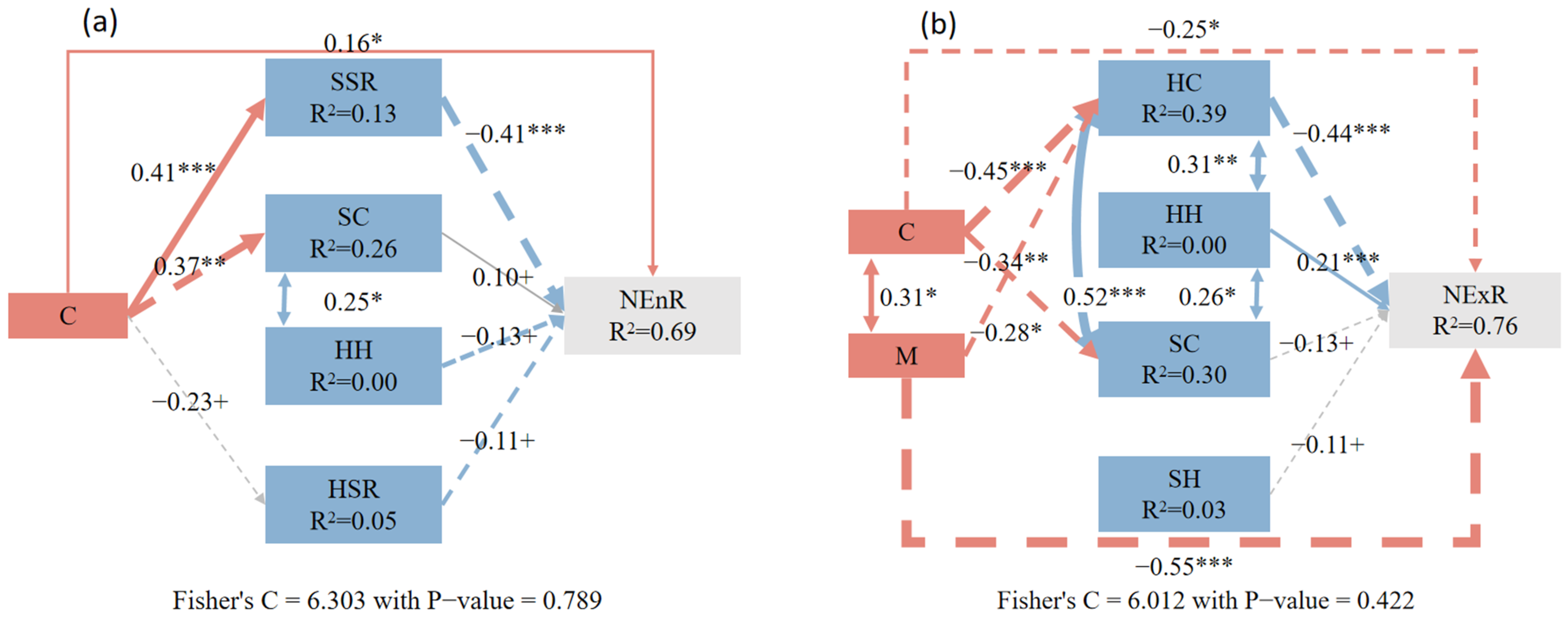
3.3. The Impact of Neighborhood Competition and Understory-Associated Vegetation on Natural Regeneration from Different Sources
4. Discussion
4.1. Impact of Environment, Climate, and Stand Structure on Natural Regeneration
4.2. Direct and Indirect Effects of Neighborhood Competition Factors and Understory-Associated Vegetation on Natural Regeneration
4.3. Effects of Neighborhood Competition on Natural Regeneration of Shrub and Herb Layers
4.4. The Responses of Endogenous and Exogenous Regeneration to Various Factors
4.5. Insights for Forestry Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.H.; Khan, M.A.S.A.; Roy, B.; Fardusi, M.J. Assessment of Natural Regeneration Status and Diversity of Tree Species in the Biodiversity Conservation Areas of Northeastern Bangladesh. J. For. Res. 2011, 22, 551–559. [Google Scholar] [CrossRef]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Restoring Natural Forests Is the Best Way to Remove Atmospheric Carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Magee, L.; Pandit, K.; Bourdon, J.; Broadbent, E.N.; Glenn, K.; Kaddoura, Y.; Machado, S.; Nieves, J.; Wilkinson, B.E.; et al. Canopy Tree Density and Species Influence Tree Regeneration Patterns and Woody Species Diversity in a Longleaf Pine Forest. For. Ecol. Manag. 2021, 490, 119082. [Google Scholar] [CrossRef]
- Pham, V.V.; Ammer, C.; Annighöfer, P.; Heinrichs, S. Tree Regeneration Characteristics in Limestone Forests of the Cat Ba National Park, Vietnam. BMC Ecol. Evol. 2022, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Shive, K.L.; Preisler, H.K.; Welch, K.R.; Safford, H.D.; Butz, R.J.; O’Hara, K.L.; Stephens, S.L. From the Stand Scale to the Landscape Scale: Predicting the Spatial Patterns of Forest Regeneration after Disturbance. Ecol. Appl. 2018, 28, 1626–1639. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Broadbent, E.N.; Rozendaal, D.M.A.; Bongers, F.; Zambrano, A.M.A.; Aide, T.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; et al. Carbon Sequestration Potential of Second-Growth Forest Regeneration in the Latin American Tropics. Sci. Adv. 2016, 2, e1501639. [Google Scholar] [CrossRef]
- Martini, F.; Zou, C.; Goodale, U.M. Intrinsic Biotic Factors and Microsite Conditions Drive Seedling Survival in a Species with Masting Reproduction. Ecol. Evol. 2019, 9, 14261–14272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shen, Y.; Liu, W.; Tan, B.; Li, Z.; Wang, Q. Quantifying the Effect of Shading and Watering on Seed Germination in Translocated Forest Topsoil at a Subtropical Karst of China. For. Ecol. Manag. 2020, 459, 117811. [Google Scholar] [CrossRef]
- Liang, W.; Wei, X. Multivariate Path Analysis of Factors Influencing Larix principis-rupprechtii Plantation Regeneration in Northern China. Ecol. Indic. 2021, 129, 107886. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Wang, X.-A.; Tu, Y.-B.; Huang, M.-Y.; Wang, C.; Zhu, Z.-H.; Guo, H. Recruitment pattern of tree populations along an altitudinal gradient: Larix chinensis beissn in qinling mountains (China). Pol. J. Ecol. 2009, 57, 451–459. [Google Scholar]
