Abstract
Recently, an outbreak of Ips sexdentatus (Börner, 1776) has caused considerable damage in the pine forests of the Czech Republic. As historical data on the biology of this pest are scarce due to its rare occurrence in recent decades, our work focused on monitoring flight activity and voltinism and investigating methods for monitoring its activity during the growing season. Observations were conducted from March to September 2021 and 2022 at three sites using 12 Theysohn traps with four types of pheromone lures (ACUMIPROTECT, ACUWIT, SEXOWIT and Pheagr IAC) together with data loggers to record weather conditions. The first beetles occurred in early May (daily mean temperatures above 13 °C). After the first egg laying stage, females re-emerged to establish a sister brood. The maximum flight activity appeared between late June and mid-July (daily mean temperatures about 20 °C), and the offspring occurred at the turn of June/July and in the first half of August. Since then, flight activity had a downward trend and quietened in September. According to the data, monitoring of I. sexdentatus should be conducted between May and September using the ACUMIPROTECT pheromone bait exhibiting the highest capturing efficacy. In future, however, the behavior of I. sexdentatus might alter due to climate change.
1. Introduction
Climate change is triggering significant changes in the bionomy of many bark beetle species. The effects are particularly pronounced in primary spruce pests such as Ips typographus (Linnaeus, 1758) and Ips duplicatus (Sahlberg, 1836). However, recent observations have also revealed dramatic changes in bark beetles previously considered to be of less importance [1,2,3,4]. Under favorable conditions, epidemic population levels are quickly reached, causing significant damage to both weakened and healthy trees [5]. In fact, even endemic species can become major pests, exacerbating the severity of infestation [6]. While outbreaks of these beetles were relatively rare in the past [1], climate change is now creating optimal living conditions such as rapid changes in their voltinism, reproduction, development rate and overwintering success [7,8,9,10,11].
Such a species is Ips sexdentatus (Börner, 1776) (Supplementary Figure S1), which is distributed across warm regions, including Portugal, Central Europe, Turkey, and Russia [12,13]. While it causes enormous damage in the forests of Caucasian spruce in Turkey (Picea orientalis (L.)) [14], its reproductive success is limited in Central Europe [15]. However, rising temperatures are creating favorable conditions for it to thrive in areas where it was previously scarce or absent. In fact, several European countries have reported an increase in occurrence and damage caused by pine bark beetles, including Austria, the Czech Republic, and Bulgaria [16,17,18,19].
In the Czech Republic before the 1990s [20], Ips sexdentatus was rare, but since the dry periods documented in the 1990s and 2000s [21,22], it has been found regularly in the original refuges and it even proliferated in central and south Bohemia where it had not been observed before [13,18,23,24] (Supplementary Figure S2). This expansion is attributed to the absence of effective pest management strategies, which contributes to delayed detection, accelerates spread of I. sexdentatus [25], and boosts heavy infestation of trees [2].
In Turkey, Ips sexdentatus populations typically begin to swarm in late April or early May when daily maximum temperatures reach 18–20 °C [14,15,26]. The pioneering sex, the males, colonize in the area near the base of trees with a bark thickness of 5–15 mm [15,27,28], create a nuptial chamber, and produce the aggregation pheromone to attract other males and lure females [29,30,31]. After mating, females bore maternal tunnels and lay eggs [32]. Environmental conditions affect the developmental time and number of generations during the season [14,29]. The higher humidity and colder climate in northern Europe extend the development time, allowing only one generation to complete its development (univoltine population) [14,15,29]. Conversely, the low humidity and warm conditions of Central and Southern Europe shorten the development time, and so two to six generations can evolve [33,34]. The flight period of Turkish populations typically ends in early September [10].
Overall, the I. sexdentatus population dynamics are influenced by environmental conditions. Understanding these factors is crucial for developing effective monitoring and prevention methods for pest control in the Czech Republic. In our work, we therefore focused on (1) mapping flight activity during the season in three affected localities in the Czech Republic, (2) revealing the number of generations under local environmental conditions, and (3) providing a preliminary insight into the typology of available pheromone lures against I. sexdentatus.
2. Materials and Methods
2.1. Study Sites
This study was conducted at three sites over two consecutive years, 2021 and 2022. All sites were located on dry sandy-gravelly soils with moderate nutrient supply and low water retention capacity [34,35,36].
Locality1 was located near Brandýs nad Labem–Stará Boleslav (GPS: 50.2109169N, 14.7128108E; Supplementary Figures S2 and S3) in a clear-cut surrounded by a stand of 80–100-year-old Scots pines (Pinus sylvestris (L.)) on podzols [36,37] with an approximate mean plant density (MPD) of 506 trees per hectare. This locality is characterized by an altitude of about 187 m above sea level, a mean annual temperature (MAT) of 9.5 °C, and a mean annual precipitation (MAP) of 575 mm [38]. Locality1 was the most recent western edge of the distribution.
Locality2 was located near Šemíkovice in the Jevišovická Highlands (GPS: 49.0487050N, 16.0878489E; Supplementary Figures S2 and S3) in an 80-year-old Scots pine stand after extensive felling of spruce (Picea abies (L.)) on cambisols–luvisols [36,37] of approximately MPD 198 trees per hectare. The locality is characterized by an altitude of about 411 m above sea level, MAT of 8.9 °C, and MAP total of 482 mm [39]. Locality2 was the area where the species spread in the 1990s.
Locality3 was located near Bzenec in South Moravia (GPS: 48.9498897N, 17.2159839E; Supplementary Figures S2 and S3) in a clear-cut surrounded by a 100-year-old Scots pine stand on cambisols [36,37] of approximately MPD 482 trees per hectare. This locality is characterized by an altitude of about 216 m above sea level, MAT of 9.7 °C, and MAP total of 537 mm [38]. Locality3 was a location where I. sexdentatus had been observed before 1995 [39].
