From Lab to Nursery: Novel Approaches of Seed Disinfection for Managing Pine Pitch Canker Propagation
Abstract
1. Introduction
2. Materials and Methods
2.1. Artificial Seed Inoculation
2.2. Treatment Selection
2.3. Treatment Application
2.4. Fungal Isolation and Identification
Morphological and Molecular Identification of Non-Fusarium Species
2.5. Nursery Germination Assays
2.6. In Vitro Germination Assay
3. Results and Discussion
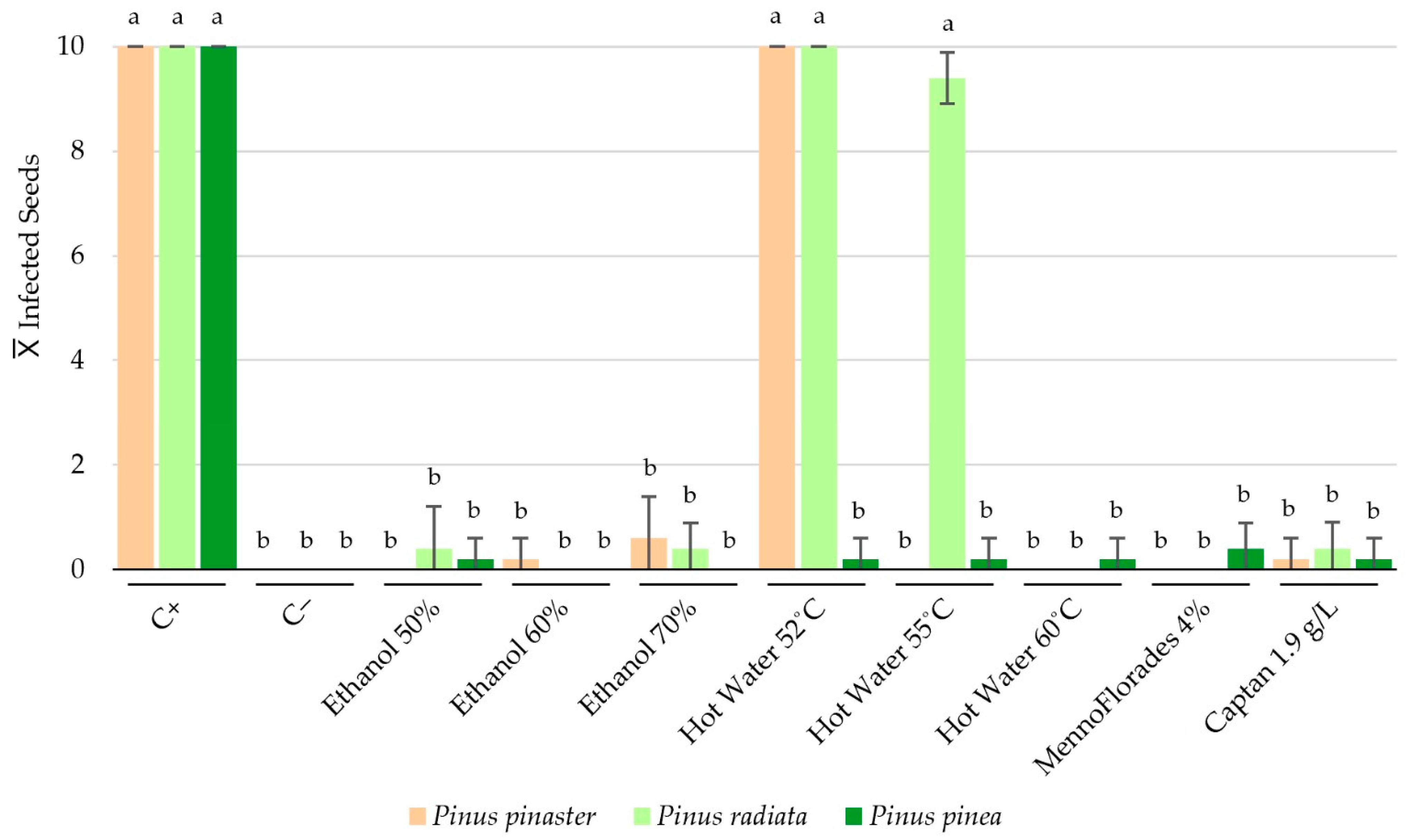
3.1. Effect of Treatments on Fusarium Circinatum-Contaminated Pinus Seeds
3.2. Isolated and Identified Non-Fusarium Species
3.3. Nursery Germination Assays
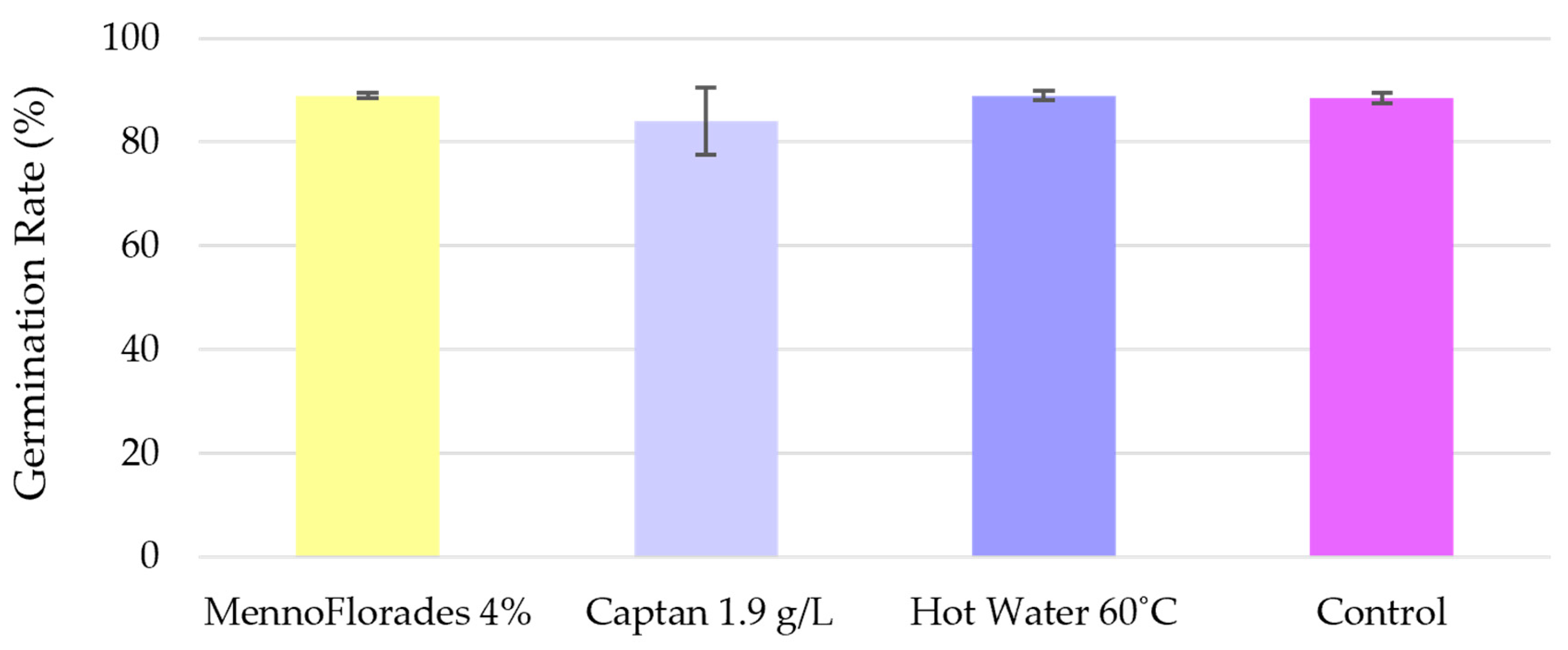
3.4. In Vitro Germination Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, T. Pitch Canker Disease of Pines. Phytopathology 2006, 96, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Bezos, D.; Martinez-Alvarez, P.; Fernandez, M.; Diez, J. Epidemiology and Management of Pine Pitch Canker Disease in Europe—A Review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Drenkhan, R.; Ganley, B.; Martín-García, J.; Vahalík, P.; Adamson, K.; Adamčíková, K.; Ahumada, R.; Blank, L.; Bragança, H.; Capretti, P.; et al. Global Geographic Distribution and Host Range of Fusarium circinatum, the Causal Agent of Pine Pitch Canker. Forests 2020, 11, 724. [Google Scholar] [CrossRef]