- Li, J.; Li, S.; Liu, C.; Guo, D.; Zhang, Q. Response of Chinese Pine Regeneration Density to Forest Gap and Slope Aspect in Northern China: A Meta-Analysis. Sci. Total Environ. 2023, 873, 162428. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Feng, Y.; Li, F.; Zhao, Y.; Zhu, J.; Yao, J.; Chang, C.; Wei, X. Drivers of Vegetation and Soil Determine Natural Regeneration of a Single Plantation at Different Slope Positions. Front. For. Glob. Chang. 2023, 6, 1167284. [Google Scholar] [CrossRef]
- Liu, H.; Shi, H.; Zhou, Q.; Hu, M.; Shu, X.; Zhang, K.; Zhang, Q.; Dang, H. Habitat Heterogeneity and Biotic Interactions Mediate Climate Influences on Seedling Survival in a Temperate Forest. For. Ecosyst. 2023, 10, 100138. [Google Scholar] [CrossRef]
- Lv, K.; Zhou, M.; Ding, Y.; Zang, R.; Yao, J.; Luo, Y.; Yan, D. Regeneration Characteristics and Influencing Factors of Woody Plant on Natural Evergreen Secondary Broad-Leaved Forests in the Subtropical, China. Glob. Ecol. Conserv. 2023, 42, e02394. [Google Scholar] [CrossRef]
- Yu, F.; Wang, D.; Shi, X.; Yi, X.; Huang, Q.; Hu, Y. Effects of Environmental Factors on Tree Seedling Regeneration in a Pine-Oak Mixed Forest in the Qinling Mountains, China. J. Mt. Sci. 2013, 10, 845–853. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Y.; Gao, Y. Potential Factors Promoting the Natural Regeneration of Larix principis-rupprechtii in North China. PeerJ 2023, 11, e15809. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Impacts of root competition in forests and woodlands: A theoretical framework and review of experiments. Ecol. Monogr. 2000, 70, 171–207. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Wang, X.; Wen, X.; Li, T.; Ye, M.; Chen, G.; Zhao, K.; Hou, G.; Li, X.; et al. Stand Structure Adjustment Influences the Biomass Allocation in Naturally Generated Pinus massoniana Seedlings through Environmental Factors. Front. Plant Sci. 2022, 13, 997795. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the Formation of Dense Understory Layers in Forests Worldwide: Consequences and Implications for Forest Dynamics, Biodiversity, and Succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Fonseca, T.F. Influence Management and Disturbances on the Regeneration of Forest Stands. Front. For. Glob. Change 2023, 6, 1123215. [Google Scholar] [CrossRef]
- Whigham, D.F. Ecology of Woodland Herbs in Temperate Deciduous Forests. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 583–621. [Google Scholar] [CrossRef]
- Salek, L.; Harmacek, J.; Jerabkova, L.; Topacoglu, O.; Machar, I. Thorny Shrubs Limit the Browsing Pressure of Large Herbivores on Tree Regeneration in Temperate Lowland Forested Landscapes. Sustainability 2019, 11, 3578. [Google Scholar] [CrossRef]
- De Lombaerde, E.; Baeten, L.; Verheyen, K.; Perring, M.P.; Ma, S.; Landuyt, D. Understorey Removal Effects on Tree Regeneration in Temperate Forests: A Meta-Analysis. J. Appl. Ecol. 2021, 58, 9–20. [Google Scholar] [CrossRef]
- O’Brien, M.J.; O’Hara, K.L.; Erbilgin, N.; Wood, D.L. Overstory and Shrub Effects on Natural Regeneration Processes in Native Pinus radiata Stands. For. Ecol. Manag. 2007, 240, 178–185. [Google Scholar] [CrossRef]
