Genetic Diversity, Mating System, and Seed Viability Reveal a Trade-Off between Outcrossing and Inbreeding in Pinus yunnanensis var. tenuifolia, an Ecologically Important Conifer Species Growing in a Hot-Dry River Basin Habitat in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample Collection
2.2. Seed Quality Measurement
2.3. DNA Extraction and Genotyping
2.4. Data Analysis
3. Results
3.1. Seed Quality
3.2. Genetic Diversity
3.3. Genetic Structure
3.4. Mating System
3.5. Relationship between the Outcrossing Rate and Seed Quality
4. Discussion
4.1. Seed Quality
4.2. Genetic Diversity and Differentiation and Its Causing and Aftereffect
4.3. Relationship between Seed Quality and Mating System and Its Highlights on Adaptive Evolution
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, S.A.; Ghazoul, J.; Ravikanth, G.; Kushalappa, C.G.; Uma Shaanker, R.; Kettle, C.J. Fragmentation Genetics of Vateria indica: Implications for Management of Forest Genetic Resources of an Endemic Dipterocarp. Conserv. Genet. 2014, 15, 533–545. [Google Scholar] [CrossRef]
- Manoel, R.O.; Alves, P.F.; Dourado, C.L.; Gaino, A.P.S.C.; Freitas, M.L.M.; Moraes, M.L.T.; Sebbenn, A.M. Contemporary Pollen Flow, Mating Patterns and Effective Population Size Inferred from Paternity Analysis in a Small Fragmented Population of the Neotropical Tree Copaifera langsdorffii Desf. (Leguminosae-Caesalpinioideae). Conserv. Genet. 2012, 13, 613–623. [Google Scholar] [CrossRef]
- Pereira, F.B.; Sebbenn, A.M.; Boshier, D.H.; Rossini, B.C.; Marino, C.L.; Freitas, M.L.M.; Rosa, J.R.B.F.; Vidal, E.; Tambarussi, E.V. Gene Flow, Mating Patterns and Inbreeding Depression in Roupala montana var. Brasiliensis, a Neotropical Timber Species. New For. 2023. [Google Scholar] [CrossRef]
- Rymer, P.D.; Sandiford, M.; Harris, S.A.; Billingham, M.R.; Boshier, D.H. Remnant Pachira Quinata Pasture Trees Have Greater Opportunities to Self and Suffer Reduced Reproductive Success Due to Inbreeding Depression. Heredity 2015, 115, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tambarussi, E.V.; Boshier, D.; Vencovsky, R.; Freitas, M.L.M.; Sebbenn, A.M. Paternity Analysis Reveals Significant Isolation and near Neighbor Pollen Dispersal in Small Cariniana Legalis Mart. Kuntze Populations in the Brazilian Atlantic Forest. Ecol. Evol. 2015, 5, 5588–5600. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; De Meester, L.; Jongejans, E.; Honnay, O. Evolutionary Changes in Plant Reproductive Traits Following Habitat Fragmentation and Their Consequences for Population Fitness. J. Ecol. 2012, 100, 76–87. [Google Scholar] [CrossRef]