2.2. Air Temperature Measurements
From the beginning of March to the end of September in both years, data loggers (Comet system, Rožnov pod Radhoštěm, the Czech Republic) were installed in all localities to measure air temperature at 30 min intervals.
2.3. Flight Activity
Theysohn pheromone traps (RIDEX, Vrbno pod Pradědem, the Czech Republic) were set up in April 2021 and 2022. Twelve traps created a transect placed approximately 20 m from the forest edge and 2 m above the ground at a distance of 30 m (Supplementary Figure S4). A set of four different synthetic pheromone baits (Table 1) was distributed in triplicate at the sites and the same bait types were distributed at 80 m intervals. The positioning of the baits was not changed during the sampling dates. The pheromone baits were replaced every eight weeks according to the manufacturer’s instructions. Samples were removed from the traps weekly and taken to the laboratory where they were separated, identified, and numbered. Observations were completed in September in both study years.

Table 1.
An overview of the synthetic pheromone baits used at selected sites during the growing season in both years of the study.
2.4. Statistical Analysis
2.4.1. Temperatures
The temperature recordings were downloaded from the data loggers at the end of September. The daily minimum (Ti) and maximum (Tm) temperatures were detected. Average temperatures (Ta) were calculated as an average of temperatures (Ti and Tm) measured in interval among two consecutive midnights.
2.4.2. The Thermal Sum
The thermal sums for certain occasions correspond to an accumulation of degree days (DD) from March 1. The daily DD were counted as the sum of the daily Tm and Ti divided by two and subtracted from the temperature threshold for the development of each day [40]. The temperature threshold (TT) for I. sexdentatus flight is considered to be 20 °C [14].
2.4.3. Cumulative Percentage of Captured Beetles
The cumulative percentage of I. sexdentatus was calculated as the division of the cumulative number of beetles in traps trapped in a one-week interval by the total number of beetles found in the season. Then the result was multiplied by one hundred.
2.4.4. Analyses in R
The analyses were performed with the statistical language R (Posit®, Boston, MA, USA; version 4.2.2; [41]) on Windows 10 x64 (build 19045) using the packages ‘Matrix’ (version 1.5.1; [42]) and ‘lme4’ (version 1.1.31; [43]). First, the D‘Agostino and Pearson omnibus normality test was applied to the data before other statistical tests were performed. Based on the result, parametric tests were used.
To test the effect of pheromone lures on catches, we fitted generalized linear mixed models for Poisson distributed data [44] using the package ‘lme4’ (version 1.1.31; [43]) and the package ‘Matrix’ (version 1.5.1; [42]). In the model, our locations and the position of the traps were included as a random effect (1|locationlocality:trap position) to account for a nested design. The season and bait type were included as fixed-effects variables. ML (maximum likelihood estimation) [45] and BOBYQA optimizer [46] were used to optimize model output. We compared our model with simplified models that included only locality as a covariate to assess treatment significance. Post hoc comparisons between bait efficacy were also performed for parametric models with simple adjustment of p values (stepwise method) provided by the ‘glht’ function from the R package ‘multcomp’ (version 1.4-23; [47]). A mixed Poisson model (estimated with ML and BOBYQA optimizer) was used to estimate differences in the abundance of catches of I. sexdentatus due to bait type.
3. Results
3.1. Flight Activity
The first captured beetles were observed in early June 2021, when the accumulated thermal sum was 56.5 ± 46.6 DD, corresponding to 35.0 ± 1.0 days of positive daily heat sum, calculated from early March above the 11 °C threshold [48]. In 2022, the first records occurred at the beginning of May, when the thermal sum amounted to 63.5 ± 16.7 DD, after 30.3 ± 15.7 days with a positive daily thermal sum.
3.1.1. Locality1
In the first decade of May in 2021, Ta reached TT suitable for flight. However, due to the cold weather, the first adults of I. sexdentatus were not captured until early June when maximum temperatures regularly reached 22 °C. Flight activity peaked in early July. About two weeks later, catches increased again. Two further increases were recorded in mid-August and early September. In the following weeks, low temperatures prevailed, and flight activity dropped to zero, so that no more beetles were caught (Figure 1).
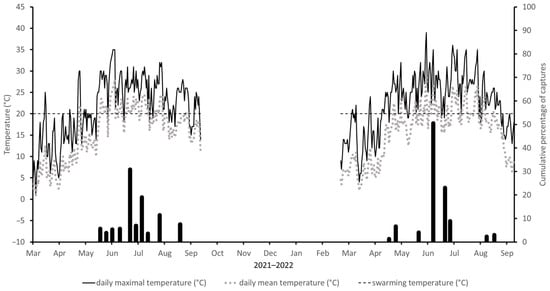
Figure 1.
Cumulative percentage of captures of Ips sexdentatus at Locality1 in period 2021–2022. The dotted line corresponds to mean daily air temperature and the solid line to maximum daily air temperature. The horizontal (dashed) line corresponds to the temperature threshold of 20 °C for the flight activity of I. sexdentatus. The sampling dates are indicated on the x-axes.
In 2022, the Ta exceeded flight TT shortly at the start of May; however, during the whole season, the temperature oscillated around TT. The first swarming adults were recorded at the beginning of May. At this time, the average daily Ta reached 16 °C. In the following weeks, daily Ta dropped and fluctuated around 15 °C, which led to an interruption in flight activity (zero catches) until the end of June, when flight activity increased again together with temperature. Due to the cold weather with daily mean temperatures of only about 17.5 °C, in mid-July, catches dropped and remained low or at zero until early September when the last flying adults were caught (Figure 1).