- Rodríguez-García, E.; Ordóñez, C.; Bravo, F. Effects of Shrub and Canopy Cover on the Relative Growth Rate of Pinus pinaster Ait. Seedlings of Different Sizes. Ann. For. Sci. 2011, 68, 337–346. [Google Scholar] [CrossRef]
- Tíscar Oliver, P. Patterns of Shrub Diversity and Tree Regeneration across Topographic and Stand-Structural Gradients in a Mediterranean Forest. For. Syst. 2015, 24, 011. [Google Scholar] [CrossRef]
- Pu, X.; Zhu, Y.; Jin, G. Effects of Local Biotic Neighbors and Habitat Heterogeneity on Seedling Survival in a Spruce-fir Valley Forest, Northeastern China. Ecol. Evol. 2017, 7, 4582–4591. [Google Scholar] [CrossRef] [PubMed]
- Metslaid, M.; Ilisson, T.; Vicente, M.; Nikinmaa, E.; Jogiste, K. Growth of Advance Regeneration of Norway Spruce after Clear-Cutting. Tree Physiol. 2005, 25, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Queenborough, S.A.; Burslem, D.F.R.P.; Garwood, N.C.; Valencia, R. Neighborhood and community interactions determine the spatial pattern of tropical tree seedling survival. Ecology 2007, 88, 2248–2258. [Google Scholar] [CrossRef]
- McAlpine, K.; Lamoureaux, S.; Timmins, S. Understory Vegetation Provides Clues to Succession in Woody Weed Stands. NZJE 2021, 45, 3418. [Google Scholar] [CrossRef]
- Douh, C.; Daïnou, K.; Joël Loumeto, J.; Moutsambote, J.-M.; Fayolle, A.; Tosso, F.; Forni, E.; Gourlet-Fleury, S.; Doucet, J.-L. Soil Seed Bank Characteristics in Two Central African Forest Types and Implications for Forest Restoration. For. Ecol. Manag. 2018, 409, 766–776. [Google Scholar] [CrossRef]
- Kurten, E.L.; Wright, S.J.; Carson, W.P. Hunting Alters Seedling Functional Trait Composition in a Neotropical Forest. Ecology 2015, 96, 1923–1932. [Google Scholar] [CrossRef]
- Peres, C.A.; Emilio, T.; Schietti, J.; Desmoulière, S.J.M.; Levi, T. Dispersal Limitation Induces Long-Term Biomass Collapse in Overhunted Amazonian Forests. Proc. Natl. Acad. Sci. USA 2016, 113, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The Biodiversity and Ecosystem Service Contributions and Trade-Offs of Forest Restoration Approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Felton, A.; Nilsson, U.; Sonesson, J.; Felton, A.M.; Roberge, J.-M.; Ranius, T.; Ahlström, M.; Bergh, J.; Björkman, C.; Boberg, J.; et al. Replacing Monocultures with Mixed-Species Stands: Ecosystem Service Implications of Two Production Forest Alternatives in Sweden. Ambio 2016, 45, 124–139. [Google Scholar] [CrossRef]
- Li, L.; Qi, Z.; Zhong, T. Forest Transition and Its Dynamics in Subtropical Chongqing, China since 1990s. Land 2021, 10, 777. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zheng, X.; Ge, X.; Li, Y.; Fang, Y.; Cui, P.; Ding, H. Neighborhood Diversity Structure and Neighborhood Species Richness Effects Differ across Life Stages in a Subtropical Natural Secondary Forest. For. Ecosyst. 2022, 9, 100075. [Google Scholar] [CrossRef]
