Abstract
Selenium is an important indicator for the evaluation of tea quality. However, the relationship between selenium uptake by tea plants (Camellia sinensis) and soil properties, as well as selenium transport and distribution in a tea plantation soil–tea plant–tea infusion production system, remain unclear. In this study, 12 tea plantations situated in a typical selenium-rich area of China were selected, and the characteristics and crucial factors influencing the uptake and transport of selenium were analyzed using a plantation soil–tea plant–tea infusion production system. The soil total selenium content ranged from 1.12 to 6.67 mg kg−1, with an average of 2.57 mg kg−1. The average available selenium content was 53.56 µg kg−1, and the activation rate of soil selenium was 2.27%. Soil-available selenium was significantly positively correlated with total selenium, available potassium, and soil organic matter contents, and was significantly negatively correlated with soil pH. The selenium content in old leaves ranged from 0.29 to 2.73 mg kg−1, which met the standard for selenium-rich tea, whereas only 33% of young leaves met this standard. The selenium enrichment factor was highest in the fibrous root and lowest in the young leaves. The average selenium transport factors from fibrous roots to main roots, from main roots to main stems, from main stems to lateral stems, from lateral stems to young leaves, and from lateral stems to old leaves were 0.53, 0.92, 0.67, 0.97, and 2.30, respectively. The selenium concentration of tea infusion ranged from 1.88 to 12.49 μg L−1, and the average selenium dissolution rate was 22.62% after one brewing. This study identified critical factors that influence soil-selenium availability. The selenium content in tea plant organs is indicated to be strongly associated with the selenium content in the main roots.
1. Introduction
Selenium is an essential trace element for many organisms and plays a crucial role in the synthesis of seleno-amino acids and enzymes [1], such as thioredoxin reductase. Selenium in the human body has multiple biological protective effects, such as the protection of the endocrine system, the inhibition of carcinogenesis, the promotion of reproductive functions, and the stability of body immunity and cell membranes [2,3]. However, an excessive ingestion of selenium may result in acute or chronic selenium toxicity [4], whereas its deficiency may cause widespread endemic diseases. Diseases induced by selenium deficiency that have been recorded in certain regions of China (e.g., Keshan disease) have been previously summarized [5]. The majority of countries and regions are deficient in selenium resources and inadequate selenium intake (<40 µg day−1) is frequent worldwide. China is among the most severely selenium-deficient countries and data from the Chinese Nutrition Association (2014) indicate that, compared with the recommended daily selenium intake for adults of 50 µg, the daily dietary intake of selenium is 43.3 µg. With regard to the ingestion of beneficial selenium, organic selenium from biological sources is more easily absorbed by the human body compared with inorganic selenium [6]. Selenium-rich agricultural products thus offer the most sustainable, safe, and effective source for selenium supplementation [7].
The dietary selenium intake in humans is mainly determined by the amount of selenium uptake by plant roots from the soil and subsequently incorporated in selenium-containing proteins [8], which accumulate in different organs of the plant, thereby entering the food chain and influencing human health [9,10]. Soil is the primary source of selenium for plants [11]. The selenium content and availability in soil are principally affected by the parent rock; for example, the Lower Cambrian black rock series contains abundant selenium and soil derived from the weathering of metamorphic rocks in the Tuoli Formation, which is high in selenium [12,13]. Soil characteristics, such as the clay mineral type, organic matter content, and soil microbial abundance and community structure, have an important influence on selenium distribution and availability in soil. In addition, environmental factors [14], such as the heavy metal content of the soil, often have ecological effects coupled with soil selenium; for example, the abnormal enrichment of cadmium was observed in selenium-rich areas of Guizhou Province in China [15]. The safe absorption of selenium from selenium-rich products is critical for human health [16]. Therefore, the development of selenium-rich resources and the promotion of selenium-rich farm produce have attracted increasing research attention. However, few studies have systematically and quantitatively analyzed the transport of selenium within a soil–crop food production system.
The tea plant (Camellia sinensis) has a great ability for selenium accumulation and is inherently well-suited for cropping in selenium-rich soils [17]. After its absorption by the tea plant, selenium interacts with nucleic acids, phenols, and pigments in plant tissues, resulting in the transformation of 80% of the inorganic selenium into organic selenium, which is readily absorbed by the human body [18,19]. Approximately 92% of the selenium in tea leaves is present in an organic form, mainly in selenium-containing proteins and amino acids, while approximately 8% is in an inorganic form [20]. In China, the selenium content of tea leaves ranges between 0.017 and 6.590 mg kg−1 and varies considerably among regions [21]. The soil selenium content is the primary factor that affects selenium accumulation in different parts of tea plants. The soil selenium status and the soil physicochemical characteristics, such as soil redox potential, pH, organic matter, and clay content, affect the bioavailability of soil selenium to tea plants [22,23]. The field management system is an important factor that influences selenium absorption by the tea plant. The selenium concentration in tea plants significantly increases in response to the application of exogenous selenate fertilizers [24]. In addition, the selenium content differs significantly among tea cultivars, tea picking seasons, and with tea leaf maturity [21,25]. The selenium content of tea in different regions of China reportedly exhibits significant variation [26]. Previous studies have presented findings from an analysis of 217 tea samples sourced from diverse regions in China, revealing that black tea exhibited the highest selenium content (0.552 μg g−1), while white tea displayed a significantly lower selenium content (0.107 μg g−1) compared with other tea varieties [27]. However, the focus of these studies primarily lay in conducting sample surveys to determine the selenium content present in tea. The interaction between selenium in processed tea leaves and the tea plant, as well as the soil, has been disregarded. Furthermore, limited research has investigated the selenium mobility from tea leaves to the tea infusion based on brewing experiments. Rather than the selenium content in tea leaves, consumer preference is more strongly concerned with the selenium content released from tea leaves after brewing. Therefore, it is critical to explore the absorption, transport, and distribution characteristics of selenium in a tea plantation soil–tea plant–tea infusion system.
Shitai County is one of three selenium-rich areas in China, with an average soil selenium content reaching 0.56 mg kg−1 [28], surpassing the established threshold for selenium rich soils (0.4 mg kg−1) [29]. It is also an important tea-producing area in southern Anhui Province. Given the long history of tea production and selenium-rich soil resources, the county represents an ideal location for the study of selenium mobility in soil and crops, and provides an opportunity to study selenium migration and transport in tea plantation soil, tea plants, and tea infusion in a selenium-rich area. With these considerations in mind, the core selenium-rich area of tea production in Shitai County was selected as the site for the present research.
The process of selenium transport in a plantation soil–tea plant–tea infusion system within naturally seleniferous regions remains insufficiently elucidated. We hypothesized that selenium distribution in the various organs of the tea plant is strongly correlated with soil selenium, whereby tea cultivated in selenium-rich soil accumulates elevated quantities of selenium. The main objectives of this study were (1) to evaluate soil-selenium availability and its relationship with soil physicochemical properties in a typical selenium-rich soil area, and (2) to quantify the accumulation and transport of selenium in a tea plantation soil–tea plant–tea infusion production system and identify the crucial factors that influence soil-selenium availability.
2. Materials and Methods
2.1. Study Area
Shitai County is located in the hinterland of the mountainous region of southern Anhui Province, China (29°59′–30°24′ N, 117°12′–117°59′ E). The topography and terrain are complex; high-elevation terrain is situated in the northern and southern parts of the county, and low-elevation terrain is situated in the eastern and western areas of the county. The area of high hills and low mountains constitutes approximately 82% of the total spatial area of the county. The annual average temperature and precipitation is 16 °C and 1626.4 mm, respectively. The annual average frost-free period is 234 d, the average sunshine duration is 1731.1 h, and average annual percentage sunshine is 39%. Thus, the study area has a humid climate typical in the central subtropical region. This area is well-suited for tea plant growth. The soil zonality shows regularity in regional distribution patterns, with Haplic Acrisols and Humic Umbrisols occurring in the mountains, and Dystric Cambisols in the low mountains and hills. The parent materials of the soils are mainly carbonate, argillaceous rock, and acid crystalline rock residual deposits on slopes. Selenium-rich strata, such as the black rock series containing coal seams and carbonate rocks, are the primary contributors to the formation of selenium-rich soil in Shitai County.