- Schmale, D.G., III; Gordon, T.R. Variation in susceptibility to pitch canker disease, caused by Fusarium circinatum, in native stands of Pinus muricata. Plant Pathol. 2003, 52, 720–725. [Google Scholar] [CrossRef]
- Gordon, T.R.; Kirkpatrick, S.C.; Aegerter, B.J.; Wood, D.L.; Storer, A.J. Susceptibility of Douglas fir (Pseudotsuga menziesii) to pitch canker, caused by Gibberella circinata (anamorph = Fusarium circinatum). Plant Pathol. 2006, 55, 231–237. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- Solel, Z.; Bruck, R.I. Relation between wilt rate and obstruction of water flow in stems of two families of loblolly pine affected by pitch canker. Eur. J. For. Pathol. 1990, 20, 317–320. [Google Scholar] [CrossRef]
- Hepting, G.H.; Roth, E.R. Pitch canker, a new disease of some southern pines. J. For. 1946, 44, 724–744. [Google Scholar]
- Berbegal, M.; Pérez-Sierra, A.; Armengol, J.; Grünwald, N.J. Evidence for Multiple Introductions and Clonality in Spanish Populations of Fusarium circinatum. Phytopathology 2013, 103, 851–861. [Google Scholar] [CrossRef]
- Landeras, E.; Garcia, P.; Fernández, Y.; Braña, M.; Fernández-Alonso, O.; Méndez-Lodos, S.; Pérez-Sierra, A.; Leon, M.; Abad-Campos, P.; Berbegal, M.; et al. Outbreak of Pitch Canker Caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- Dwinell, D. Global Distribution of the