3.1.2. Locality2
In the second week of 2021, the Ta reached TT values for flight. The swarming of I. sexdentatus began in early June after a six-week period in which the daily Tm rose to 31 °C, even though the Ta at that time was around 11 °C. The beetles flew out when the daily Ta exceeded 19 °C and the daily Tm reached 23.8 °C. At this point, in the following weeks, catches decreased with temperature, and an increase in flight activity was observed in mid-July when daily Ta exceeded 23 °C. A further increase in activity was recorded in early August. From this point onwards, the daily Ta remained below 20 °C, so that the catches showed a downward trend. The last flying adults were detected in early September (Figure 2).
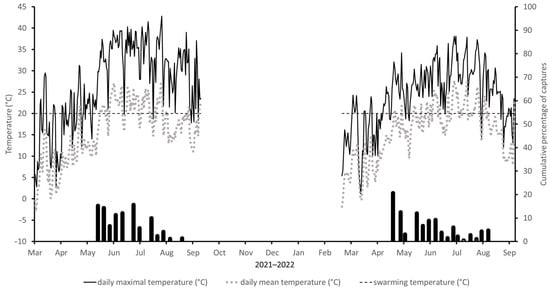
Figure 2.
Cumulative percentage of captures of Ips sexdentatus at Locality2 in period 2021–2022. The dotted line corresponds to mean daily air temperature and the solid line to maximum daily air temperature. The horizontal (dashed) line corresponds to the temperature threshold of 20 °C for the flight activity of I. sexdentatus. The sampling dates are indicated on the x-axes.
In 2022, flight activity started about six weeks after a week in March with a Tm of about 24 °C and the second half of April with a daytime Tm of 20 °C. However, the Ta necessary for flight occurred no earlier than in the second half of May. The first bark beetles emerged in early May when daily Ta reached 17 °C and daily Tm reached 22.5 °C. Thereafter, the temperature decreased, and so only small numbers of beetles were caught until early June. The next time any flight activity was observed was in the second half of July and at the end of August. In September, the temperature dropped, and flight activity came to a standstill and no beetles were caught (Figure 2).
3.1.3. Locality3
In 2021, although the Tm was regularly above 20 °C, Ta oscillated only around 11 °C; however, the flight TT was exceeded for a short time in the second decade of May. Moreover, the week-long cold snap in mid-April probably led to a shift in spring swarming, which did not begin until early June with a maximum in the first June decade, when daily Ta fluctuated around 20 °C and daily Tm was 32 °C. Thereafter, temperatures dropped, and catches were low until mid-July. Since then, the number of beetles caught tended to decrease with temperature, and the last catches were monitored in the first half of September (Figure 3).
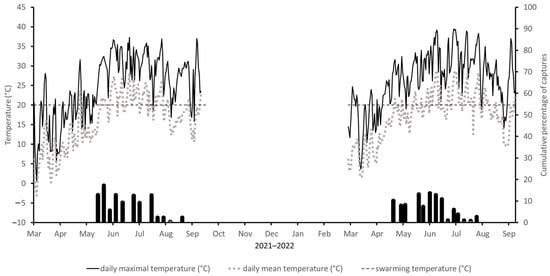
Figure 3.
Cumulative percentage of captures of Ips sexdentatus at Locality3 in period 2021–2022. The dotted line corresponds to mean daily air temperature and the solid line to maximum daily air temperature. The horizontal (dashed) line corresponds to the temperature threshold of 20 °C for the flight activity of I. sexdentatus. The sampling dates are indicated on the x-axes.
In 2022, a warm week at the end of March (daily Tm up to 23 °C, Ta about 10 °C), followed by a drop in daily Tm to 3 °C, and a five-week period with a mean daily Ta of about 8 °C caused the start of swarming that year in early May, when daily Ta fluctuated around 18 °C and the maximum Ta reached 23.9 °C. Daily Ta was low in May and June, so the next catches were not recorded until the second half of June. In the second half of July and in August, flight activity remained at a minimum. Flight activity decreased thereafter and came to a halt in September (Figure 3).
3.2. The Efficiency of Pheromone Lures
A total of 5538 individuals of I. sexdentatus were caught at all our sites in both years (Table 2). From other representatives of the genus Ips, I. typographus, I. duplicatus and I. acuminatus were found in the traps in quantities of 19,832, 218, and 2768 individuals, respectively.

Table 2.
The total number of Ips sexdentatus caught at our sites in 2021 and 2022 with four different pheromone baits.
3.3. Lure Attractiveness
The number of I. sexdentatus in the traps in 2021 and 2022 (df = 1, χ2 = 1974.3, p < 0.001) differed significantly between the sites (Table 2; df = 1, χ2 = 3614.4, p < 0.001). In general, the lowest number was caught at site 1 in 2021 (Table 2), and the highest number was observed at Locality2 in 2021 (Table 2). Comparing the abundance of first- and second-generation beetles caught, large differences can be observed at Locality1 (Figure 4). The number of beetles caught depending on the type of bait differed significantly for I. sexdentatus (df = 23, χ2 = 2518.9, p < 0.001). The highest number of catches was observed in traps with ACP bait (3775 individuals), and conversely, the lowest number of catches was in traps with PH bait (563 individuals). However, the less effective baits SX, PH and AW had similar attractiveness values, with a significant difference observed between AW and PH (p < 0.05) (Figure 4). On the other hand, I. typographus and I. duplicatus responded most positively to PH (19,227 and 171 captured individuals, respectively). In addition, the number of catches was influenced by the minimum temperatures in the week prior to sampling (df = 4, χ2 = 55.19, p < 0.001).