2.2. Tea Plant Sampling and Analysis
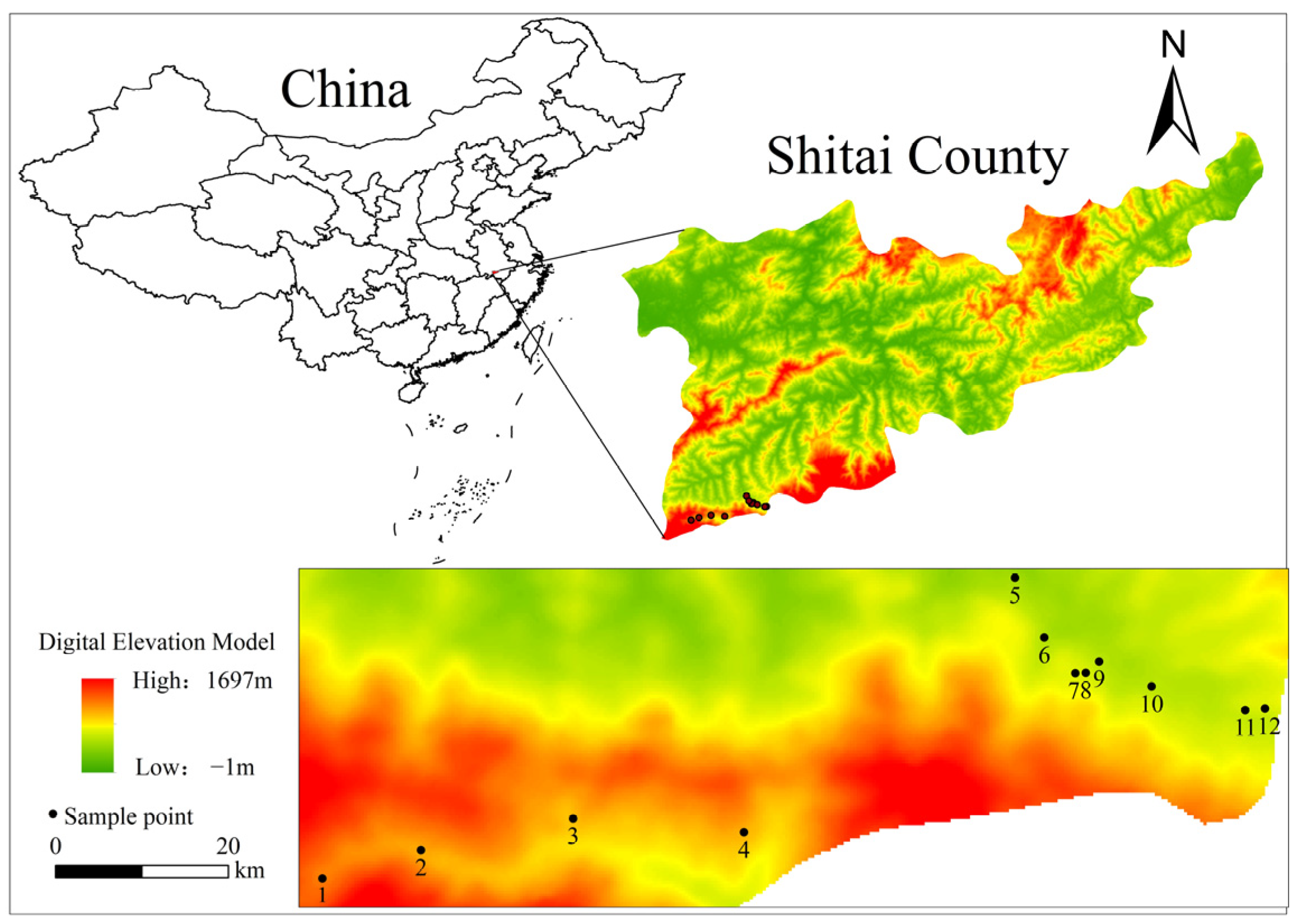
Tea plant samples were collected in April 2022 in the core selenium-rich tea-producing area of Xianyu Mountain, south of Shitai. Based on the spatial distribution of tea plantations on selenium-rich soil in Shitai County, 12 typical tea plantations were selected based on a field investigation and were designated plantations no. 1 to no. 12, respectively (Figure 1). In the tea-producing regions selected for this study, the prevailing cultivars of tea plants grown comprise ‘Zhuye’, ‘Jiuhan’ and a small proportion of ‘Liuye’. All three varieties are Qihong tea cultivars with similar characteristics. Consistent with their prevalence in the tea-producing regions, the predominant cultivars grown in the 12 tea plantations selected for this study were Zhuye and Jiuhan. Basic information on each plantation is listed in Table S1.
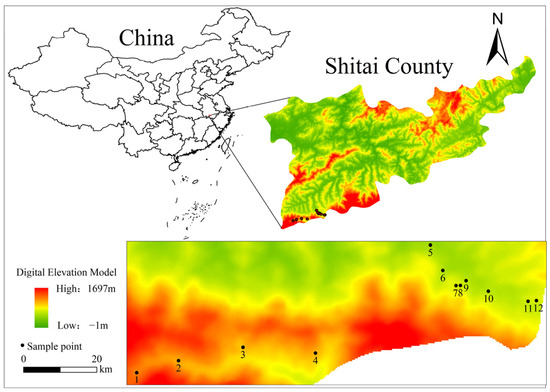
Figure 1.
Sampling points for tea plantations in the research survey.
Three typical plots were selected in each tea plantation with each plot measuring 5 m × 8 m in area. Samples were collected from tea plants using the five-point sampling method within each plot. Samples were collected from six parts of each tea plant: fibrous root, main root, main stem, lateral stem, young leaves (one bud with two leaves each measuring less than 1 cm in width), and old leaves (mature leaves exceeding 2.5 cm in width), of which young leaves were collected for analysis of the selenium content and for preparation of a tea infusion. In each plot, the samples from the five sampling points were combined for each of the six organ samples. Thus, a total of 216 tea plant samples (12 × 3 × 6) were collected.
The tea plant samples were washed with deionized water, dried, and crushed, and then sieved through a 0.18 mm mesh sieve. The tea plant samples used for determination of the selenium contents were digested with 5 mL nitric acid and then analyzed using inductively coupled plasma–mass spectrometry (ICP-MS). The young leaves used for preparation of tea infusion were degreened for 30 min at 105 °C, then processed into green tea by local artisans in accordance with traditional tea-making techniques practiced in the region. For selenium detection in tea infusion, a sample of fresh tea leaves was placed in a 200 mL triangular bottle, 100 mL boiling deionized water was added, allowed to brew for 10 min, and then was filtered and detected using ICP-MS.
2.3. Soil Sampling and Analysis
Soil samples were gathered concurrently with the tea plant samples. Near the rhizosphere of the sampled tea plants, surface soil (0–20 cm depth) samples were collected using a soil drill (5 cm diameter) at five sampling points per plot and mixed thoroughly to form a single composite soil sample. A total of 1 kg soil was taken from each composite sample. Thus, in total, 36 soil samples were collected.
The soil samples were air dried, then ground to pass through 1.0 mm and 0.15 mm mesh sieves. Available selenium was extracted with 0.5 mol L−1 sodium bicarbonate (pH 8.5) and then detected using ICP-MS. The soil pH was determined using the potentiometric method with a soil/water ratio of 1:2.5 (w/w). The soil organic matter content was determined using the K2Cr2O7 oxidation capacity method, while the soil total nitrogen content was analyzed using the Kjeldahl method. The soil-available phosphorus content was assessed with the NH4F–HCl method. The total phosphorus content was determined using the antimony and molybdenum resistance colorimetric method, whereas available potassium was measured following neutral ammonium acetate extraction [30].
2.4. Statistical Analysis
The activation rate of selenium in the soil (ASeR) is the percentage of available selenium to total selenium in a soil sample and was calculated using the formula:
where ASe is the available selenium content in the soil, and Se is the total selenium content in the soil.
ASeR(%) = ASe/Se × 100
The selenium enrichment factor (EF) is the ratio of selenium content in different organs of the tea plant to selenium content in the soil, and represents the ability for selenium enrichment in different organs of the plant. This index was calculated as follows:
where Corgan is the selenium content in the plant organ (mg kg−1), and Csoil is the selenium content in the soil (mg kg−1).
EF = Corgan/Csoil
The selenium translocation factor (TF) refers to the ability for acropetal selenium transport from the base to the top between adjacent organs of the plant. This index was calculated as follows:
where Yj and Yi are the selenium content (mg kg−1) of two organs of the tea plant from the base to the top, respectively.
TF = Yi/Yj × 100
The selenium dissolution rate (DR) represents the mobility of selenium from tea leaves into the tea infusion, indicating the proportion of selenium leaching from the leaves during brewing. The dissolution rate was calculated using the formula:
where Ctea infusion is the selenium content in the tea infusion (mg kg−1) and Cyoung leaf is the selenium content in the young leaf (mg kg−1).
DR = Ctea infusion/Cyoung leaf × 100%
Data are presented as the mean ± standard deviation of three independent replicates. Statistical analyses were performed using IBM SPSS Statistics 26.0 (Chicago, IL, USA). Univariate analysis of variance (ANOVA) was used to assess the significance of differences among the indicators, followed by Duncan’s new multiple range test (p < 0.05). Microsoft Excel 2003, ArcGIS 10.2, Origin 2021, and GraphPad Prism 9.3 software were used for graphical visualization of the data.
3. Results
3.1. Soil Physicochemical Properties
The soil physicochemical properties of the tea plantations are summarized in Table S2. The soil texture for all tea plantations was classified as loamy clay. The mean soil bulk density was recorded as 1.19 g cm−3. The soil pH in the tea plantation ranged from 5.58 to 6.86. The coefficient of variation (CV) ranged from 1.6% to 7.7%, indicating there was limited variation in the soil pH. The soil in the tea plantation was rich in organic matter content, which ranged from 7.11 to 99.8 g kg−1. However, the CV for soil organic matter content at the different sampling sites varied greatly. Soil total nitrogen content in the tea plantation varied between 1.78 and 5.27 mg kg−1. The total phosphorus content of the soil ranged from 0.04% to 0.17%, and the average available phosphorus content in the soil was 4.19 mg kg−1. These results indicated that the total phosphorus content was high and the soil-available phosphorus content was low. The soil-available potassium content varied between 72 and 142 mg kg−1, and the CV ranged from 5.3% to 25.6%. In summary, the soil in the study area was high in organic matter content and low in available phosphorus content, and exhibited notable spatial variation in soil physicochemical properties.