Pitch Canker Fungus. In Current and Potential Impacts of Pitch Canker in Radiata Pine; IMPACT Monterey Workshop: Monterey, CA, USA; Citeseer: Princeton, NJ, USA, 1999; Volume 30, pp. 54–57. [Google Scholar]
- European and Mediterranean Plant Protection Organization (EPPO) Global Database. First Report of Gibberella circinata in France; EPPO: Paris, France, 2006; p. 104. [Google Scholar]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First report of Pitch Canker caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.; Moniz, F.; Amparo, P. First report of Pitch canker on Pines caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- 6º Inventário Florestal Nacional—IFN6. Available online: https://inforcna.pt/Media/Files/201979_Ifn6PrincipaisResultadosJun2019.pdf (accessed on 13 May 2024).
- Instituto Nacional de Estatística—Statistics Portugal, Estatísticas do Comércio Internacional. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=280862956&PUBLICACOESmodo=2 (accessed on 13 May 2024).
- Vettraino, A.; Potting, R.; Raposo, R. EU legislation on forest plant health: An overview with a focus on Fusarium circinatum. Forests 2018, 9, 568. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO) Global Database. EPPO Datasheet: Fusarium circinatum. Available online: https://gd.eppo.int/taxon/GIBBCI/datasheet (accessed on 13 May 2024).