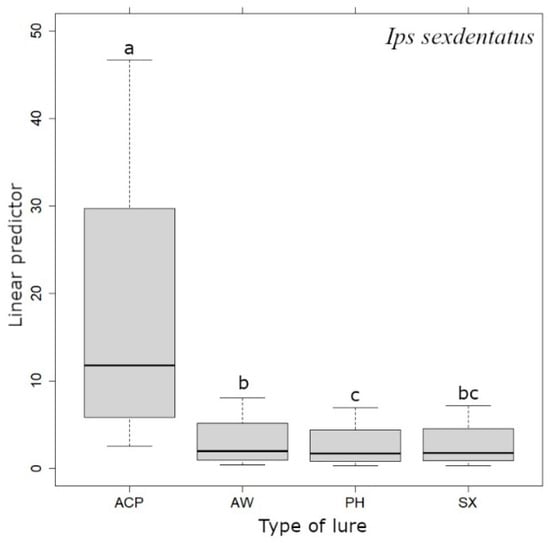
Figure 4.
Differences in catching Ips sexdentatus with different pheromone lures. Different letters above the boxplots indicate statistically significant differences (p < 0.05). The type of bait is shown on the x-axes: ACP (ACUMIPROTECT), AW (ACUWIT), PH (Pheagr IAC) and SX (SEXOWIT). The linear predictor shown on the y-axes refers to a combination of the factors considered in the model (location, season, trap position).
4. Discussion
Compared to the bark beetle species on pine trees (e.g., Tomicus piniperda (Linnaeus, 1758), Tomicus minor (Hartig, 1834), Orthotomicus erosus (Wollaston 1857)), Ips sexdentatus is considered “the late swarmer”, as the first active beetles are usually caught in June [49,50,51]. The flight activity of I. sexdentatus at the studied sites follows the pattern previously reported in northern Italy, Turkey, eastern Romania, and France [14,48,52,53,54,55]. In contrast, I. sexdentatus only emerged at our sites when daily temperatures exceeded 13 °C. Similarly to Turkish populations, mass flight occurred at temperatures above 20 °C [14]. The first increase in abundance occurred two to three weeks after the peak in the spring swarming (mid-July), which could indicate that females are re-emerge and establish a sister brood, similar to I. typographus and I. duplicatus; however, due to few observations, further studies are needed to confirm this theory. The second increase occurred in the first half of August, which indicates the emergence of offspring; therefore, it is certain that I. sexdentatus is univoltine in the Czech Republic. Flight activity lasts 3–4 months and begins when the mean daily temperature reaches 13 °C (early May), similarly to I. typographus and I. duplicatus [56]. Conversely, the flight activity of I. sexdentatus already ceased in August and ended in early September, about one month earlier than that of I. duplicatus and I. typographus [3,57,58,59,60,61,62]. This significantly shortens the period during which the pines are exposed to bark beetle infestation, which considerably reduces the damage. Although the current flight activity and population dynamics have been mapped, some changes could be observed in the future due to progressive warming. For example, the shift in spring swarming to an earlier date and a clearly recognizable increase in flight activity could be expected, similar to what has been predicted for I. typographus [63,64,65,66]; or permanent sister broods could develop, similar to I. sexdentatus in France [47]; and finally, additional generations could appear at the end of the growing season. Therefore, the monitoring period could be extended to capture I. sexdentatus in April and September. In addition, surveys could be increased at the time of female re-emergence and the emergence of offspring.
Pheromone baits are considered the most practical means of monitoring and controlling bark beetle outbreaks [67,68]. Our results show that their effectiveness varies considerably. Traps baited with ACP lures showed the highest attractiveness for both species studied. In general, the number of I. sexdentatus caught was about 4.7–8.1 times higher when ACP baits were used than when other baits (AW, PH or SX) were used. The high affinity of I. sexdentatus to ACP baits, which were originally developed for the capture of I. acuminatus, is not surprising as interspecific inhibition between Ips genera [69] towards pheromone compounds is eminent [29,31,70,71]. Thus, similar to I. acuminatus, I. sexdentatus is also attracted to the substances ipsenol, ipsdienol, and verbenol contained in ACP [72]. In contrast, our data contradict Knížek [18], who found PH and SX baits to be very effective for catching I. sexdentatus. Considering that the chirality of ipsdienol varies considerably between species and populations within the same species [73,74] and that the exclusion or inappropriate enantiomer drastically reduces the attractiveness of the aggregation pheromone [71], a plausible explanation for the low efficacy of the SX lure could be the use of an inappropriate optical enantiomer of ipsdienol in the mixture. Similarly, the low efficacy of PH could be due to the solvent 2-methylbut-3-en-2-ol (MB) [75]. However, MB is a dominant component of the aggregation pheromone of I. typographus, I. duplicatus, O. erosus, Pityogenes spp. or Ips aminitus (Eichhoff, 1871) [76,77,78,79,80,81], it is minimally attractive to pine bark beetles [71]. Therefore, the ACP bait is an optimal mixture of suitable enantiomers with potentially high attractiveness and thus a suitable tool for monitoring I. sexdentatus.