- Hui, G.; Wang, Y.; Zhang, G.; Zhao, Z.; Bai, C.; Liu, W. A Novel Approach for Assessing the Neighborhood Competition in Two Different Aged Forests. For. Ecol. Manag. 2018, 422, 49–58. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, R.; Wang, N.; Xie, L.; Feng, Y.; Li, Y.; Ding, H.; Fang, Y. Spatial Distributions of Intra-Community Tree Species under Topographically Variable Conditions. J. Mt. Sci. 2023, 20, 391–402. [Google Scholar] [CrossRef]
- Hui, G.; Zhang, G.; Zhao, Z.; Yang, A. Methods of Forest Structure Research: A Review. Curr. For. Rep. 2019, 5, 142–154. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Pan, L.; Sun, Y. Hegyi Competition Index Decomposition to Improve Estimation Accuracy of Larix olgensis Crown Radius. Ecol. Indic. 2022, 143, 109322. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Guimarães-Steinicke, C.; Weigelt, A.; Proulx, R.; Lanners, T.; Eisenhauer, N.; Duque-Lazo, J.; Reu, B.; Roscher, C.; Wagg, C.; Buchmann, N.; et al. Biodiversity Facets Affect Community Surface Temperature via 3D Canopy Structure in Grassland Communities. J. Ecol. 2021, 109, 1969–1985. [Google Scholar] [CrossRef]
- García-Palacios, P.; Gross, N.; Gaitán, J.; Maestre, F.T. Climate Mediates the Biodiversity–Ecosystem Stability Relationship Globally. Proc. Natl. Acad. Sci. USA 2018, 115, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, R.; Yang, L.; Tan, T.; Li, H.; Liu, M. Elevation Gradient Distribution of Indices of Tree Population in a Montane Forest: The Role of Leaf Traits and the Environment. For. Ecosyst. 2022, 9, 100012. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. Glmm.Hp: An R Package for Computing Individual Effect of Predictors in Generalized Linear Mixed Models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Atsbha, T.; Desta, A.B.; Zewdu, T. Woody Species Diversity, Population Structure, and Regeneration Status in the Gra-Kahsu Natural Vegetation, Southern Tigray of Ethiopia. Heliyon 2019, 5, e01120. [Google Scholar] [CrossRef] [PubMed]
- Grace, J. Structural Equation Modeling and Natural Systems-All Databases; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Liang, W.; Wei, X. Factors Promoting the Natural Regeneration of Larix principis-rupprechtii Plantation in the Lvliang Mountains of Central China. PeerJ 2020, 8, e9339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Cao, Y.; Bai, Z.; Qin, Q. Effects of Soil and Topographic Factors on Vegetation Restoration in Opencast Coal Mine Dumps Located in a Loess Area. Sci. Rep. 2016, 6, 22058. [Google Scholar] [CrossRef]
- Gastauer, M.; Massante, J.C.; Ramos, S.J.; Da Silva, R.D.S.S.; Boanares, D.; Guedes, R.S.; Caldeira, C.F.; Medeiros-Sarmento, P.S.; De Castro, A.F.; Prado, I.G.D.O.; et al. Revegetation on Tropical Steep Slopes after Mining and Infrastructure Projects: Challenges and Solutions. Sustainability 2022, 14, 17003. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Wu, J.; An, Z.; Suo, L.; Ding, J.; Li, S.; Wei, D.; Jin, L. Effects of the Rainfall Intensity and Slope Gradient on Soil Erosion and Nitrogen Loss on the Sloping Fields of Miyun Reservoir. Plants 2023, 12, 423. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, T.; Yu, L.; Li, Y.; Zhang, G. Spatial Patterns of Community-Level Sprouting in a Karst Old-Growth Forest of Southwest China. For. Ecol. Manag. 2022, 523, 120516. [Google Scholar] [CrossRef]