- Winn, A.A.; Elle, E.; Kalisz, S.; Cheptou, P.O.; Eckert, C.G.; Goodwillie, C.; Johnston, M.O.; Moeller, D.A.; Ree, R.H.; Sargent, R.D. and Analysis of Inbreeding Depression in Mixed-Mating Plants Provides Evidence for Selective Interference and Stable Mixed Mating. Evolution 2011, 65, 3339–3359. [Google Scholar] [CrossRef] [PubMed]
- Ahlinder, J.; Giles, B.E.; García-Gil, M.R. Life Stage-Specific Inbreeding Depression in Long-Lived Pinaceae Species Depends on Population Connectivity. Sci. Rep. 2021, 11, 8834. [Google Scholar] [CrossRef]
- Breed, M.F.; Ottewell, K.M.; Gardner, M.G.; Marklund, M.H.K.; Dormontt, E.E.; Lowe, A.J. Mating Patterns and Pollinator Mobility Are Critical Traits in Forest Fragmentation Genetics. Heredity 2015, 115, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Viso, F.; Young, A.G.; Pannell, J.R. The Loss of Self-incompatibility in a Range Expansion. J. Evol. Biol. 2020, 33, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, A.L.; Eckert, C.G. Evolution of Dispersal and Mating Systems along Geographic Gradients: Implications for Shifting Ranges. Funct. Ecol. 2014, 28, 5–21. [Google Scholar] [CrossRef]
- Restoux, G.; Silva, D.E.; Sagnard, F.; Torre, F.; Klein, E.; Fady, B. Life at the Margin: The Mating System of Mediterranean Conifers. Web Ecol. 2008, 8, 94–102. [Google Scholar] [CrossRef]
- Durel, C.E.; Bertin, P.; Kremer, A. Relationship between Inbreeding Depression and Inbreeding Coefficient in Maritime Pine (Pinus pinaster). Theor. Appl. Genet. 1996, 92, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Mullin, T.J.; Persson, T.; Abrahamsson, S.; Andersson Gull, B. Effects of Inbreeding Depression on Seed Production in Scots Pine (Pinus sylvestris). Can. J. For. Res. 2019, 49, 854–860. [Google Scholar] [CrossRef]
- Williams, C.G. Selfed Embryo Death in Pinus taeda: A Phenotypic Profile. New Phytol. 2008, 178, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.D.; Aitken, S.N. Mating System and Inbreeding Depression in Whitebark Pine (Pinus albicaulis Engelm.). Tree Genet. Genomes 2007, 3, 379–388. [Google Scholar] [CrossRef]