5. Conclusions
Characteristics of flight activity, such as spring emergence and re-emergence of females, sister broods and termination of flight, are crucial information for monitoring and mapping the population dynamics of Ips sexdentatus. Under the climatic conditions of the Czech Republic, the optimal survey period begins in early May, when the overwintering generation begins to swarm, and lasts until early September, when activity comes to ahalt. However, it is important to consider the possibility of extending the monitoring period due to global warming. It may also be useful to increase protection measures before the females re-emerge and establish a sister brood and before callows emerge in search of new hosts. Theysohn traps with ACP lures containing an optimal combination of the relevant enantiomers have proven to be a suitable tool for monitoring the population dynamics of I. sexdentatus.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15060961/s1, Figure S1: Six-toothed pine bark beetle Ips sexdentatus adult and damage caused on Scots pine. The scale bar for head (A) and back part of elytra (B) is 250 µm, and for maternal gallery (C) is 2.5 cm. Figure S2: Distribution map of Ips sexdentatus in the Czech Republic and location of experimental localities ( ). Distribution data were extracted from following literature sources: (
). Distribution data were extracted from following literature sources: ( ) Knížek et al. [18]; (
) Knížek et al. [18]; ( ) Knížek and Zahradník [21]; (■) Knížek and Liška [22]; (
) Knížek and Zahradník [21]; (■) Knížek and Liška [22]; ( ) Nature Conservation Agency of the Czech Republic [24]; (●) Jelínek [39]. Figure S3: A set of maps: (A) a map with the location of the Czech Republic in Europe; (B) a map with the location of the research area in the Czech Republic; and detailed maps with the location of the sites (C—Locality1; D—Locality2, E—Locality3). Figure S4: Illustrative photos of monitored localities. A—Locality1; B—Locality2; C—Locality3.
) Nature Conservation Agency of the Czech Republic [24]; (●) Jelínek [39]. Figure S3: A set of maps: (A) a map with the location of the Czech Republic in Europe; (B) a map with the location of the research area in the Czech Republic; and detailed maps with the location of the sites (C—Locality1; D—Locality2, E—Locality3). Figure S4: Illustrative photos of monitored localities. A—Locality1; B—Locality2; C—Locality3.
 ). Distribution data were extracted from following literature sources: (
). Distribution data were extracted from following literature sources: ( ) Knížek et al. [18]; (
) Knížek et al. [18]; ( ) Knížek and Zahradník [21]; (■) Knížek and Liška [22]; (
) Knížek and Zahradník [21]; (■) Knížek and Liška [22]; ( ) Nature Conservation Agency of the Czech Republic [24]; (●) Jelínek [39]. Figure S3: A set of maps: (A) a map with the location of the Czech Republic in Europe; (B) a map with the location of the research area in the Czech Republic; and detailed maps with the location of the sites (C—Locality1; D—Locality2, E—Locality3). Figure S4: Illustrative photos of monitored localities. A—Locality1; B—Locality2; C—Locality3.
) Nature Conservation Agency of the Czech Republic [24]; (●) Jelínek [39]. Figure S3: A set of maps: (A) a map with the location of the Czech Republic in Europe; (B) a map with the location of the research area in the Czech Republic; and detailed maps with the location of the sites (C—Locality1; D—Locality2, E—Locality3). Figure S4: Illustrative photos of monitored localities. A—Locality1; B—Locality2; C—Locality3.Author Contributions
Conceptualization, P.D.; methodology, P.D.; validation, P.D.; formal analysis, D.H.; investigation, P.D., M.D., J.K. and D.H.; resources, P.D.; data curation, D.H.; writing—original draft preparation, D.H. and P.D.; writing—review and editing, P.D. and D.H.; visualization, D.H.; supervision, P.D.; project administration, P.D.; funding acquisition, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Forests of the Czech Republic, state enterprises [project 05/2019]. Petr Doležal and Markéta Davídková acknowledge support from the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO0123.
Data Availability Statement
Data available on request.
Acknowledgments
Authors would like to thank to Jan Okrouhlík for consultation with statistical analyses and Pavel Doležal for his support in the field. Sincere thanks come to three anonymous reviewers for their comments that considerably improved the quality of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Etxebeste, I.; Lencina, J.L.; Pajares, J. Saproxylic community, guild and species responses to varying pheromone components of a pine bark beetle. Bull. Entomol. Res. 2013, 103, 497–510. [Google Scholar] [CrossRef]
- Foit, J.; Čermák, V. Colonization of disturbed Scots pine trees by bark- and wood-boring beetles. Agric. For. Entomol. 2014, 16, 184–195. [Google Scholar] [CrossRef]
- Wermelinger, B.; Schneider Mathis, D.; Knížek, M.; Forster, B. Tracking the spread of the northern bark beetle (Ips duplicatus [Sahlb.]) in Europe and first records from Switzerland and Liechtenstein. Alp. Entomol. 2020, 4, 179–184. [Google Scholar] [CrossRef]
- Holuša, J.; Foit, J.; Knížek, M.; Schovankova, J.; Lukášová, K.; Vanicka, H.; Trombik, J.; Kula, E. The bark beetles Orthotomicus laricis and Orthotomicus longicollis are not pests in Central Europe: A case study from the Czech Republic. Bull. Insectology 2019, 72, 253–260. [Google Scholar]
- Guérard, N.; Dreyer, E.; Lieutier, F. Interactions between Scots pine, Ips acuminatus (Gyll.) and Ophiostoma brunneo-ciliatum (Math.): Estimation of the critical thresholds of attack and inoculation densities and effects on hydraulic properties in the stem. Ann. For. Sci. 2000, 57, 681–690. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Anderegg, W.R.L.; Das, A.J.; Stephenson, N.L. Why is tree drought mortality so hard to predict? Trends Ecol. Evol. 2021, 36, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Wermelinger, B.; Seifert, M. Analysis of temperature dependent development of the spruce bark beetle Ips typographus (L.) (Col., Scolytidae). J. Appl. Entomol. 1998, 122, 185–191. [Google Scholar] [CrossRef]