3.2. Soil Selenium Content
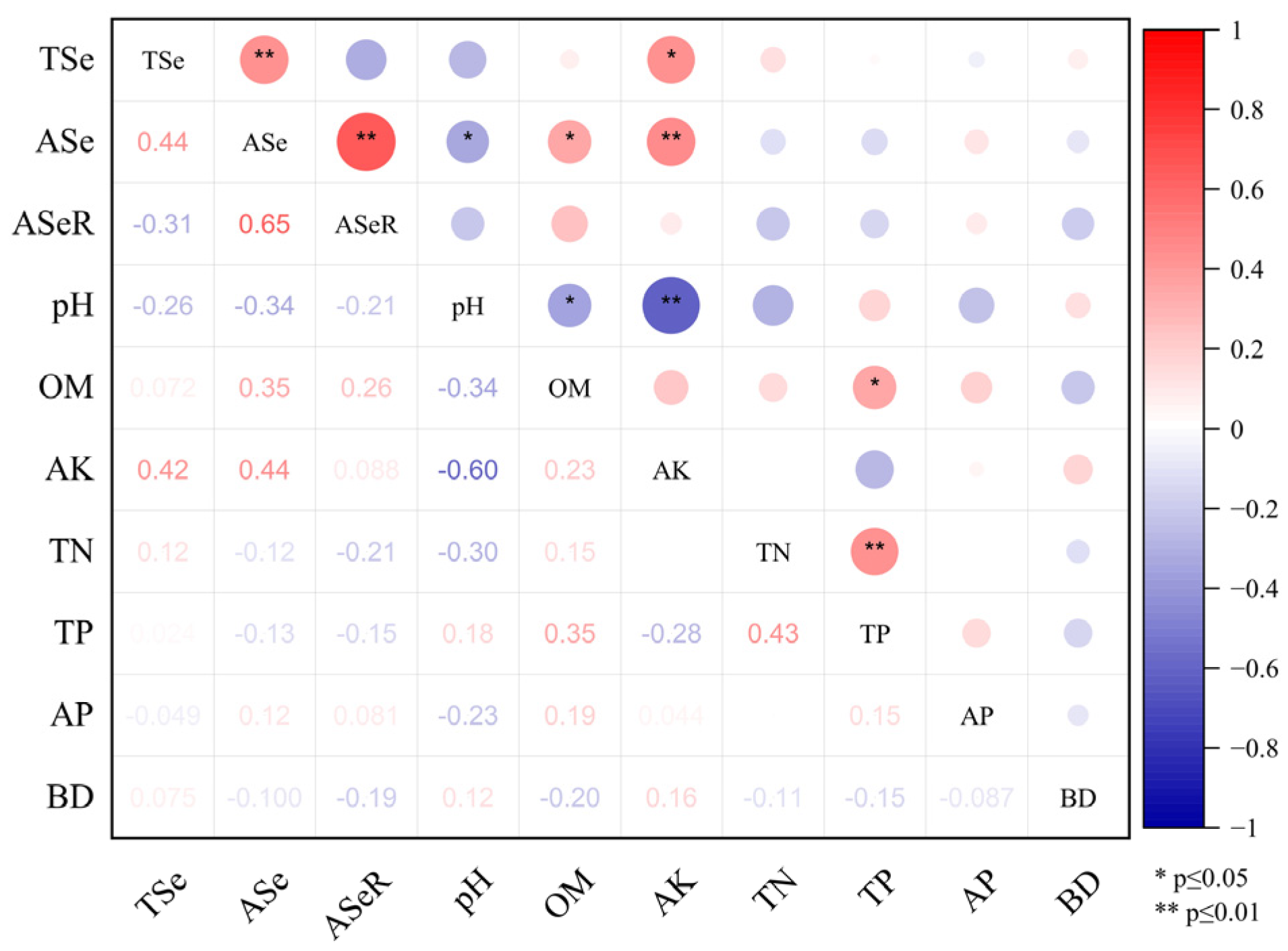
The topsoil total selenium content in the tea plantations ranged from 1.12 to 6.67 mg kg−1, with an average selenium content of 2.58 mg kg−1. The soil selenium contents of the no. 9 and no. 10 plantations were greater than 3 mg kg−1, exceeding the selenium toxicity soil standard (≥3 mg kg−1) [31]. The available selenium content in the plantation soil ranged from 10.4 to 140 µg kg−1, with an average of 53.56 µg kg−1. The ASeR ranged from 0.32% to 4.96%, with an average of 2.27%. The range of CVs for total selenium content, available selenium content, and ASeR in the soil were 1.13%–45.32%, 6.64%–55.2%, and 11.9%–66.67%, respectively (Table 1). Thus, the distribution of the selenium content in the tea plantation soil showed a degree of regional spatial heterogeneity. Correlation analysis indicated that the soil-available selenium content was positively correlated with soil-available potassium content (p < 0.01) and soil organic matter content (p < 0.05) and was negatively correlated with soil pH (p < 0.05; Figure 2). These results indicated that soil-available potassium, soil organic matter content, and soil pH might be crucial factors that influence soil-selenium availability.

Table 1.
Total and available Se in tea plantation soils.
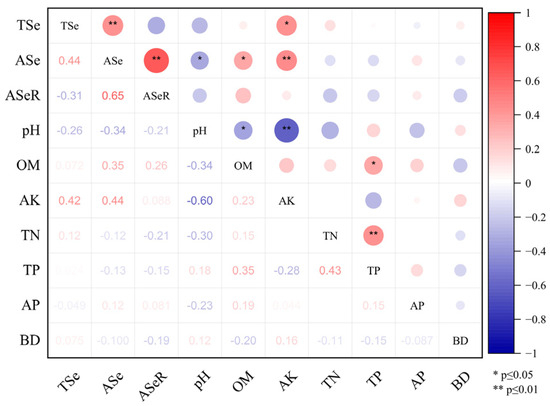
Figure 2.
Correlation analysis of soil selenium and soil physicochemical indicators. Note: TSe, ASe, ASeR, OM, AK, TN, TP, AP, BD represent total selenium, available selenium, the activation rate of selenium in the soil, organic matter, available potassium, total nitrogen, total phosphorus, available phosphorus, and bulk density, respectively.
3.3. Distribution of Selenium in Tea Plants
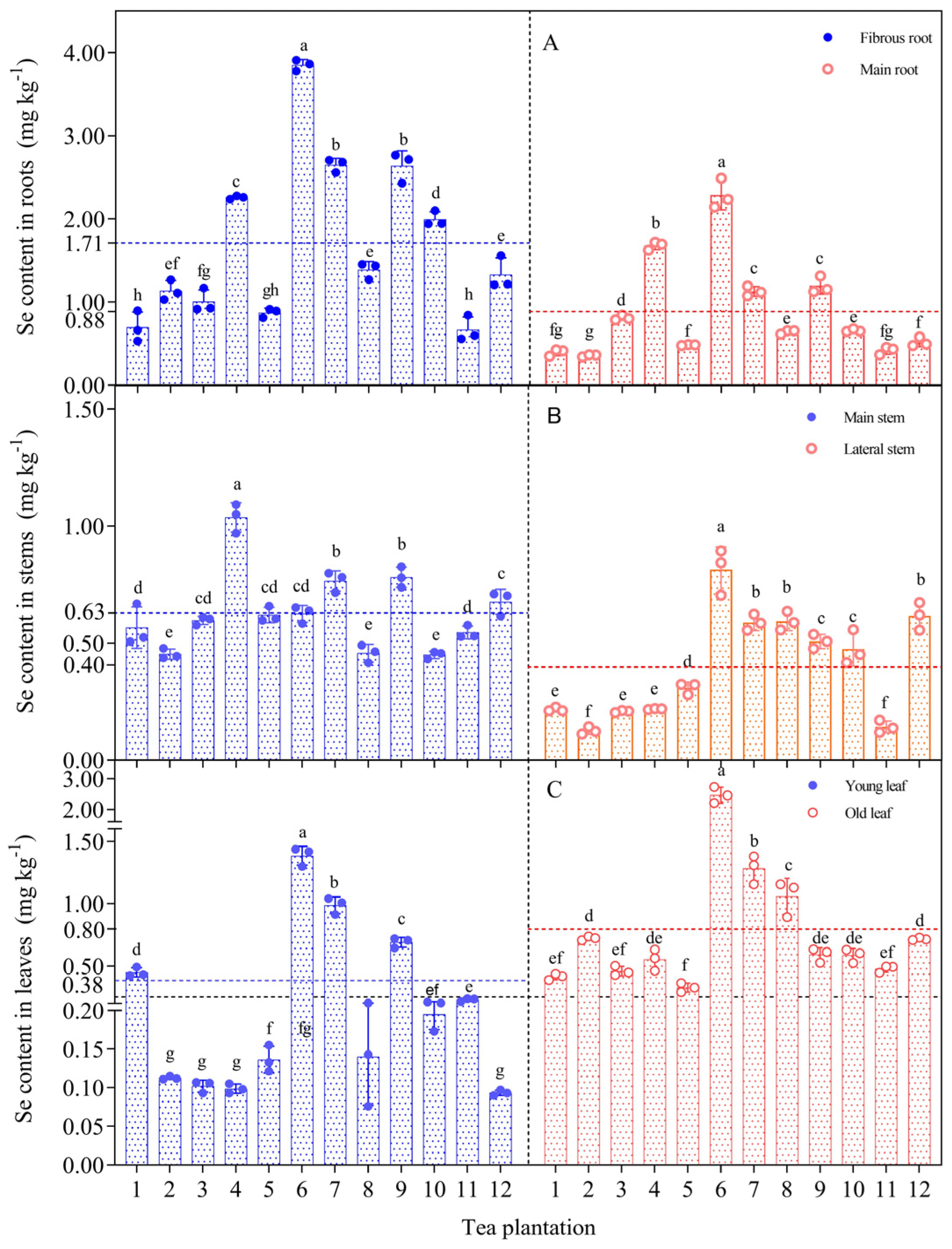
The ability to accumulate and transport selenium differed significantly among different organs of the tea plants (Figure 3). The selenium content in the fibrous root was significantly higher than that of the other organs. The selenium content showed a trend to decrease from the fibrous root (0.53–3.91 mg kg−1) to the main root (0.34–2.49 mg kg−1), from the main stem (0.42–1.09 mg kg−1) to the lateral stem (0.11–0.89 mg kg−1), and from old leaves (0.29–2.73 mg kg−1) to young leaves (0.08–1.44 mg kg−1).