- European Food and Environment Safety Agency (EFSA) Panel on Plant Health (PLH). Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. EFSA J. 2010, 8, 1620. [Google Scholar] [CrossRef]
- Fernandes, L.; Paiva, D.; Roxo, I.; Fernandes, A.R.; Ribeiro, D.; Ribeiro, H.; Portugal, A. Development of New Preventive Strategies for Pine Pitch Canker Caused by Fusarium circinatum in Irrigation Water and Evaluation in a Real Nursery Context. Forests 2023, 14, 443. [Google Scholar] [CrossRef]
- Silva, A.C.; Diogo, E.; Bragança, H. Effect of Substrate Solarization for the Control of Fungi: The Case Study of Fusarium circinatum, the Quarantine Agent of Pine Pitch Canker. Silva Lusit. 2021, 29, 161–175. [Google Scholar] [CrossRef]
- Chemetova, C.; Quilhó, T.; Braga, S.; Fabião, A.; Gominho, J.; Ribeiro, H. Aged Acacia melanoxylon bark as an organic peat replacement in container media. J. Clean. Prod. 2019, 232, 1103–1111. [Google Scholar] [CrossRef]
- Chemetova, C.; Mota, D.; Fabião, A.; Gominho, J.; Ribeiro, H. Low-temperature hydrothermally treated Eucalyptus globulus bark: From by-product to horticultural fiber-based growing media viability. J. Clean. Prod. 2021, 319, 128805. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). 91 (2) Fusarium circinatum (formerly Giberella circinata). EPPO Bull. 2019, 49, 228–247. [Google Scholar] [CrossRef]
- Krebs, H.A.; Wigginst, D.; Stubbs, M. Studies on the mechanism of the antifungal action of benzoate. Biochem. J. 1983, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.D. Action of the Fungicides Captan and Folpet on Rat Liver Mitochondria. Biochem. Pharmacol. 1971, 20, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Dantigny, P. Control of food spoilage fungi by ethanol. Food Control 2011, 22, 360–368. [Google Scholar] [CrossRef]
- Crous, P.; Swart, L.; Coertze, S. The effect of hot-water treatment on fungi occurring in apparently healthy grapevine cuttings. Phytopathol. Mediterr. 2001, 40, S464–S466. [Google Scholar]
- Agustí-Brisach, C.; Pérez-Sierra, A.; Armengol, J.; García-Jiménez, J.; Berbegal, M. Efficacy of hot water treatment to reduce the incidence of Fusarium circinatum on Pinus radiata seeds. Forestry 2012, 85, 629–635. [Google Scholar] [CrossRef]
- Liao, T.; Ye, J.; Chen, J.; Han, Y.; Wu, C.; An, Y. Biological characteristics of Fusarium circinatum. J. Nanjing For. Univ. 2008, 32, 83–86. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Paiva, D.S.; Fernandes, L.; Trovão, J.; Mesquita, N.; Tiago, I.; Portugal, A. Uncovering the Fungal Diversity Colonizing Limestone Walls of a Forgotten Monument in the Central Region of Portugal by High-Throughput Sequencing and Culture-Based Methods. Appl. Sci. 2022, 12, 10650. [Google Scholar] [CrossRef]
- Paiva, D.S.; Fernandes, L.; Pereira, E.; Trovão, J.; Mesquita, N.; Tiago, I.; Portugal, A. Exploring Differences in Culturable Fungal Diversity Using Standard Freezing Incubation—A Case Study in the Limestones of Lemos Pantheon (Portugal). J. Fungi 2023, 9, 501. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- Robert, V.; Stegehuis, G.; Stalpers, J. The MycoBank Engine and Related Databases. 2005. Available online: https://www.MycoBank.org/ (accessed on 13 May 2024).