- Régnière, J.; Bentz, B. Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. J. Insects Physiol. 2007, 53, 559–572. [Google Scholar] [CrossRef]
- Powell, J.A.; Bentz, B.J. Connecting phenological predictions with population growth rates for mountain pine beetle, an outbreak insect. Landsc. Ecol. 2009, 24, 657–672. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the Western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Jonášová, M.; Prach, K. Central-European Mountain spruce (Picea abies (L.) Karst.) forests: Regeneration of tree species after a bark beetle outbreak. Ecol. Eng. 2004, 23, 15–27. [Google Scholar] [CrossRef]
- Knížek, M. Lýkožrout borový (Ips sexdentatus). Lesn. Práce 2020, 99, 14–17. (In Czech) [Google Scholar]
- Özcan, G.E. Use of pheromone-baited traps for monitoring Ips sexdentatus (Boerner) (Coleoptera: Curculionidae) in oriental spruce stands. Afr. J. Biotechnol. 2011, 10, 16351–16360. [Google Scholar]
- Bakke, A. Ecological studies on bark beetles (Coleoptera: Scolytidae) associated with Scots pine (Pinus sylvestris L.) in Norway with particular reference to the influence of temperature. Medd. Fra Det Nor. Skogsforsoksvesen 1968, 21, 443–602. [Google Scholar]
- Barna, M.; Ferezliev, A.; Tsakov, H.; Mihál, I. Investigations of mature Scots pine stands in wind-throw areas in Norway spruce forests in Western Rhodopes. Folia Oecol. 2020, 47, 1–9. [Google Scholar] [CrossRef]
- Georgieva, M.; Belilov, S.; Dimitrov, S.; Iliev, M.; Trenkin, V.; Mirchev, P.; Georgiev, G. Application of remote sensing data for assessment of bark beetle attacks in pine plantations in Kirkovo region, the Eastern Rhodopes. Forests 2022, 13, 620. [Google Scholar] [CrossRef]
- Knížek, M.; Liška, J.; Véle, A. Efficacy of synthetic lures for pine bark beetle monitoring. J. For. Sci. 2022, 68, 19–25. [Google Scholar] [CrossRef]
- Kovac, M.; Rigling, D.; Pernek, M. Ophiostomatales associated with Mediterranean pine engraver, Orthotomicus erosus (Coleoptera, Curculionidae) in Dalmatia, Croatia. J. Fungi 2022, 8, 788. [Google Scholar] [CrossRef]
- Pfeffer, A. Fauna ČSR: Kůrovci—Scolytoidea, 1st ed.; Československá Akademie Věd: Prague, Czech Republic, 1995; p. 324. (In Czech) [Google Scholar]
- Knížek, M.; Zahradník, P. Bark and wood boring beetles in the pine stands. In Methodology of Forest Insect and Disease Survey in Central Europe, Proceedings of the Second Workshop of the IUFRO Working Party 7.03.10, Sion-Châteauneuf, Switzerland, 20–23 April 1999; Forster, B., Knížek, M., Grodzki, W., Eds.; Swiss Federal Institute for Forest, Snow and Landscape Research: Sion-Châteauneuf, Switzerland, 1999; pp. 54–59. [Google Scholar]
- Knížek, M.; Liška, J. Výskyt lesních škodlivých činitelů v roce 2020 a jejich očekávaný stav v roce 2021. In Zpravodaj Ochrany Lesa, Supplementum; Forestry and Game Management Research Institute: Jíloviště, Czech Republic, 2021; p. 76. Available online: https://www.vulhm.cz/files/uploads/2021/06/ZOL_Suppl_2021.pdf (accessed on 11 August 2022).
- Liška, J.; Knížek, M.; Véle, A. Evaluation of insect pest occurrence in areas of calamitous mortality of Scots pine. Cent. Eur. For. J. 2021, 67, 85–90. [Google Scholar] [CrossRef]
- Nature Conservation Agency of the Czech Republic. Available online: https://portal.nature.cz/w/druh-6250#/ (accessed on 18 May 2024).
- Lubojacký, J.; Lorenc, F.; Samek, M.; Knížek, M.; Liška, J. Main problems in forest protection in the Czech Republic in 2021 and forecast for 2022. Zprav. Ochr. Lesa 2022, 25, 17–26. (In Czech) [Google Scholar]
- Özcan, G.E. Assessment of Ips sexdentatus population considering the capture in pheromone traps and their damages under non-epidemic conditions. Šumarski List 2017, 141, 47–56. [Google Scholar] [CrossRef][Green Version]
- Bouhot, L.; Lieutier, F.; Debouzie, D. Spatial and temporal distribution of attacks by Tomicus piniperda L. and Ips sexdentatus Boern. (Col., Scolytidae) on Pinus sylvestris. J. Appl. Entomol. 1987, 106, 356–371. [Google Scholar] [CrossRef]
- Markalas, S. Frequency and distribution of insect species on trunks in burnt pine forests of Greece. Schweiz. Entomol. Ges. 1997, 70, 57–61. [Google Scholar] [CrossRef]
- Vité, J.P.; Bakke, A.; Renwick, J.A.A. Pheromones in Ips (Coleoptera: Scolytidae): Occurrence and production. Can. Entomol. 1972, 104, 1967–1975. [Google Scholar] [CrossRef]
- Kohnle, U.; Meyer, M.; Kluber, J. Formulation of population attractant for the pine bark beetle, Ips sexdentatus (Col, Scolytidae). Allg. Forst Jagdztg. 1992, 163, 81–87. [Google Scholar]
- Etxebeste, I.; Pajares, J.A. Verbenone protects pine trees from colonization by the six-toothed pine bark beetle, Ips sexdentatus Boern. (Col.: Scolytinae). J. Appl. Entomol. 2011, 135, 258–268. [Google Scholar] [CrossRef]
- Jactel, H.; Lieutier, F. Effects of attack density on fecundity of the Scots pine beetle Ips sexdentatus Boern (Col.; Scolytidae). J. Appl. Entomol. 1987, 104, 190–204. [Google Scholar] [CrossRef]
- Sierra, J.M.; Martín, A.B. Efectividad de trampas de feromona en la captura masiva de Ips sexdentatus Boern. (Coleoptera: Scolytidae), escoítido perforador de los pinos. Bol. San. Veg. Plagas 2004, 30, 745–752. (In Spanish) [Google Scholar]
- Péter, S. Effect of Climate Change on Population Dynamics of Bark Beetles: Relationships between Temperature and Development Rate of Ips sexdentatus (Boern.). Ph.D. Dissertation, Université de Lorraine, Lorraine, Italy, 2014. [Google Scholar]
- ÚVT, BENETA.cz. Taxonomický Klasifikační Systém půd České Republiky. Available online: https://klasifikace.pedologie.czu.cz (accessed on 17 April 2023).