- Stoehr, M.; Ott, P.; Woods, J. Inbreeding in Mid-Rotation Coastal Douglas-Fir: Implications for Breeding. Ann. For. Sci. 2015, 72, 195–204. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H. The Distribution of Pinus yunnanensis var. Tenuifolia in Relation to the Environmental Conditions. Acta Phytoecol. Geobot. Sin. 1981, 5, 28–37. [Google Scholar]
- Wang, H. A Preliminary Study of Pinus yunnanensis var. Tenuifolia Formation of Guangxi. Bull. Bot. Res. 1987, 7, 127–150. [Google Scholar]
- Xu, X. Geographical Distribution and Growth Characteristics of Pinus yunnanensis var. Tenuifolia Forest in Guizhou Province. Guizhou Sci. 1983, 1, 94–98. [Google Scholar]
- Bai, W.; Chen, B.; Chen, D.; Huang, T.; Liu, X. Drying Characteristics of Pinus yunnanensis var. Tenuifolia Wood. J. Fujian For. Sci. Tech. 2016, 43. [Google Scholar] [CrossRef]
- Qin, L.; Liu, X.; Lan, L.; Fu, Y.; Yang, L. Green Wood Properties of Pinus yunnanensis via Tenuifolia. J. Northwest For. Univ. 2015, 30, 217–223. [Google Scholar] [CrossRef]
- Bai, T.; Yu, C.; Gan, Z.; Lai, H.; Yang, Y.; Huang, H.; Jiang, W. Association of Cone and Seed Traits of Pinus yunnanensis var. Tenuifolia with Geo-Meteorological Factors. Chin. J. Plant Ecol. 2020, 44, 1224–1235. [Google Scholar] [CrossRef]
- Feng, S.-S.; Huang, C.-H.; Tang, M.-Y.; Jiang, W.-X.; Bai, T.-D. Geographical Variation of Needles Phenotypic and Anatomic Traits between Populations of Pinus yunnanensis var. Tenuifolia and Its Environmental Interpretation. Chin. J. Plant Ecol. 2023, 47, 1116–1130. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Ming, A.; Wang, H.; Yu, S.; Ye, S. Spatial Pattern Dynamics among Co-Dominant Populations in Early Secondary Forests in Southwest China. J. For. Res. 2021, 32, 1373–1384. [Google Scholar] [CrossRef]
- Devi, V. The Germinationmetrics Package: A Brief Introduction; ICAR-National Bureau of Plant Genetic Resources: New Delhi, India, 2021. [Google Scholar]
- Kuang, M.; Yang, W.; Xu, H.; Wang, Y.; Zhou, D.; Feng, X. A Rapid Method of DNA Extraction from Single Cotton Seed. Mol. Plant Breed. 2010, 8, 827–831. [Google Scholar]
- Chen, M. Study on Seed Fruit Traits and Genetic Diversity of Pinus massoniana Tugong Provenance. Master’s Thesis, Guangxi University, Nanning, China, 2018. [Google Scholar]
- Schmidtling, R.C.; Hipkins, V. Genetic Diversity in Longleaf Pine (Pinus palustris): Influence of Historical and Prehistorical Events. Can. J. For. Res. 1998, 28, 1135–1145. [Google Scholar] [CrossRef]