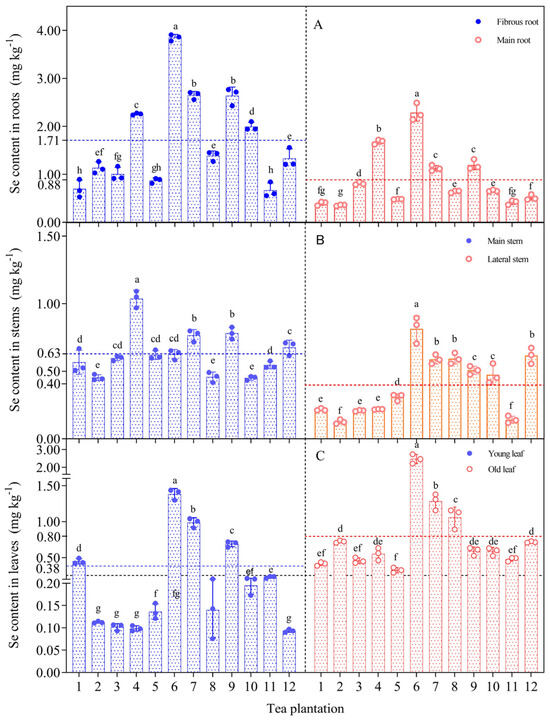
Figure 3.
Distribution of selenium content in roots (A), stems (B) and leaves (C) of tea plants. Different letters indicate significant differences between the corresponding indices across various sampling point, p < 0.05. Note: The red and blue dashed lines represent the mean values of different tea plant organs, while the black dashed lines represent the standard selenium content in selenium-rich tea (0.25 mg kg−1).
The uneven distribution of selenium in the tea plants indicated that the intake and transport capacity of selenium differed significantly among the organs of the tea plant. The selenium content of old leaves exceeded the standard value for selenium-rich tea of 0.25 mg kg−1, and only 33% of the selenium content in young leaves exceeded the standard value. In plantation no. 6, the selenium content of tea plant organs was generally higher than that at the other sampling sites. Tea plantation no. 11 had the lowest selenium content in the main roots and lateral stems, but the selenium content of young leaves and old leaves approached or exceeded the standard value for selenium-rich tea.
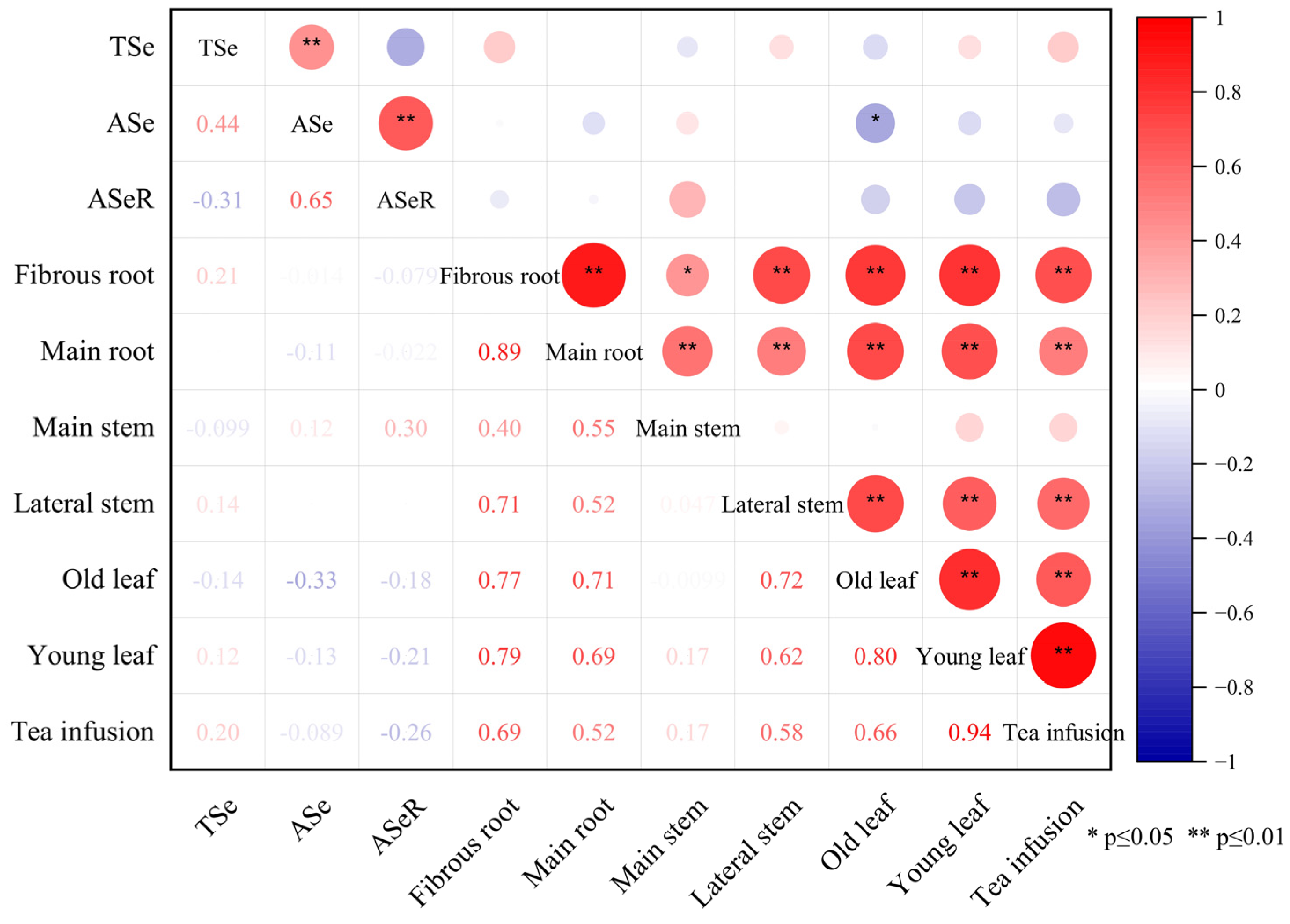
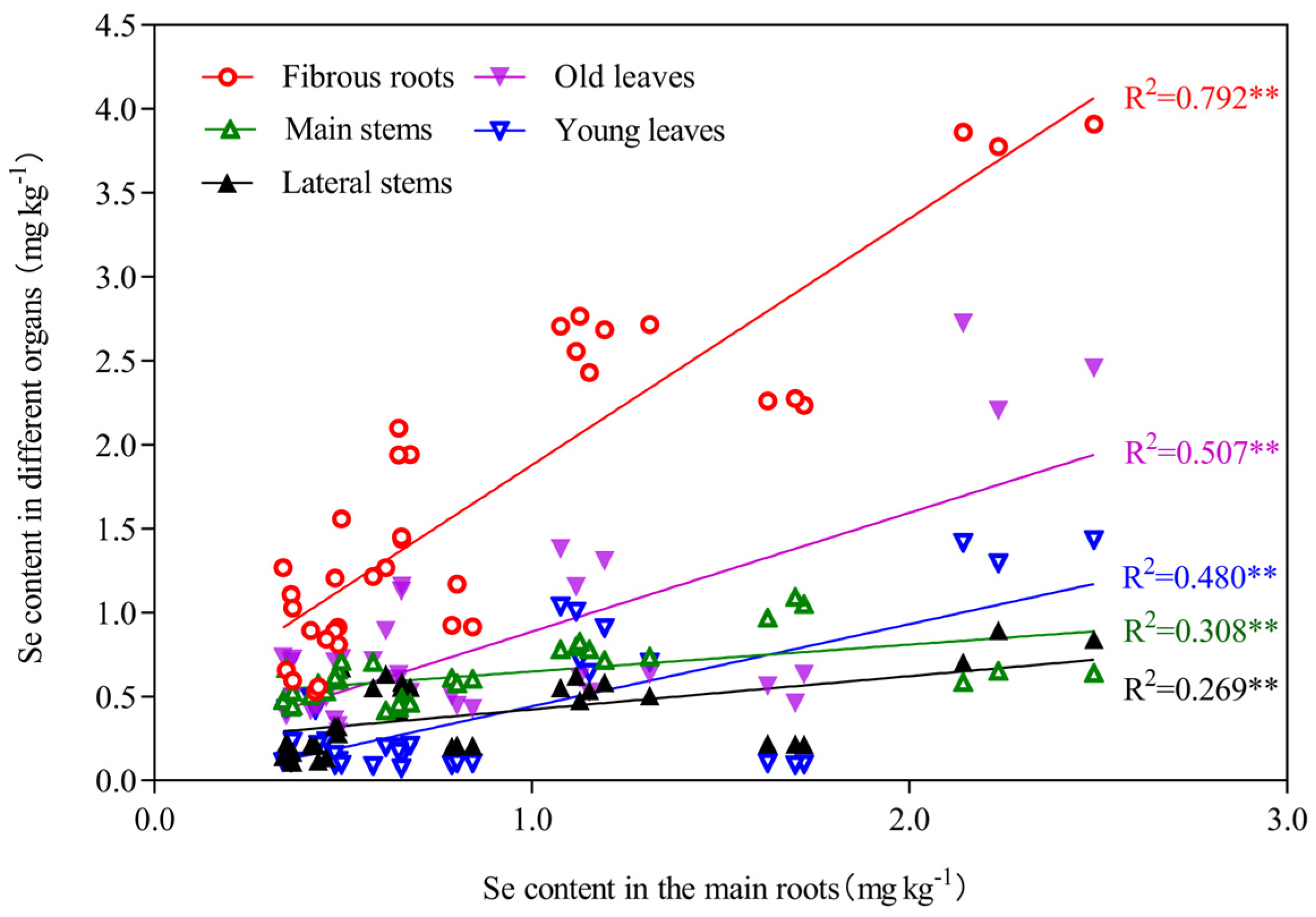
We found that there was no significant correlation between the selenium content in the tea plantation soil and the content in different organs of tea plants (Figure 4). However, the different organs of tea plants exhibited relatively consistent selenium distribution characteristics, selenium accumulation in young leaves, old leaves, main stems, lateral stems, and fibrous roots was strongly associated with the selenium content in the main roots (Figure 5).
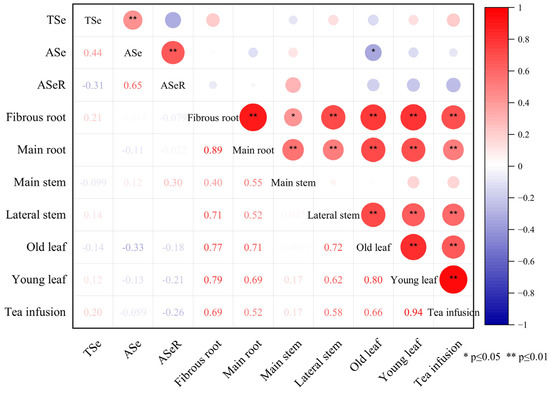
Figure 4.
Correlation heatmap between Se content in tea plantation soil and different organs of tea plants. Note: TSe, ASe, ASeR represent total selenium, available selenium, the activation rate of selenium in the soil, respectively.
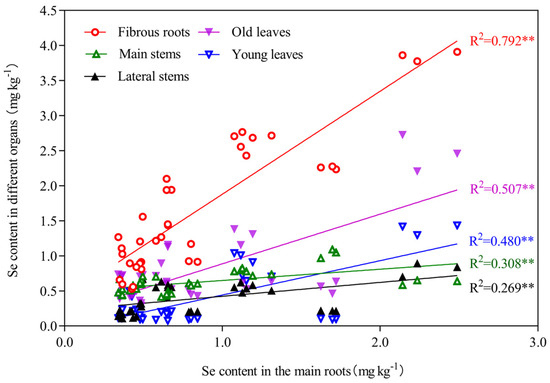
Figure 5.
Linear regression analysis between the main roots and other organs of tea plants. Note: ** represent the significance level of 1% in statistical analysis (p < 0.01).
3.4. Selenium Enrichment Factor in Tea Plants