- Czech Geological Survey, Soil Map 1: 50 000. Available online: https://mapy.geology.cz/pudy/#; https://mapy.geology.cz/geo/ (accessed on 17 April 2023).
- Forests of the Czech Republic, State Enterprises: Porostní Mapy—Informační Portál Stáří Lesních Porostů na Území České Republiky. Available online: https://geoportal.lesycr.cz/itc/?serverconf=default&wmcid=882 (accessed on 17 April 2023).
- METEOBLUE (2006–2023): Pozorované Historické Údaje o Klimatu a počasí. Available online: www.meteoblue.com (accessed on 18 April 2023).
- Jelínek, J. Check List of Czechoslovak Insects IV (Coleoptera). Folia Heyrovskyana 1993, 1, 5–172. [Google Scholar]
- Bessin, R.; Villanueva, R. Predicting Insect Development Using Insect Degree Days; Cooperative Extension Service University of Kentucky College of Agriculture, Food and Environment: Lexington, KY, USA, 2019; Available online: https://entomology.ca.uky.edu/ef123 (accessed on 18 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 18 April 2023).
- Bates, D.; Maechler, M.; Jagan, M. Matrix: Sparse and Dense Matrix Classes and Methods. R Package Version 1.5-1. 2022. Available online: https://CRAN.R-project.org/package=Matrix (accessed on 17 April 2023).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.M.; Ardia, D.; Gil, D.L.; Windover, D.; Cline, J. DEoptim: An R Package for Global Optimization by Differential Evolution. J. Stat. Softw. 2011, 40, 1–26. [Google Scholar] [CrossRef]
- Powell, M.J.D. The BOBYQA Algorithm for Bound Constrained Optimization without Derivatives; Technical Report NA2009/06; Department of Applied Mathematics and Theoretical Physics: Cambridge, UK, 2009. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Pineau, X.; David, G.; Peter, Z.; Sallé, A.; Baude, M.; Lieutier, F.; Jactel, H. Effect of temperature on the reproductive success, developmental rate and brood characteristics of Ips sexdentatus (Boern.). Agric. For. Entomol. 2016, 19, 23–33. [Google Scholar] [CrossRef]
- Långström, B. Life cycles and shoot-feeding of pine shoot beetles. Stud. For. Suec. 1983, 163, 1–29. [Google Scholar]
- Tribe, G.D. Phenology of Pinus radiata log colonization and reproduction by the European bark beetle Orthotomicus erosus (Wollaston) (Coleoptera: Scolytidae) in the south-western Cape Province. J. Entomol. Soc. South. Afr. 1990, 53, 117–126. [Google Scholar]
- Mendel, Z.; Boneh, O.; Shenhar, Y.; Riov, J. Diurnal flight patterns of Orthotomicus erosus and Pityogenes calcaratus in Israel. Phytoparasitica 1991, 19, 23–31. [Google Scholar] [CrossRef]
- Lozzia, G.C. Monitoring and control of Ips sexdentatus Boerner using synthetic pheromones. DiPSA 1998, 27, 71–84. [Google Scholar]
- Isaia, G.; Manea, A.; Paraschiv, M. Study on the effect of pheromones on the bark beetles of the Scots pine. Bull. Transilv. Univ. Bras. 2010, 3, 67–72. [Google Scholar]
- Sarikaya, O.; Avci, M.; Yildirim, S. Flight activity and biology of Ips sexdentatus Boerner in black pine (Pinus nigra Arnold) forests of Isparta, Turkey. In Proceedings of the International Science Conference, Forests in the Future—Sustainable Use, Risks and Challenges, Belgrade, Serbia, 4–5 October 2012; Rakonjac, L., Ed.; Institute of Forestry: Belgrade, Serbia, 2012; pp. 595–604. [Google Scholar]
- López, S.; Goldarazena, A. Flight dynamics and abundance of Ips sexdentatus (Coleoptera: Curculionidae: Scolytinae) in different sawmills from Northern Spain: Differences between local Pinus radiate (Pinales: Pinaceae) and Southern France incoming P. pinaster timber. Psyche A J. Entomol. 2012, 2012, 145930. [Google Scholar] [CrossRef]
- Holuša, J.; Lukášová, K.; Lubojacký, J. Comparison of seasonal flight activity of Ips typographus and Ips duplicatus. Sci. Agric. Bohem. 2012, 43, 109–115. [Google Scholar]
- Bakke, A.; Austara, O.; Pettersen, H. Seasonal flight activity and attack pattern of Ips typographus in Norway under epidemic conditions. Medd. Norsk I Skogfor 1977, 33, 257–268. [Google Scholar]
- Harding, S.; Ravn, H. Seasonal activity of Ips typographus L. (Col., Scolytidae) in Denmark. Z. Angew. Entomol. 1985, 99, 123–131. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Ips typographus (L.) pheromone trapping in south Alps: Spring catches determine damage thresholds. J. Appl. Entomol. 2004, 128, 307–311. [Google Scholar] [CrossRef]