- Bai, T.-D.; Xu, L.-A.; Xu, M.; Wang, Z.-R. Characterization of Masson Pine (Pinus massoniana Lamb.) Microsatellite DNA by 454 Genome Shotgun Sequencing. Tree Genet. Genomes 2014, 10, 429–437. [Google Scholar] [CrossRef]
- Kutil, B.L.; Williams, C.G. Triplet-Repeat Microsatellites Shared Among Hard and Soft Pines. J. Hered. 2001, 92, 327–332. [Google Scholar] [CrossRef]
- Ni, Z.X.; Bai, T.; Cai, H.; Chen, S.; Xu, L.A. The Transferability of Pinus massoniana SSR in Other Pinus Species. Mol. Plant Breed. 2015, 13, 2811–2817. [Google Scholar]
- Xu, Y. Genetic Variation of Natural Populations in Pinus yunnanensis Franch. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Sabin, T.E.; Stafford, S.G. Assessing the Need for Transformation of Response Variables; Forest Research Laboratory: Riverside, CA, USA; Oregon State University: Corvallis, OR, USA, 1990; Volume Special Publication 20. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Goudet, J. Hierfstat, a Package for r to Compute and Test Hierarchical F-Statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes: Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, T.; Dunn, D.W.; Zhang, P.; Sun, H.; Li, B. A Generalized Framework for AMOVA with Multiple Hierarchies and Ploidies. Integr. Zool. 2021, 16, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Frichot, E.; Mathieu, F.; Trouillon, T.; Bouchard, G.; François, O. Fast and Efficient Estimation of Individual Ancestry Coefficients. Genetics 2014, 196, 973–983. [Google Scholar] [CrossRef]
- Frichot, E.; François, O. LEA: An R Package for Landscape and Ecological Association Studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y. Determine the Number of Unknown Targets in Open World Based on Elbow Method. IEEE Trans. Fuzzy Syst. 2021, 29, 986–995. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.-Y. Ggtree: An r Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Ritland, K. Extensions of Models for the Estimation of Mating Systems Using n Independent Loci. Heredity 2002, 88, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ritland, K.; Miscampbell, A.; Van Niejenhuis, A.; Brown, P.; Russell, J. Selfing and Correlated Paternity in Relation to Pollen Management in Western Red Cedar Seed Orchards. Botany 2020, 98, 353–359. [Google Scholar] [CrossRef]
- Zieffler, A.S.; Harring, J.R.; Long, J.D. Comparing Groups: Randomization and Bootstrap Methods Using R, 1st ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-62169-1. [Google Scholar]