The ability to enrich selenium differed significantly among organs of the tea plants (Table 2). The fibrous root had the strongest ability to enrich selenium and the average selenium EF was 0.79. The soil-available selenium was taken up by the fibrous roots and then translocated to the main root. The selenium EF of the main root was 0.42, which was approximately half of that of the fibrous root. Most of the selenium absorbed by the tea plant was concentrated in the root, and the selenium transported to the aboveground parts gradually decreased. The EFs for the main stem, lateral stem, and young leaves were 0.30, 0.19, and 0.17, respectively. The EF of old leaves was 0.40. Therefore, the selenium enrichment ability of the analyzed organs of the tea plant in selenium-rich tea plantations was ranked as fibrous root, main root, main stem, lateral stem, and young leaves, and eventually accumulated in old leaves. With regard to the selenium enrichment ability in different organs of the three tea varieties, Zhuye had the strongest selenium enrichment ability, whereas Jiuhan had the weakest selenium enrichment ability. The lowest EF values were observed in young leaves of Liuye (Table S3).

Table 2.
Se enrichment factor (EF) in different organs of tea plants.
3.5. Selenium Translocation Factor for Different Organs of Tea Plants
The average selenium TFs are listed in Table S4. Approximately 53% of selenium migrated from the fibrous roots to the main roots, 92% from the main roots to the main stem, 67% from the main stem to the lateral stem, 97% from the lateral stem to young leaves, and 203% from the lateral stem to old leaves. Translocation factors from the main roots to the main stem were >100% at plantations no. 1, no. 2, no. 5, no. 11, and no. 12, <30% at plantation no. 6, and between 60% and 100% at the other plantations. Translocation factors from the main stem to the lateral stem were ≥100% at plantations no. 6, no. 8, no. 10, and no. 12, from the lateral stem to young leaves were ≥100% at plantations no. 1, no. 6, no. 7, no. 9, and no. 11, and from the lateral stem to old leaves was ≥100% at all plantations.
3.6. Selenium Concentration in Tea Infusion
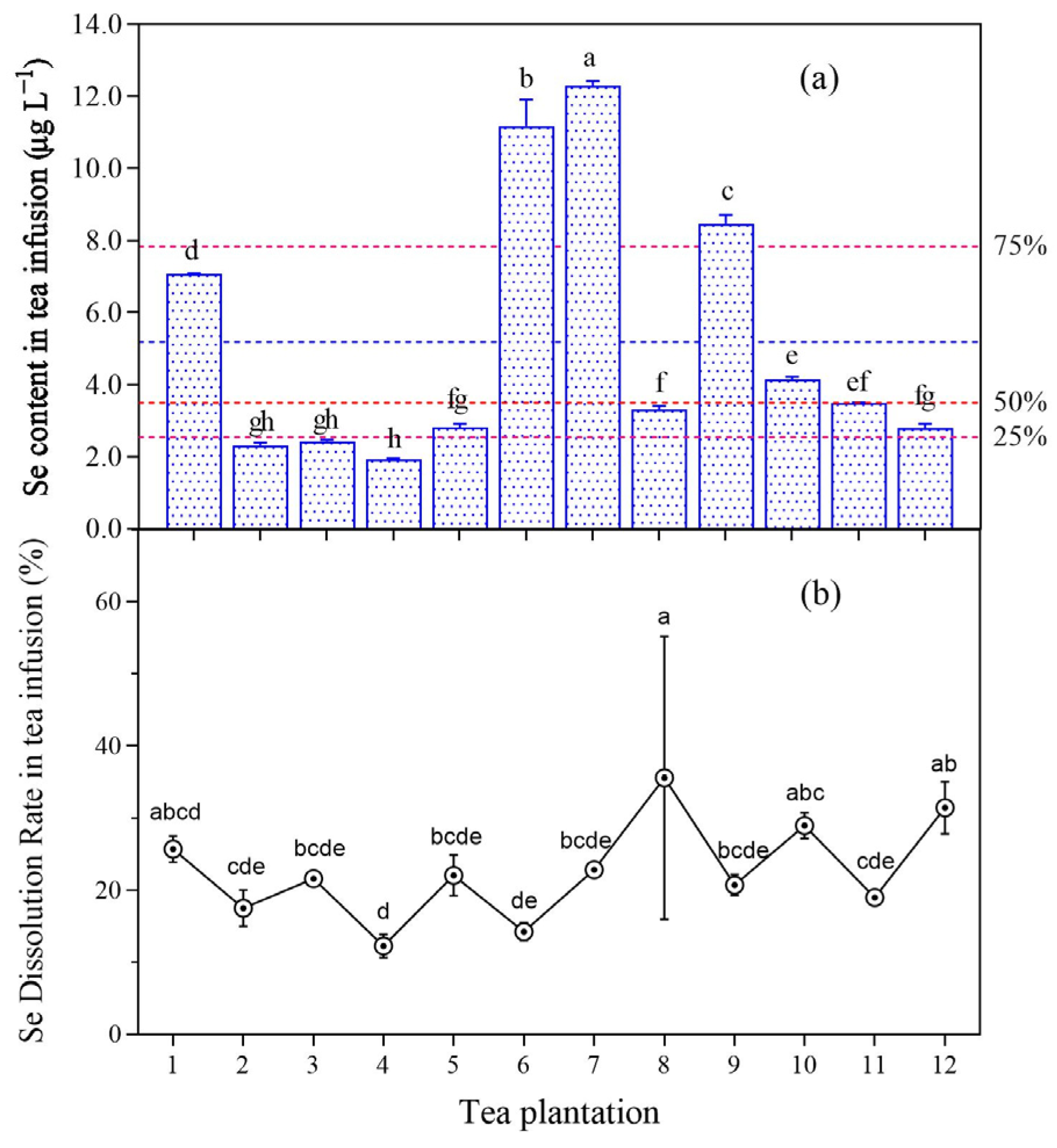
The selenium concentration in tea infusion ranged from 1.88 to 12.49 μg L−1 (Figure 6), the mean was 5.18 μg L−1, the median was 3.49 μg L−1, the 25% quantile was 2.53 μg L−1, the standard deviation was 3.55 μg L−1, the average standard error was 0.592 μg L−1, and the CV was 69%. The selenium concentration exceeded the average value for plantations no. 1, no. 6, no. 7, and no. 9, among which the selenium concentration in tea infusion for plantations no. 6 and no. 7 exceeded 10 μg L−1. The selenium concentration in tea infusion for plantations no. 1, no. 6, no. 7, no. 9, no. 10, and no. 11 was similar to or greater than the 50% quantile.