- Dwinell, L.D. Contamination of Pinus radiata seeds in California by Fusarium circinatum. In Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, States Department of Agriculture (USDA) Forest Service, San Diego, CA, USA; 1999; pp. 1–4. [Google Scholar]
- Šerá, B.; Zahoranová, A.; Bujdáková, H.; Šerý, M. Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O’Donnell using non-thermal plasma treatment. Rom. Rep. Phys 2019, 71, 701. [Google Scholar]
- Iturritxa, E.; Desprez-Loustau, M.L.; García-Serna, I.; Quintana, E.; Mesanza, N.; Aitken, J. Effect of alternative disinfection treatments against fungal canker in seeds of Pinus radiata. Seed Technol. 2011, 33, 88–110. [Google Scholar]
- Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum. Forests 2020, 11, 837. [Google Scholar] [CrossRef]
- Gioia, T.; Galinski, A.; Lenz, H.; Müller, C.; Lentz, J.; Heinz, K.; Nagel, K.A. GrowScreen-PaGe, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply. Funct. Plant Biol. 2016, 44, 76–93. [Google Scholar] [CrossRef]
- Capieau, K.; Stenlid, J.; Stenström, E. Potential for biological control of Botrytis cinerea in Pinus sylvestris seedlings. Scand. J. For. Res. 2004, 19, 312–319. [Google Scholar] [CrossRef]
- Jiao, Y.; Huang, J. Allelopathic effects of aqueous extracts from uncomposted and composted Mexican devil (Ageratina adenophora) plants on forest fungal growth and soil nitrogen and phosphorus mobilization. Weed Sci. 2024, 72, 76–85. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskaite, I.; Matelis, A.; Pliūra, A.; Vasaitis, R. Fungi in living and dead stems and stumps of Pinus mugo on coastal dunes of the Baltic Sea. Plant Protect. Sci. 2014, 50, 221–226. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskaite, I.; Stenlid, J.; Vasaitis, R. Impact of forest fire on occurrence of Heterobasidion annosum ss root rot and other wood–inhabiting fungi in roots of Pinus mugo. Forestry 2010, 83, 83–92. [Google Scholar] [CrossRef]
- Stępniewska, S.; Mańka, M. Fungi inhabiting Scots pine (Pinus sylvestris) seeds in Wielkopolska region. Phytopathol. Pol. 2004, 31, 73–76. [Google Scholar]
- Sanz–Ros, A.V.; Müller, M.M.; San Martín, R.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol. 2015, 119, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Hui, S.; Choi, J.; Asiegbu, F.O.; Valkonen, J.P.; Lee, Y.H. Secret lifestyles of Neurospora crassa. Sci. Rep. 2014, 4, 5135. [Google Scholar] [CrossRef] [PubMed]
- Botella, L.; Diez, J.J. Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers. 2011, 47, 9–18. [Google Scholar] [CrossRef]
- Garay–Serrano, E.; del Pilar Ortega–Larrocea, M.; Reverchon, F.; Suárez–Quijada, I. Persistence of ecto– and ectendomycorrhizal fungi associated with Pinus montezumae in experimental microcosms. Symbiosis 2018, 74, 67–78. [Google Scholar] [CrossRef]
- Jones, J.P.; Sun, X.; Eckhardt, L.; Webber, A.; Hess, N.; Barnett, J.; McGilvary, J. Longleaf seedling production: Some problems and their solutions. LA Agric. 2002, 45, 4–11. [Google Scholar]