- Michalakis, Y.; Excoffier, L. A Generic Estimation of Population Subdivision Using Distances Between Alleles With Special Reference for Microsatellite Loci. Genetics 1996, 142, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Tíscar Oliver, P.; Lucas Borja, M. Seed Mass Variation, Germination Time and Seedling Performance in a Population of Pinus nigra Subsp. Salzamannii. For. Syst. 2010, 19, 344. [Google Scholar] [CrossRef]
- Tumpa, K.; Vidaković, A.; Drvodelić, D.; Šango, M.; Idžojtić, M.; Perković, I.; Poljak, I. The Effect of Seed Size on Germination and Seedling Growth in Sweet Chestnut (Castanea sativa Mill.). Forests 2021, 12, 858. [Google Scholar] [CrossRef]
- Wei, W.; Chen, M.-X.; Li, X.-Q.; Jiang, W.-X.; Bai, T.-D. How Does Population Outcrossing Rate Influence Seed Quality? A Case Study from a Seed Tree Stand of Pinus massoniana. New For. 2023. [Google Scholar] [CrossRef]
- Kärkkäinen, K.; Savolainen, O.; Koski, V. Why Do Plants Abort so Many Developing Seeds: Bad Offspring or Bad Maternal Genotypes? Evol. Ecol. 1999, 13, 305–317. [Google Scholar] [CrossRef]
- Hall, D.; Zhao, W.; Heuchel, A.; Gao, J.; Wennström, U.; Wang, X.-R. The Effect of Gene Flow on Frost Tolerance in Scots Pine—Latitudinal Translocation of Genetic Material. For. Ecol. Manag. 2023, 544, 121215. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Jiang, H. A Study on Comparative Anatomy of Pinus yunnanensis Needles under Different Habitats. J. Southwest Forestry Coll. 2004, 24, 1–5. [Google Scholar]
- Huang, R. The Population Genetics and Evolution of Pinus yunnanensis. J. Yunnan Univ. 1993, 15, 50–63. [Google Scholar]
- Huang, B.; Zhao, Y.; Huo, D.; Su, W.; Zhang, G. Effect of Temperature on Cone-Opening Time and Seed Germination of Pinus yunanensis. Seed 2016, 35, 19–21. [Google Scholar]
- Yang, W.; Li, L.; Wang, Y.; Ou, Y.; Ling, L.; Xu, T.; Wu, S. Effects of Temperature and Exogenous Hormone Presoaking Seeds on Seed Germination of Pinus yunnanensis. Seed 2017, 36, 10–19. [Google Scholar]
- Zhang, H.; Meng, L.; Yu, T.; Yu, J.; Si, H.; Wu, X.; Ma, X.; Su, W.; Zhang, G. Effect of Short-Term Heating Pretreatment on Seed Germination in Four Pine Species. Seed 2018, 37, 32–37. [Google Scholar]
- Cram, W.H. Some Effects of Self-, Cross-, and Open-Pollinations in Picea pungens. Can. J. Bot. 1984, 62, 392–395. [Google Scholar] [CrossRef]