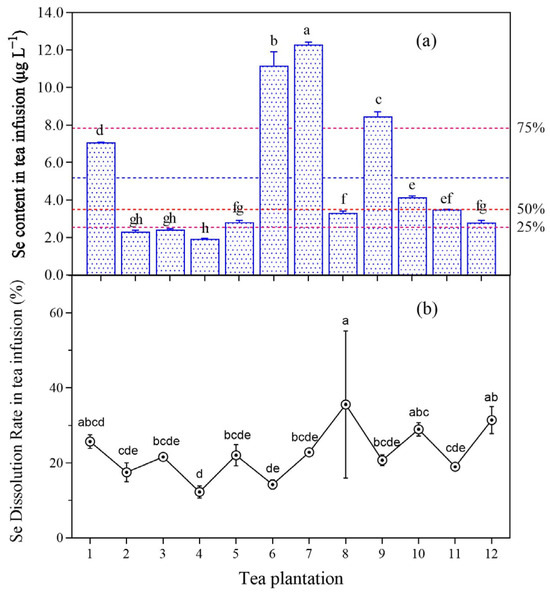
Figure 6.
Selenium content (a) and dissolution rate (b) of tea infusion after one brewing of young leaves. Different letters indicate significant differences between the corresponding indices across various sampling point, p < 0.05. Note: The red dashed lines in figure (a) represent the respective quantile lines, while the blue dashed lines depict the mean values.
4. Discussion
4.1. Availability and Factors Influencing Soil Selenium in Tea Plantations
Owing to the exposure of coal-bearing strata in the Lower Cambrian Hetang Formation [23,32], the topsoil of tea plantations in Shitai County is rich in selenium, which is approximately 11 times that of the background selenium value of surface soil in Anhui Province, China (0.23 mg kg−1). The ASeR in the soil of the same region was previously reported to range from 2.47% to 6.05% [33], which was consistent with the present results. Selenite, selenate, and organic selenium are the main plant-available forms of selenium [34]. A significant positive correlation was observed between the available selenium and total selenium contents in the soil (Figure 2). The available selenium content was strongly related to the soil chemical properties. The soil-selenium availability was significantly negatively correlated with soil pH (p < 0.05). This finding was supported by previous research [35]. Soil pH is associated with the redox potential and soil mineral ion content, which strongly affects the adsorption and desorption abilities of the soil [36]. In neutral or alkaline soil, selenium exists as selenate (SeO42−), which has weaker adsorb ability in a clay soil [37]. In acidic soil, selenium mainly exists in the form of selenite (SeO32−), which is more readily immobilized by absorption to soil iron and aluminum oxides [35,38]. The soil pH in the tea plantation ranged between 5.58 and 6.8, and may be the primary reason that the soil ASeR was relatively low. Therefore, an appropriate increase in the soil pH through agronomic practices would increase the availability of selenite in acidic soils.
The soil-available selenium content was greatly influenced by organic matter [39]. A significant positive correlation was observed between the selenium and organic matter contents in agricultural soils [40]. However, the effect of organic matter on soil-selenium availability remains controversial. Organic matter adsorbed and immobilized soil selenium in a bound state, thereby reducing soil-selenium availability [23]. The present results showed that soil-available selenium content was significantly positively correlated with soil organic matter content (p < 0.05), which may be associated with the rich content of organic matter in the local soil and the high enrichment of selenium. Through the activation of soil organic matter and microbial metabolism, soil selenium was activated into available selenium. Simultaneously, soil organic matter mineralization released organic selenium, thus improving the availability of selenium.
Soil properties and selenium content may be affected by the elevation of the tea plantation. The total and available selenium contents were significantly higher in tea plantations located at moderate and high elevations (500–800 m) than at low elevation (<500 m) [41]. However, in the present study, tea plantations no. 1, no. 2, no. 3, and no. 4 situated at moderate and high elevations had average (or median) total and available selenium contents. In contrast, plantations no. 9 and no. 10 at low elevations had high selenium contents, with total selenium contents of 3.99 and 5.5 mg kg−1, respectively, and available selenium contents of 75 and 78 μg kg−1, respectively. These results indicated that the elevation of the tea plantation affects the distribution of selenium in the soil, but the decisive factor in the selenium content lies in the soil parent material. The coal-bearing strata in the parent rock in plantations no. 9 and no. 10 are extensively exposed. Selenium is extremely active in strata containing coal and is readily released to the local soil environment [42].
4.2. Selenium Enrichment and Migration in the Soil–Tea Plant System
As an important trace element to improve the quality of tea, selenium enters tea plant tissues by way of atmospheric deposition and root uptake. Root uptake from the soil is the main selenium source for plants in terrestrial ecosystems [11,43]. Significant differences in the capacity for the absorption of soil selenium by different organs of tea plants were observed in this study, and the selenium EF showed a decreasing trend from fibrous roots, main roots, old leaves, main stems, lateral stems, and young leaves. The root system absorbs and enriches a large amount of soil selenium, especially fibrous roots, which was consistent with the findings of previous research [44]. The absorption of selenium by roots is controlled by various factors, especially by the bioavailability of soil selenium [37]. In this study, despite the low bioavailability of selenium (ASeR = 2.27%), the EF of fibrous roots reached 0.78, indicating an extremely strong selenium enrichment ability, which may be associated with the tea plant cultivars grown in the study region. The selenium EFs of the main stem and lateral stem were average, and reflected the lack of a significant correlation between the main stem and other organs (Figure 4). The leaves of tea plants have not evolved specialized tissues for selenium absorption [45], and the weakest selenium enrichment ability observed was that for young leaves (EF = 0.17). The EF value for old leaves was substantially higher than that for young leaves, indicating that selenium continued to accumulate in the old leaves over time.
Understanding the distribution of selenium in tea plants solely from the perspective of its enrichment and absorption is inadequate. More importantly, the knowledge of how selenium is transported and redistributed after entering the tea plant is required. The highest selenium content in the fibrous roots and main roots in the 12 tea plantations was 24.5 times and 8.6 times that of the young leaves, respectively, indicating that the selenium transport ability is low in the tea plant. From the perspective of selenium migration in the soil–tea plant system, selenium is mainly transferred between the soil and the root system. The tea plant root system rapidly converts selenite adsorbed from the soil through phosphate transporter proteins into organic selenium, which obstructs the efficiency of selenium transport from the fibrous root to the main root [46]. In the present study, the selenium TF from the fibrous root to the main root was only 52.85%. The selenium is more highly concentrated in the root epidermis and root cortex tissues of alfalfa (Medicago sativa) [47], and the binding of selenium in the root epidermis and root cortex further limits the efficiency of selenium transport from the main root to the aboveground parts. Although the migration efficiency between the adjacent organs of the tea plant gradually increases with the development of autonomous roots, the proportion of selenium content reaching the leaves is still not high. This may be a physiological stress–response mechanism in tea plants growing in selenium-rich soils to effectively inhibit selenium toxicity. In addition, selenium transport is constrained by factors such as selenium transport proteins and ion channels in tea plants [48]. To further enhance the selenium content in young leaves, tea cultivars that exhibit high selenium enrichment and migration transport ratios should be preferentially planted. This approach will enhance the mobility of selenium from the root system to young leaves and improve the utilization efficiency of selenium-rich soil resources. Further studies focused on the metabolic and molecular levels are required to elucidate the underlying mechanisms of selenium absorption and transport in tea plants.