- Baier, P.; Pennerstorfer, J.; Schopf, A. PHENIPS – a comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For. Ecol. Manag. 2007, 249, 171–186. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.-H.; Wang, Y.; Liu, G.-T.; Zhou, X.; Niu, J.; Schlyter, F. Catching Ips duplicatus (Sahlberg) (Coleoptera: Scolytidae) with pheromone-baited traps: Optimal trap type, colour, height and distance to infestation. Pest. Manag. Sci. 2009, 66, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Öhrn, P.; Långström, B.; Lindelöw, Å.; Björklund, N. Seasonal flight patterns of Ips typographus in southern Sweden and thermal sums required for emergence. Agric. For. Entomol. 2014, 16, 147–157. [Google Scholar] [CrossRef]
- Lobinger, G. Die Lufttemperatur als limitierender Faktor für die Schwärmaktivität zweier rindenbrütender Fichtenborkenkäferarten, Ips typographus L. und Pityogenes chalcographus L. (Col., Scolytidae). Anz. Fär Schädlingskunde Pflanzenschutz Umweltschutz 1994, 67, 14–17. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Appelberg, G.; Harding, S.; Bärring, L. Spatio-temporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Glob. Chang. Biol. 2009, 15, 486–499. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Harding, S.; Krokene, P.; Lange, H.; Lindelöw, Å.; Økland, B.; Raven, H.P.; Schroeder, L.M. Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Clim. Chang. 2011, 109, 695–718. [Google Scholar] [CrossRef]
- Jakoby, O.; Lischke, H.; Wermelinger, B. Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus). Glob. Chang. Biol. 2019, 25, 4048–4063. [Google Scholar] [CrossRef] [PubMed]
- Vité, J.P.; Baader, E. Present and future use of semiochemicals in pest management of bark beetles. J. Chem. Ecol. 1990, 16, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A. Using Pheromones in the Management of Bark Beetle Outbreaks. Forest insect guilds: Patterns of interaction with host trees. In Report No. NE-153; Baranchikov, Y.N., Mattson, W.J., Hain, F.P., Payne, T.L., Eds.; Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991. [Google Scholar]
- Byers, J.A. Chemical ecology of bark beetles. Experientia 1989, 45, 271–283. [Google Scholar] [CrossRef]
- Francke, W.; Pan, M.L.; Bartels, J.; König, W.A.; Vité, J.P.; Krawielitzki, S.; Kohnle, U. The odour bouquet of three pine engraver beetles (Ips spp.). J. Appl. Entomol. 1986, 101, 453–461. [Google Scholar] [CrossRef]
- Birgersson, G.; Dalusky, M.J.; Espelie, K.E.; Berisford, C.W. Pheromone production, attraction, and interspecific inhibition among four species of Ips bark beetles in the southeastern USA. Psyche A J. Entomol. 2012, 2012, 532652. [Google Scholar] [CrossRef]
- Paiva, M.R.; Pessoa, M.F.; Vité, J.P. Reduction in the pheromone attractant response of Orthotomicus erosus (Woll.) and Ips sexdentatus Boern. (Col., Scolytidae). J. Appl. Entomol. 1988, 106, 198–200. [Google Scholar] [CrossRef]
- Seybold, S. The Role of Chirality in the Olfactory-Directed Aggregation Behavior of Pine Engraver Beetles in the Genus Ips (Coleoptera: Scolytidae). Ph.D. Dissertation, University of California, CSU, Berkeley, CA, USA, 1992. [Google Scholar]
- Ivarsson, P. The Pheromone Systems of the Spruce Bark Beetles, Ips duplicatus and I. typographus: Biosynthesis and Regulations. Ph.D. Dissertation, Göteborg University, Göteborg, Sweden, 1995. [Google Scholar]
- Doležal, P.; Davídková, M.; Vovesný, P.; Drašar, P. Pheromone dispenser ACUMIPROTECT for mass trapping of the sharp-dentated bark beetle, Ips acuminatus (Coleoptera; Curculionidae). Chem. Listy 2023, 117, 13–16. [Google Scholar] [CrossRef]
- Bakke, A.; Froyen, P.; Skattebol, L. Field response to a new pheromonal compound isolated from Ips typographus. Naturwissenschaften 1977, 64, 98–99. [Google Scholar] [CrossRef]
- Giesen, H.; Kohnle, U.; Vité, J.P.; Pan, M.-L.; Francke, W. Aggregationspheromon des mediterranen Kiefernborkenkäfers Ips (Orthotomicus) erosus. J. Appl. Entomol. 1984, 98, 95–97. [Google Scholar] [CrossRef]
- Baader, E.J. Pityogenes spp. (Col., Scolytidae): Investigations on semiochemicals and their application in forest protection. J. Appl. Entomol. 1989, 107, 1–31. [Google Scholar] [CrossRef]
- Zuber, M. Racemat und Enantiomere von Ipsdienol zur Anlockung von Ips amitinus (Eich.) (Col., Scolytidae). Anz. Für Schädlingskunde Pflanzenschutz Umweltschutz 1994, 67, 92–93. [Google Scholar] [CrossRef]
- Seybold, S.J.; Huber, D.P.W.; Lee, J.C.; Graves, A.D.; Bohlmann, J. Pine monoterpenes and pine bark beetles: A marriage of convenience for defense and chemical communication. Phytochem. Rev. 2006, 5, 143–178. [Google Scholar] [CrossRef]
- Duduman, M.-L.; Beránková, K.; Jakuš, R.; Hradecký, J.; Jirošová, A. Efficiency and sustainability of Ips duplicatus (Coleoptera: Curculionidae) pheromone dispensers with different designs. Forests 2022, 13, 511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).