- Callejas-Díaz, M.; Chambel, M.R.; San-Martín-Lorén, J.; Gea-Izquierdo, G.; Santos-Del-Blanco, L.; Postma, E.; Climent, J.M. The Role of Maternal Age, Growth, and Environment in Shaping Offspring Performance in an Aerial Conifer Seed Bank. Am. J. Bot. 2022, 109, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, N.H.; Picó, F.X. Seed Germination, Seedling Traits, and Seed Bank of the Tree Moringa peregrina (Moringaceae) in a Hyper-Arid Environment. Am. J. Bot. 2011, 98, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Vidal, E.; Sampedro, L.; Zas, R. Is the Benefit of Larger Seed Provisioning on Seedling Performance Greater under Abiotic Stress? Environ. Exp. Bot. 2017, 134, 45–53. [Google Scholar] [CrossRef]
- Cai, C.; Xiao, J.; Ci, X.; Conran, J.G.; Li, J. Genetic Diversity of Horsfieldia tetratepala (Myristicaceae), an Endangered Plant Species with Extremely Small Populations to China: Implications for Its Conservation. Plant Syst. Evol. 2021, 307, 50. [Google Scholar] [CrossRef]
- Hoban, S.; Archer, F.I.; Bertola, L.D.; Bragg, J.G.; Breed, M.F.; Bruford, M.W.; Coleman, M.A.; Ekblom, R.; Funk, W.C.; Grueber, C.E.; et al. Global Genetic Diversity Status and Trends: Towards a Suite of Essential Biodiversity Variables (EBVs) for Genetic Composition. Biol. Rev. 2022, 97, 1511–1538. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Genetic Diversity Analysisi of Pinus yunnanensis Franch. var. Tenuifolia Cheng et Law Based on cpSSR and nSSR Makers. Master’s Thesis, Guangxi University, Nanning, China, 2021. [Google Scholar]
- Miao, Y.; Gao, C.; Li, J.; Liu, Z.; Cui, K. Genetic Diversity, Population Structure and a Core Collection Establishment of Pinus yunnanensis Using Microsatellite Markers. Eur. J. For. Res. 2023, 142, 1439–1451. [Google Scholar] [CrossRef]
- Schwendemann, A.B.; Wang, G.; Mertz, M.L.; McWilliams, R.T.; Thatcher, S.L.; Osborn, J.M. Aerodynamics of Saccate Pollen and Its Implications for Wind Pollination. Am. J. Bot. 2007, 94, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.R.; Lindgren, D. Effect of Inbreeding on Production of Filled Seed in Pinus radiata—Experimental Results and a Model of Gene Action. Theoret. Appl. Genet. 