- Fernandes, A.R.; Silva, A.C.; Portugal, A.; Fernandes, C.; Ribeiro, D.; Bragança, H.; Ribeiro, H.; Barbosa, J.N.; Fernandes, L.; Martins, L.; et al. Prevenção do Cancro-Resinoso-do-Pinheiro (Fusarium circinatum)-Manual Técnico para Fornecedores de Materiais Florestais de Reprodução, 1st ed.; Centro PINUS: Viana do Castelo, Portugal, 2021. [Google Scholar]


| Treatment | Concentration/Temperature | Time (min) | No. of Treated Seeds (per Pinus Species) |
|---|---|---|---|
| MennoFlorades | 4% (v/v) | 60 | 100 |
| Captan | 1.9 g/L | 5 | 100 |
| Ethanol | 50% (v/v) | 5 | 100 |
| 60% (v/v) | 5 | 100 | |
| 70% (v/v) | 5 | 100 | |
| Hot Water | 52 °C | 30 | 100 |
| 55 °C | 30 | 100 | |
| 60 °C | 15 | 100 |
| Nursery | Treatment | No. of Containers per Treatment | No. of Cells per Container | Total No. of Cells per Treatment | |
|---|---|---|---|---|---|
| A | MennoFlorades | 4%—1 h | 25 | 54 | 1350 |
| Control | N/A | 25 | 54 | 1350 | |
| B | Captan | 1.9 g/L—5 min | 24 | 60 | 1440 |
| Hot Water | 60 °C—15 min | 24 | 60 | 1440 | |
| Control | N/A | 24 | 60 | 1440 | |
| Germination Rate Post-Sowing (%) | ||||
|---|---|---|---|---|
| Nursery | A | B | ||
| Treatment | 1 Month | 2 Months | 1 Month | 2 Months |
| MennoFlorades at 4% for 60 m | 54.30 a | 63.19 a | - | - |
| Captan at 1.9 g/L | - | - | 15.63 a | 48.54 a |
| Hot Water at 60 °C for 15 m | - | - | 16.32 a | 59.03 b |
| Control | 59.41 a | 66.96 a | 17.5 a | 55.21 b |
| Certification | |||||
|---|---|---|---|---|---|
| Nursery | A | B | |||
| Treatment | 4% MennoFlorades | Control | Hot Water at 60 °C | 1.9 g/L Captan | Control |
| Alveoli (total) | 1350 | 1350 | 1440 | 1440 | 1440 |
| No. of plants/lot | 853 | 904 | 1236 | 1163 | 1156 |
| No. of certified plants | 0 | 10 | 1150 | 1000 | 1075 |
| % of certified plants | 0.00 | 1.11 | 93.04 | 85.98 | 92.99 |
| Mean height (cm) | 4.88 | 4.63 | 17.11 | 16.78 | 18.33 |
| Mean diameter (mm) | 1.04 | 0.96 | 2.03 | 2.00 | 2.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, L.; Paiva, D.S.; Silva, A.C.; Fernandes, C.; Fernandes, A.R.; Ribeiro, D.; Martins, L.; Bragança, H.; Portugal, A. From Lab to Nursery: Novel Approaches of Seed Disinfection for Managing Pine Pitch Canker Propagation. Forests 2024, 15, 1154. https://doi.org/10.3390/f15071154
Fernandes L, Paiva DS, Silva AC, Fernandes C, Fernandes AR, Ribeiro D, Martins L, Bragança H, Portugal A. From Lab to Nursery: Novel Approaches of Seed Disinfection for Managing Pine Pitch Canker Propagation. Forests. 2024; 15(7):1154. https://doi.org/10.3390/f15071154
Chicago/Turabian StyleFernandes, Luís, Diana S. Paiva, Ana C. Silva, Cláudia Fernandes, Ana Rita Fernandes, Dina Ribeiro, Luís Martins, Helena Bragança, and António Portugal. 2024. "From Lab to Nursery: Novel Approaches of Seed Disinfection for Managing Pine Pitch Canker Propagation" Forests 15, no. 7: 1154. https://doi.org/10.3390/f15071154
APA StyleFernandes, L., Paiva, D. S., Silva, A. C., Fernandes, C., Fernandes, A. R., Ribeiro, D., Martins, L., Bragança, H., & Portugal, A. (2024). From Lab to Nursery: Novel Approaches of Seed Disinfection for Managing Pine Pitch Canker Propagation. Forests, 15(7), 1154. https://doi.org/10.3390/f15071154