- Warren, R.J. Mechanisms Driving Understory Evergreen Herb Distributions across Slope Aspects: As Derived from Landscape Position. Plant Ecol. 2008, 198, 297–308. [Google Scholar] [CrossRef]
- Badano, E.I.; Sánchez-Montes de Oca, E.J. Seed Fate, Seedling Establishment and the Role of Propagule Size in Forest Regeneration under Climate Change Conditions. For. Ecol. Manag. 2022, 503, 119776. [Google Scholar] [CrossRef]
- Rank, R.; Maneta, M.; Higuera, P.; Holden, Z.; Dobrowski, S. Conifer Seedling Survival in Response to High Surface Temperature Events of Varying Intensity and Duration. Front. For. Glob. Chang. 2022, 4, 731267. [Google Scholar] [CrossRef]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased Vapor Pressure Deficit Due to Higher Temperature Leads to Greater Transpiration and Faster Mortality during Drought for Tree Seedlings Common to the Forest–Grassland Ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Chmura, D.; Sierka, E. The Invasibility of Deciduous Forest Communities after Disturbance: A Case Study of Carex Brizoides and Impatiens Parviflora Invasion. For. Ecol. Manag. 2007, 242, 487–495. [Google Scholar] [CrossRef]
- Lyon, J.; Sharpe, W.E. Impacts of Hay-Scented Fern on Nutrition of Northern Red Oak Seedlings. J. Plant Nutr. 2003, 26, 487–502. [Google Scholar] [CrossRef]
- Jensen, A.M.; Gotmark, F.; Lof, M. Shrubs Protect Oak Seedlings against Ungulate Browsing in Temperate Broadleaved Forests of Conservation Interest: A Field Experiment. For. Ecol. Manag. 2012, 266, 187–193. [Google Scholar] [CrossRef]
- Liu, N.; Wang, D.; Guo, Q. Exploring the Influence of Large Trees on Temperate Forest Spatial Structure from the Angle of Mingling. For. Ecol. Manag. 2021, 492, 119220. [Google Scholar] [CrossRef]
- Dong, L.; Bettinger, P.; Liu, Z. Optimizing Neighborhood-Based Stand Spatial Structure: Four Cases of Boreal Forests. For. Ecol. Manag. 2022, 506, 119965. [Google Scholar] [CrossRef]
- Fichtner, A.; Härdtle, W.; Li, Y.; Bruelheide, H.; Kunz, M.; Von Oheimb, G. From Competition to Facilitation: How Tree Species Respond to Neighbourhood Diversity. Ecol. Lett. 2017, 20, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Beckage, B.; Lavine, M.; Clark, J.S. Survival of Tree Seedlings across Space and Time: Estimates from Long-term Count Data. J. Ecol. 2005, 93, 1177–1184. [Google Scholar] [CrossRef]
- Gavinet, J.; Prévosto, B.; Fernandez, C. Do Shrubs Facilitate Oak Seedling Establishment in Mediterranean Pine Forest Understory? For. Ecol. Manag. 2016, 381, 289–296. [Google Scholar] [CrossRef]
- Zhu, Y.; Queenborough, S.A.; Condit, R.; Hubbell, S.P.; Ma, K.P.; Comita, L.S. Density-Dependent Survival Varies with Species Life-History Strategy in a Tropical Forest. Ecol. Lett. 2018, 21, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Kaproth, M.A.; McGraw, J.B. Seed Viability and Dispersal of the Wind-Dispersed Invasive Ailanthus altissima in Aqueous Environments. For. Sci. 2008, 54, 490–496. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, S.; Lei, X.; Frank, S.C.; Tian, D.; Wang, G.; Deng, X. Secondary Forest Floristic Composition, Structure, and Spatial Pattern in Subtropical China. J. For. Res. 2013, 18, 111–120. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt Stress Inhibits Photosynthesis and Destroys Chloroplast Structure by Downregulating Chloroplast Development–Related Genes in Robinia pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Hui-hui, Z.; Guang-liang, S.; Jie-yu, S.; Xin, L.; Ma-bo, L.; Liang, M.; Nan, X.; Guang-yu, S. Photochemistry and Proteomics of Mulberry (Morus alba L.) Seedlings under NaCl and NaHCO3 Stress. Ecotoxicol. Environ. Saf. 2019, 184, 109624. [Google Scholar] [CrossRef]
- Bhatt, A.; Gairola, S.; Govender, Y.; De Moura Souza-Filho, P.R. The Invasive Melia Azedarach in Durban (South Africa): Impacts on Tree Community Structure. Folia Geobot. 2021, 56, 139–147. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Lee, S.; Yi, K.; Kim, H.-S.; Chung, S.; Chung, J.; Kim, H.S.; Yoon, T.K. Seed Dispersal Models for Natural Regeneration: A Review and Prospects. Forests 2022, 13, 659. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.; Xin, Z.; Liu, Z.; Schurr, F.M. A Trade-off between Primary and Secondary Seed Dispersal by Wind. Plant Ecol. 2019, 220, 541–552. [Google Scholar] [CrossRef]



| Categories | No. | Explanatory Variables | Equation | Note |
|---|---|---|---|---|
| Environmental factors | (1) | Slope (SLO, °) | Multiple measurements were averaged using a compass in the plot. | |
| (2) | Altitude (ALT, m) | The altitude of permanent plots was measured using GPS within the plot, | ||
| (3) | Transformation aspect (TRASP) | where ASPi denotes the aspect of the plot i. After conversion, 0 is a sunny slope and 1 is a shady slope. | ||
| Climate factors | (4) | Mean annual temperature (MAT, °C) | Mean annual temperature data at 1 km resolution from WorldClim 2.1 https://worldclim.org/ (accessed on 10 February 2024). | |
| (5) | Mean annual precipitation (MAP, mm) | Mean annual precipitation data at 1 km resolution from WorldClim 2.1 https://worldclim.org/ (accessed on 10 February 2024). | ||
| Stand structure | (6) | Tree density (TDEN, stems/plot) | Tree density for which DBH > 5 cm. | |
| (7) | Average tree diameter at breast height (DBH, cm) | Using vernier calipers, the diameter at breast height (DBH > 5 cm) of each tree at 1.3 m was measured, and the average DBH was calculated. | ||
| (8) | Average tree height (ATH, m) | Using a laser rangefinder (Tianjin Lookout Photoelectric 3M02–600), the height of each tree (DBH > 5 cm) was measured, and the mean ATH was calculated. | ||
| (9) | Tree species richness (TSS) | Tree species richness for which DBH > 5 cm. | ||
| Neighborhood competition | (10) | The uniform angle index (W) | αij represents the angle formed between the reference tree and its two neighboring trees out of the four nearest trees. i denotes the nth reference tree, while j denotes the nth of the four nearest trees to the reference tree, with n = 1, 2, 3, 4 [37]. | |