1985, 71, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Husband, B.C.; Schemske, D.W. Evolution of the Magnitude and Timing of Inbreeding Depression in Plants. Evolution 1996, 50, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Tambarussi, E.V.; Boshier, D.; Vencovsky, R.; Freitas, M.L.M.; Sebbenn, A.M. Inbreeding Depression from Selfing and Mating between Relatives in the Neotropical Tree Cariniana legalis Mart. Kuntze. Conserv. Genet. 2017, 18, 225–234. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Inbreeding Depression and the Cost of Inbreeding on Seed Germination. Seed Sci. Res. 2015, 25, 355–385. [Google Scholar] [CrossRef]
- Capblancq, T.; Munson, H.; Butnor, J.R.; Keller, S.R. Genomic Drivers of Early-Life Fitness in Picea rubens. Conserv. Genet. 2021, 22, 963–976. [Google Scholar] [CrossRef]
- Del Castillo, R.F.; Trujillo, S. Effect of Inbreeding Depression on Outcrossing Rates among Populations of a Tropical Pine. New Phytol. 2008, 177, 517–524. [Google Scholar] [CrossRef]
- Levin, D.A. Mating System Shifts on the Trailing Edge. Ann. Bot. 2012, 109, 613–620. [Google Scholar] [CrossRef]
- Gao, J.; Tomlinson, K.W.; Zhao, W.; Wang, B.; Lapuz, R.S.; Liu, J.; Pasion, B.O.; Hai, B.T.; Chanthayod, S.; Chen, J.; et al. Phylogeography and Introgression between Pinus kesiya and Pinus yunnanensis in Southeast Asia. J. Sytematics Evol. 2023, 62, 120–134. [Google Scholar] [CrossRef]
- Woods, J.H.; Heaman, J.C. Effect of Different Inbreeding Levels on Filled Seed Production in Douglas-Fir. Can. J. For. Res. 1989, 19, 54–59. [Google Scholar] [CrossRef]


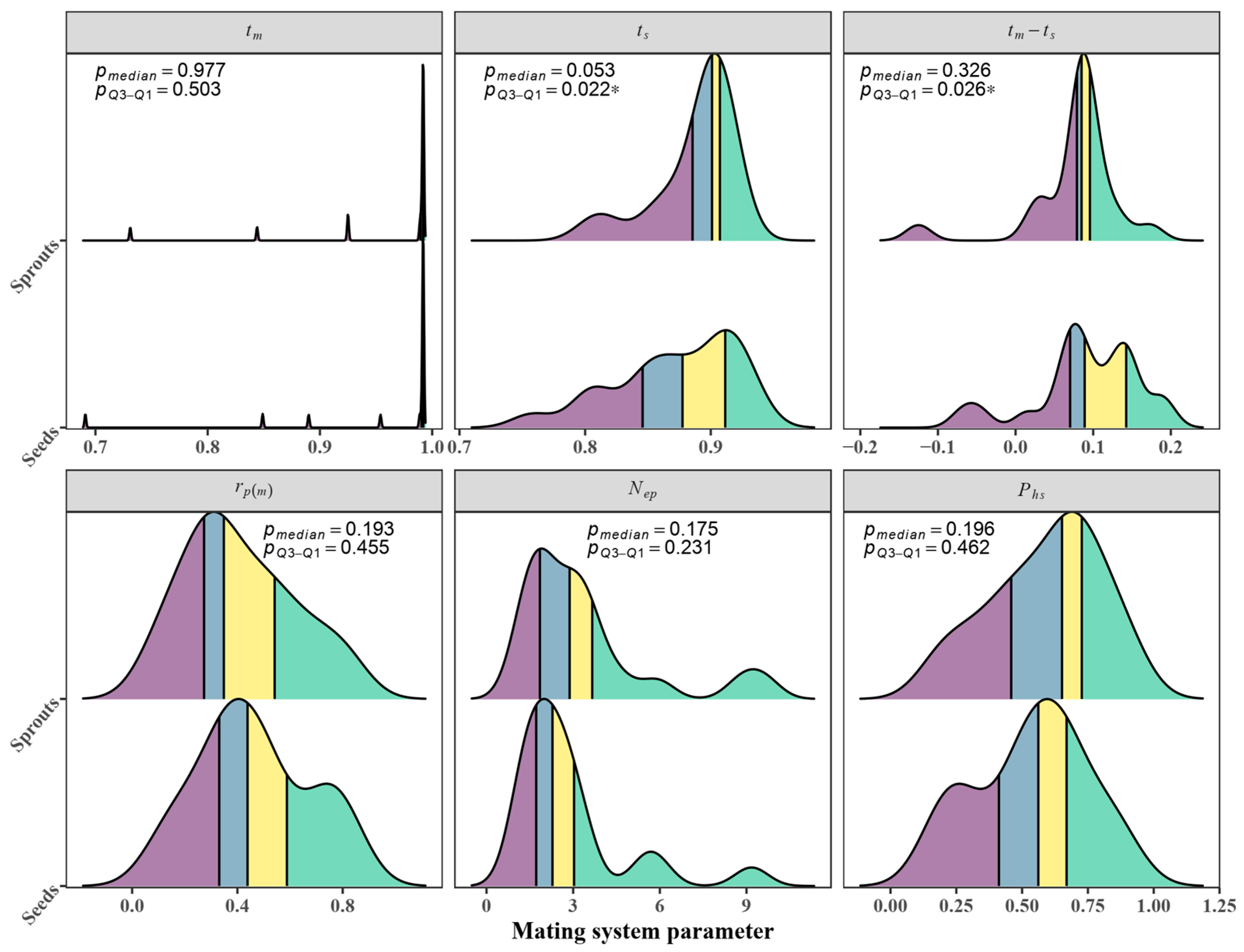
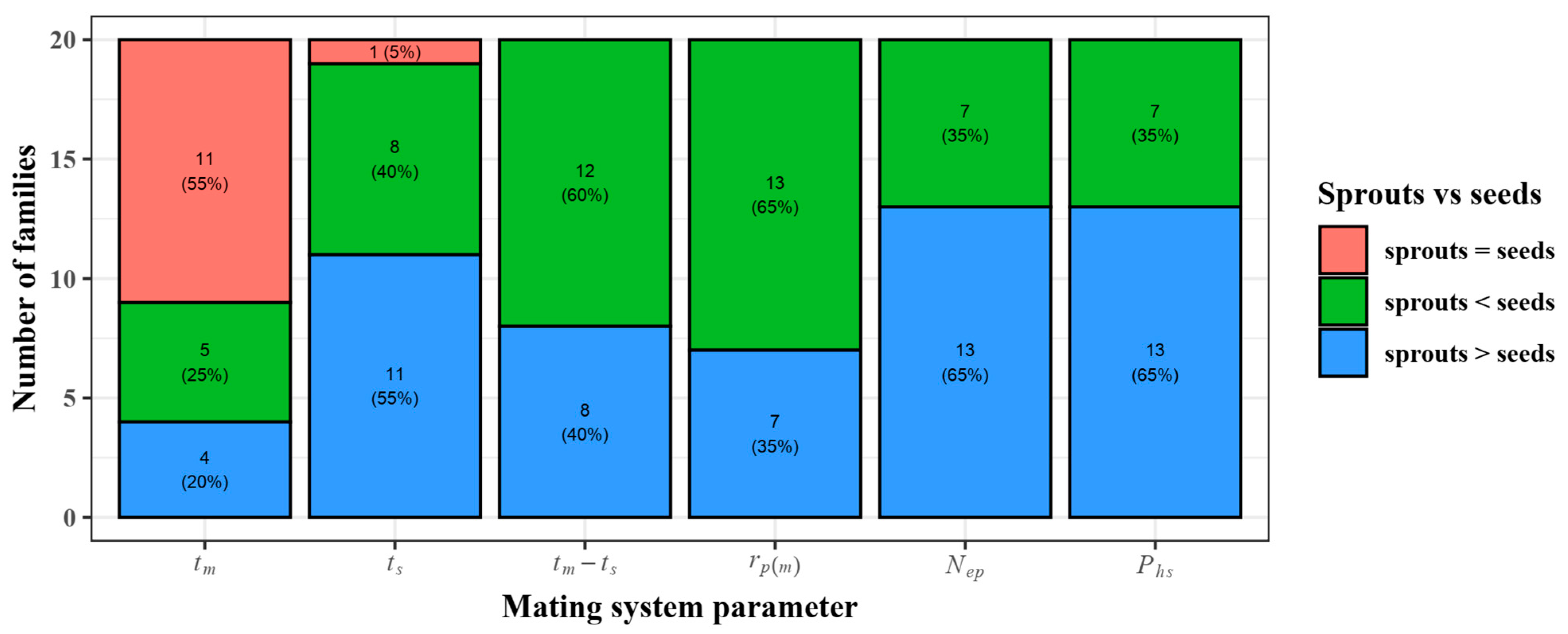

| Family ID | Sample Size | Aobs | Arich | Hobs | Hs | SH | F |
|---|---|---|---|---|---|---|---|
| BY-24 | 24/24 | 3.8/4.0 | 3.6/3.8 | 0.528/0.496 | 0.477/0.472 | 0.838/0.826 | −0.070/0.003 |
| BY-23 | 24/24 | 3.9/4.2 | 3.8/4.0 | 0.497/0.433 | 0.440/0.503 | 0.801/0.912 | −0.125/0.174 |
| BY-37 | 24/24 | 4.3/5.5 | 4.2/5.3 | 0.479/0.336 | 0.506/0.547 | 0.926/1.076 | 0.093/0.369 |
| BY-47 | 24/24 | 5.0/6.1 | 4.8/5.8 | 0.496/0.465 | 0.554/0.582 | 1.057/1.151 | 0.103/0.220 |
| CJ-45 | 24/24 | 5.0/5.3 | 4.9/5.0 | 0.604/0.586 | 0.598/0.552 | 1.131/1.039 | −0.02/−0.028 |
| QX-41 | 24/24 | 5.2/6.4 | 5.0/6.1 | 0.520/0.556 | 0.560/0.595 | 1.079/1.208 | 0.074/0.058 |
| QX-14 | 24/24 | 6.3/5.9 | 6.0/5.7 | 0.487/0.506 | 0.582/0.552 | 1.186/1.100 | 0.150/0.057 |
| BW-26 | 24/24 | 6.3/6.0 | 6.0/5.8 | 0.498/0.560 | 0.606/0.590 | 1.219/1.193 | 0.191/0.049 |
| XQ-1 | 24/24 | 4.0/4.8 | 3.8/4.6 | 0.455/0.472 | 0.421/0.480 | 0.769/0.935 | −0.008/0.047 |