Selenium absorption and transport by tea plants is constrained by multiple factors, such as the selenium forms in the soil, soil properties, and tea plant cultivars [49]. No significant correlation was observed between the selenium content in the tea plantation soil and the content in different organs of tea plants in the present study (Figure 4). However, different organs of tea plants exhibited relatively consistent selenium distribution characteristics. The selenium content in selenium-rich soil may not be the only factor that determines the migration and distribution of selenium in tea plants. Selenium accumulation in young leaves, old leaves, main stems, lateral stems, and fibrous roots was strongly associated with the selenium content in the main roots. Among the six organs of tea plants studied, a significant correlation with selenium content was only observed for the main root (p < 0.01; Figure 4). The goodness-of-fit for a linear regression of the selenium content between the main root and other organs of the tea plant was relatively high (p < 0.01; Figure 5). Therefore, the main roots of tea plants and not the soil selenium content are the dominant factor that determines the redistribution of selenium in different organs of tea plants grown in selenium-rich soils.
4.3. Characteristics of Selenium Release from Young Leaves
The selenium dissolution rate in tea infusion was significantly affected by the temperature, duration, and frequency of tea brewing [50,51]. A strong correlation was observed between selenium content in young leaves and selenium concentration in tea infusion (p < 0.01). Using the generally applied tea-brewing method, the average selenium dissolution rate in the tea infusion after one brewing was 22.6% (Figure S1), which was higher than the average selenium dissolution rate of 4.72% previously reported for nine types of Chinese tea [27]. Based on a survey by the World Health Organization, the upper limit of selenium intake for healthy adults is 400 µg day−1 [52]. A recommended standard for the dietary intake of selenium by humans of 57 µg day−1 was proposed [53], and drinking selenium-rich tea is an effective means to supplement selenium intake. The average daily tea consumption by adults is 11.4 µg [54]. On this basis, the average selenium intake would be 0.98 µg day−1 from the studied tea after one brewing. After three brewings, the total dissolution rate of selenium can reach 60% [55], from which the daily consumption of selenium-rich tea could provide approximately 2.60 µg day−1 of selenium. The selenium content in all old leaves exceeded the lower limit (0.25 mg kg−1) of the selenium-rich tea standard [56]. However, young leaves from only four tea plantations met this standard, despite the average selenium content in young leaves from 12 plantations of 0.43 mg kg−1. These results inferred that the tea produced in selenium-rich areas may not qualify as genuine selenium-rich tea.
The study area was limited to a specific region within Shitai County, necessitating a further expansion of the investigation scope for selenium-rich tea plantations. Our findings revealed significant disparities in the capacity for soil-selenium enrichment among the three tea cultivars, with significant variations in selenium transport capacity observed among different tea plant varieties (Table S5). Nevertheless, an examination of the biological mechanisms governing soil selenium absorption and transportation across different tea cultivars was not undertaken. The further investigation of the mechanism underlying the low selenium mobility from the fibrous roots to the main roots of tea plants grown in selenium-rich areas is required. In addition, a molecular-level analysis of selenium enrichment and transport among different organs of tea plants should be undertaken. Tea cultivars with a high selenium EF and TF should be selected and cultivated. It will be essential to cultivate high-quality selenium-rich tea and continue to optimize soil nutrient supply, cultivation techniques, and harvesting management practices.
5. Conclusions
The soil selenium content of selenium-rich tea plantations ranged from 1.16 to 5.50 mg kg−1, of which 30.56% of the studied plantations points had excessive selenium contents. The available selenium content in the soil ranged from 10.4 to 140 µg kg−1 and the activation rate ranged from 1.31% to 4.25%. The soil total selenium content was positively correlated with available selenium, and, in addition, soil pH, soil organic matter content, and available potassium were important factors that affected soil-selenium availability. The average selenium EF was highest in fibrous roots and lowest in young leaves, and the EF of old leaves was substantially higher than that for young leaves. The selenium content of the main roots was positively correlated with the selenium content in other organs, which is the dominant factor that determines the redistribution of selenium in tea plants. The TF from fibrous roots to main roots was 53%, from main roots to main stems was 72%, from main stems to lateral stems was 64%, and from lateral stems to young leaves was 96%. The average dissolution rate of selenium from young leaves to a tea infusion was 22.62%. Drinking selenium-rich tea is an effective and healthy means to supplement selenium intake. In general, the selenium content of soils in Shitai County is adequate, but only one-third of young leaves met the selenium-rich tea standard. Additional measures should be used to produce selenium-rich tea, such as soil amendments, planting tea varieties with high selenium enrichment and transport factors, and an optimization of the picking period.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15060914/s1, Figure S1: Linear regression analysis of selenium in young leaves and tea infusion; Table S1: Basic properties of sampling sites in selenium-rich tea plantations; Table S2: Physical and chemical indices of selenium-rich tea plantation soil in Shitai County; Table S3: Selenium Enrichment Factor (EF) among various tea plant varieties; Table S4: Selenium Translocation Factor (TF) between different organs of tea plants; Table S5: Selenium Translocation Factor (TF) among different varieties of tea plants.
Author Contributions
H.W. Data curation, formal analysis, funding acquisition, writing—review and editing; D.Z. investigation, visualization, writing—original draft; X.W. visualization, writing—original draft; X.T. visualization, writing—original draft; G.H. visualization, writing—original draft; S.L. visualization, writing—original draft; X.J. supervision; D.W. funding acquisition, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (grant number 2021YFD1700903) and the Key Natural Science Research Projects in Universities of Anhui Province (grant number KJ2021A1137).
Data Availability Statement
Data are contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dinh, Q.T.; Cui, Z.W.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.X.; Zhou, F.; Wang, M.K.; Yu, D.S.; Liang, D.L. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.F.; Ning, Z.P.; Kwon, S.Y.; Li, M.L.; Tack, F.G.; Kwon, E.E.; Rinklebe, J.; Yin, R.S. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Qin, H.B.; Zhu, J.M.; Liang, L.; Wang, M.S.; Su, H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013, 52, 66–74. [Google Scholar] [CrossRef]
- Ekumah, J.N.; Ma, Y.; Akpabli-Tsigbe, N.D.K.; Kwaw, E.; Ma, S.M.; Hu, J. Global soil distribution, dietary access routes, bioconversion mechanisms and the human health significance of selenium: A review. Food Biosci. 2021, 41, 100960. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, M.; Zhou, F.; Zhai, H.; Qi, M.X.; Liu, Y.; Li, Y.N.; Zhang, N.C.; Ma, Y.Z.; Huang, J.; et al. Selenium bioavailability in soil-wheat system and its dominant influential factors: A field study in Shaanxi province, China. Sci. Total Environ. 2021, 770, 144664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Ren, J.G.; Xue, C.Z.; Lin, E.D. Study on the relationship between soil selenium and plant selenium uptake. Plant Soil 2005, 277, 197–206. [Google Scholar] [CrossRef]
- Gong, J.J.; Yang, J.Z.; Wu, H.; Fu, Y.A.; Gao, J.W.; Tang, S.X.; Ma, S.M. Distribution of soil selenium and its relationship with parent rocks in Chengmai County, Hainan Island, China. Appl. Geochem. 2022, 136, 105147. [Google Scholar] [CrossRef]
- Ni, R.X.; Luo, K.L.; Tian, X.L.; Yan, S.G.; Zhong, J.T.; Liu, M.Q. Distribution and geological sources of selenium in environmental materials in Taoyuan County, Hunan Province, China. Environ. Geochem. Health 2016, 38, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chen, C.Q.; Yin, R.S.; Shen, Y.; Mao, K.; Yang, Z.G.; Feng, X.B.; Zhang, H. Bioaccumulation of Hg in rice leaf facilitates selenium bioaccumulation in rice (Oryza sativa L.) leaf in the Wanshan mercury mine. Environ. Sci. Technol. 2020, 54, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Yuan, L.X.; Qi, S.H.; Yin, X.B. The threshold effect between the soil bioavailable molar Se: Cd ratio and the accumulation of Cd in corn (Zea mays L.) from natural Se-Cd rich soils. Sci. Total Environ. 2019, 688, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Song, H.X.; Guo, Y.B.; Fan, B.; Huang, Y.T.; Mao, X.F.; Liang, K.H.; Hu, Z.Q.; Sun, X.D.; Fang, Y.; et al. Benefit–risk assessment of dietary selenium and its associated metals intake in China (2017–2019): Is current selenium-rich agro-food safe enough? J. Hazard. Mater. 2020, 398, 123224. [Google Scholar] [CrossRef]