| (11) | The diameter dominance index (U) | Di represents the diameter at breast height (DBH) of the reference tree, while Dj represents the DBH of one of the four nearest trees to the reference tree. Here, i indicates the nth tree within the subplot, and j indicates the nth of the four nearest trees to the reference tree, where n = 1, 2, 3, 4 [38]. | ||
| (12) | The mingling index (M) | speciesi represents the species of the reference tree, while speciesj represents the species of one of the four nearest trees to the reference tree. Here, i denotes the nth tree within the subplot, and j denotes the nth of the four nearest trees to the reference tree, where n = 1, 2, 3, 4 [39]. | ||
| (13) | The crowdedness index (C) | i represents the nth tree within the subplot, and j represents the nth of the four nearest trees to the reference tree, where n = 1,2,3,4. CW denotes the average canopy width of the trees, and dij represents the distance between the reference tree and its adjacent tree [40]. | ||
| (14) | Hegyi competition index (H) | i represents the nth tree within the subplot, and j represents the nth of the four nearest trees to the reference tree, where n = 1,2,3,4. D denotes the diameter at breast height (DBH) of the tree, and dij is the distance between the reference tree and its adjacent tree [41]. | ||
| Understory-associated vegetation | (15) | Shrub height (SH, m) | The average height of plants between 1.2 and 5 m, excluding natural regeneration saplings. | |
| (16) | Shrub cover (SC, %) | The coverage of plants between 1.2 and 5 m in height, excluding natural regeneration saplings. | ||
| (17) | Shrub species richness (SSR) | The species richness of all plants excluding natural regeneration saplings with heights between 1.2 and 5 m. | ||
| (18) | Herb height (HH, m) | The average height of plants below 1.2 m, excluding naturally regenerated seedlings. | ||
| (19) | Herb cover (HC, %) | The coverage of plants below 1.2 m in height, excluding natural regeneration seedlings. | ||
| (20) | Herb species richness (HSR) | The species richness of plants below 1.2 m in height, excluding naturally regenerated seedlings. |
| Mean (Stems/Plot) | S.D. | CV (%) | Max | Min | |
|---|---|---|---|---|---|
| NTR | 10.6 | 11.4 | 108.0 | 54.0 | 0.0 |
| NHS | 7.9 | 10.8 | 136.7 | 46.0 | 0.0 |
| NSS | 2.7 | 2.9 | 108.2 | 14.0 | 0.0 |
| NEnR | 4.6 | 6.8 | 145.9 | 30.0 | 0.0 |
| NExR | 6.0 | 8.9 | 150.3 | 54.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Qin, K.; Fang, W.; Wang, H. Neighborhood Competition and Understory-Associated Vegetation Are Important Factors Influencing the Natural Regeneration of Subtropical Mountain Forests. Forests 2024, 15, 1017. https://doi.org/10.3390/f15061017
Wang Z, Qin K, Fang W, Wang H. Neighborhood Competition and Understory-Associated Vegetation Are Important Factors Influencing the Natural Regeneration of Subtropical Mountain Forests. Forests. 2024; 15(6):1017. https://doi.org/10.3390/f15061017
Chicago/Turabian StyleWang, Zizhuo, Kunrong Qin, Wen Fang, and Haiyang Wang. 2024. "Neighborhood Competition and Understory-Associated Vegetation Are Important Factors Influencing the Natural Regeneration of Subtropical Mountain Forests" Forests 15, no. 6: 1017. https://doi.org/10.3390/f15061017