- Sentkowska, A. Content of selenoaminoacids and catechins in Chinese green teas. Eur. Food Res. Technol. 2021, 247, 613–622. [Google Scholar] [CrossRef]
- Chen, S.Z.; Zhu, S.P.; Lu, D.B. Solidified floating organic drop microextraction for speciation of selenium and its distribution in selenium-rich tea leaves and tea infusion by electrothermal vapourisation inductively coupled plasma mass spectrometry. Food Chem. 2015, 169, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Q.; Liu, K.L. Selenium and selenoproteins: Their function and development of selenium-rich foods. Int. J. Food Sci. Technol. 2022, 57, 7026–7037. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, Y.Q.; Ruan, J.Y.; Yin, J.F. Selenium affects the activity of black tea in preventing metabolic syndrome in high-fat diet-fed Sprague–Dawley rats. J. Sci. Food Agric. 2020, 100, 225–234. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Song, X.W.; Luo, J.L.; Yu, Z.; Ni, D.J. Natural variation of selenium concentration in diverse tea plant (Camellia sinensis) accessions at seedling stage. Sci. Hortic. 2016, 198, 163–169. [Google Scholar] [CrossRef]
- Liu, X.W.; Zhao, Z.Q.; Duan, B.H.; Hu, C.X.; Zhao, X.H.; Guo, Z.H. Effect of applied sulphuron the uptake by wheat of selenium applied as selenite. Plant Soil 2015, 386, 35–45. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.L.; Peng, Q.; Cui, Z.W.; Huang, J.; Lin, Z.Q. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Hu, Q.H.; Xu, J.; Pang, G.X. Effect of selenium on the yield and quality of green tea leaves harvested in early spring. J. Agric. Food Chem. 2003, 51, 3379–3381. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Meng, Q.; Shi, J.; Zhou, M.X.; Zhu, Y.; You, Q.S.; Xu, P.; Wu, W.L.; Lin, Z.; Lv, H.P.; et al. Special tea products featuring functional components: Health benefits and processing strategies. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1686–1721. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.Y.; Yan, W.; Peng, L.J.; Zhou, J.J.; He, J.L.; Zhang, N.; Cheng, S.Y.; Cai, J. Insights into the key quality components in Se-Enriched green tea and their relationship with Selenium. Food Res. Int. 2023, 165, 112460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Deng, Y.L.; Wu, X.L.; Zhang, D.; Wang, F.; Liu, K.C.; Lu, S.Y. The levels of selenium in tea from China and associated human exposure. J. Food Compos. Anal. 2022, 110, 104567. [Google Scholar] [CrossRef]
- Long, Z.D.; Yuan, L.X.; Hou, Y.Z.; Bañuelos, G.S.; Liu, Y.X.; Pan, L.P.; Liu, X.D.; Yin, X.B. Spatial variations in soil selenium and residential dietary selenium intake in a selenium-rich county, Shitai, Anhui, China. J. Trace Elem. Med. Biol. 2018, 50, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.C.; Ren, R.; Wang, L.X.; Zhi, Q.; Yu, T.; Hou, Q.Y.; Yang, Z.F. Using machine learning to predict selenium and cadmium contents in rice grains from black shale-distributed farmland area. Sci. Total Environ. 2024, 912, 168802. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agricultural Chemistry Analysis; Chinese Agriculture and Technology Press: Beijing, China, 2000; pp. 13–14, 108–109, 147–149, 168–169, 181, 194–195. (In Chinese) [Google Scholar]
- Cui, Z.W.; Huang, J.; Peng, Q.; Yu, D.S.; Wang, S.S.; Liang, D.L. Risk assessment for human health in a seleniferous area, Shuang’an, China. Environ. Sci. Pollut. Res. 2017, 24, 17701–17710. [Google Scholar] [CrossRef]
- Yang, R.Y.; He, Y.H.; Luo, L.F.; Zhu, M.; Zan, S.T.; Guo, F.Y.; Wang, B.; Yang, B.B. The interaction between selenium and cadmium in the soil-rice-human continuum in an area with high geological background of selenium and cadmium. Ecotoxicol. Environ. Saf. 2021, 222, 112516. [Google Scholar] [CrossRef]
- Wen, B.Y.; Zhang, T.L.; Li, X.Z.; Xie, Z.D. A feasibility study of selenium-rich soil development in Longnan County of Jiangxi Province. Geol. China 2014, 41, 256–263, (In Chinese with English Abstract). [Google Scholar]
- Zhang, M.; Xing, G.F.; Tang, S.H.; Pang, Y.W.; Yi, Q.; Huang, Q.Y.; Huang, X.; Huang, J.F.; Li, P.; Fu, H.T. Improving soil selenium availability as a strategy to promote selenium uptake by high-Se rice cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Shao, Y.; Cai, C.F.; Zhang, H.T.; Fu, W.; Zhong, X.M.; Tang, S. Controlling factors of soil selenium distribution in a watershed in Se-enriched and longevity region of South China. Environ. Sci. Pollut. Res. 2018, 25, 20048–20056. [Google Scholar] [CrossRef]
- Zhai, H.; Kleawsampanjai, P.; Wang, M.; Qi, M.X.; Liu, Y.; Liu, N.N.; Zhou, F.; Wang, M.K.; Liang, D.L. Effects of soil moisture on aging of exogenous selenate in three different soils and mechanisms. Geoderma 2021, 390, 114966. [Google Scholar] [CrossRef]
- Constantino, L.V.; Quirino, J.N.; Monteiro, A.M.; Abrao, T.; Parreira, P.S.; Urbano, A.; Santos, M.J. Sorption-desorption of selenite and selenate on Mg-Al layered double hydroxide in competition with nitrate, sulfate and phosphate. Chemosphere 2017, 181, 627–634. [Google Scholar] [CrossRef]
- Gavrilenko, N.A.; Saranchina, N.V.; Fedan, D.A.; Gavrilenko, M.A. Solid-phase spectrophotometric iodometric determination of nitrite and selenium (IV) using a polymethacrylate matrix. J. Anal. Chem. 2017, 72, 546–550. [Google Scholar] [CrossRef]
- Smazíková, P.; Praus, L.; Száková, J.; Tremlová, J.; Hanc, A.; Tlustos, P. Effects of organic matter-rich amendments on selenium mobility in soils. Pedosphere 2019, 29, 740–751. [Google Scholar] [CrossRef]
- Yamada, H.; Kamada, A.; Usuki, M.; Yanai, J. Total selenium content of agricultural soils in Japan. Soil Sci. Plant Nutr. 2009, 55, 616–622. [Google Scholar] [CrossRef]
- Yu, W.Q.; Wang, F.; Chen, Y.Z.; Shang, R.Y.; You, Z.M.; Zang, C.R.; Chen, C.S. Study on soil selenium content and its influencing factors in typical tea garden of fujian province. J. Tea Sci. 2020, 40, 173–185, (In Chinese with English Abstract). [Google Scholar]
- Qin, H.B.; Zhu, J.M.; Su, H. Selenium fractions in organic matter from Se-rich soils and weathered stone coal in selenosis areas of China. Chemosphere 2012, 86, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.J.; Carignan, J. Reviews on atmospheric selenium: Emissions, speciation and fate. Atmos. Environ. 2007, 41, 7151–7165. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Yu, Y.; Wang, Q.; Luo, Z.; Jiang, R.F.; Li, H.F. Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta 2015, 241, 907–916. [Google Scholar] [CrossRef]
- Kaiser, H. Stomatal uptake of mineral particles from a sprayed suspension containing an organosilicone surfactant. J. Plant Nutr. Soil Sci. 2014, 177, 869–874. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Bai, B.Q.; Chen, W.; Zhang, J.L.; Shen, Y.X. Growth effects and distribution of selenite in Medicago sativa. Plant Soil 2018, 425, 527–538. [Google Scholar] [CrossRef]
- Galeas, M.L.; Zhang, L.H.; Freeman, J.L.; Wegner, M.; Pilon-Smits, E.H. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators. New Phytol. 2007, 173, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.Q.; Zhang, D.; Shen, J.C.; Gong, W.R.; Wu, X.L.; Wang, F.; Chen, Y.N.; Li, X.Y.; Zheng, Q.Z.; Luo, D.H.; et al. Trace elements in successive tea infusions made via a brewing method widespread in China: Implications for human exposure. J. Food Compos. Anal. 2023, 115, 104989. [Google Scholar] [CrossRef]
- Ye, Y.Y.; He, J.L.; He, Z.J.; Zhang, N.; Liu, X.Q.; Zhou, J.J.; Cheng, S.Y.; Cai, J. Evaluation of the brewing characteristics, digestion profiles, and neuroprotective effects of two typical Se-enriched green teas. Foods 2022, 11, 2159. [Google Scholar] [CrossRef]
- Burk, R.F. Selenium, an antioxidant nutrient. Nutr. Clin. Care 2002, 5, 75–79. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Peng, C.Y.; Zhu, X.H.; Hou, R.Y.; Ge, G.F.; Hua, R.M.; Wan, X.C.; Cai, H.M. Aluminum and heavy metal accumulation in tea leaves: An interplay of environmental and plant factors and an assessment of exposure risks to consumers. J. Food Sci. 2018, 83, 1165–1172. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhang, J.; Guo, G.Y. Effect of brewing conditions on the leaching rule of Xinyang black tea soup contents. Food Res. Dev. 2014, 35, 15–19, (In Chinese with English Abstract). [Google Scholar]
- NY/T600-2002; Selenium Rich Tea. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2002. (In Chinese)
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).