| XQ-13 | 24/24 | 5.3/6.9 | 5.1/6.7 | 0.470/0.401 | 0.567/0.638 | 1.100/1.333 | 0.185/0.335 |
| XQ-34 | 24/24 | 3.7/4.2 | 3.5/4.0 | 0.542/0.540 | 0.451/0.518 | 0.779/0.921 | −0.122/−0.044 |
| XQ-7 | 24/24 | 4.7/7.0 | 4.5/6.8 | 0.502/0.460 | 0.507/0.637 | 0.949/1.317 | −0.009/0.241 |
| WJ-18 | 24/24 | 5.0/5.7 | 4.8/5.4 | 0.506/0.482 | 0.572/0.594 | 1.081/1.162 | 0.156/0.213 |
| WJ-24 | 24/24 | 4.7/5.5 | 4.5/5.3 | 0.368/0.377 | 0.467/0.538 | 0.893/1.072 | 0.195/0.187 |
| WJ-28 | 24/24 | 4.9/3.8 | 4.8/3.7 | 0.436/0.392 | 0.505/0.406 | 0.982/0.732 | 0.110/0.030 |
| WJ-41 | 24/24 | 6.1/5.2 | 5.9/5.0 | 0.521/0.500 | 0.603/0.531 | 1.223/1.021 | 0.031/0.025 |
| DT-17 | 24/24 | 3.8/3.9 | 3.7/3.8 | 0.457/0.394 | 0.462/0.438 | 0.817/0.809 | 0.045/0.194 |
| DT-4 | 24/24 | 4.7/5.0 | 4.5/4.8 | 0.468/0.478 | 0.500/0.499 | 0.948/0.982 | 0.039/0.044 |
| DT-1 | 24/24 | 4.4/4.5 | 4.2/4.4 | 0.553/0.470 | 0.524/0.540 | 0.937/1.004 | −0.046/0.095 |
| DT-40 | 24/24 | 4.2/5.3 | 4.0/5.0 | 0.521/0.515 | 0.491/0.545 | 0.899/1.058 | −0.096/−0.013 |
| mean | 24/24 | 4.8/5.3 *** | 4.6/5.0 *** | 0.495/0.471 ** | 0.520/0.538 * | 0.981/1.043 ** | 0.044/0.113 *** |
| Source of Variation | DF | Sum Sq | Mean Sq | Var Comp | Diff Coef (Φst) |
|---|---|---|---|---|---|
| Between populations | 6 | 1181.381/1082.009 | 196.897/180.335 | 1.034/0.883 ** | 0.122/0.102 |
| Between families within population | 13 | 785.455/829.850 | 60.420/63.835 | 1.121/1.175 | 0.151/0.151 |
| Between individuals within family | 460 | 3036.305/3428.562 | 6.601/7.453 | 0.294/0.833 ** | 0.047/0.126 |
| Within individual | 480 | 2885.913/2777.849 | 6.012/5.787 | 6.012/5.787 ** | |
| Total | 959 | 7889.054/8118.271 | 8.226/8.465 | 8.462/8.677 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-Q.; Wen, Y.-Z.; Huang, C.-H.; Tang, M.-Y.; Jiang, W.-X.; Bai, T.-D. Genetic Diversity, Mating System, and Seed Viability Reveal a Trade-Off between Outcrossing and Inbreeding in Pinus yunnanensis var. tenuifolia, an Ecologically Important Conifer Species Growing in a Hot-Dry River Basin Habitat in Southwest China. Forests 2024, 15, 982. https://doi.org/10.3390/f15060982
Li X-Q, Wen Y-Z, Huang C-H, Tang M-Y, Jiang W-X, Bai T-D. Genetic Diversity, Mating System, and Seed Viability Reveal a Trade-Off between Outcrossing and Inbreeding in Pinus yunnanensis var. tenuifolia, an Ecologically Important Conifer Species Growing in a Hot-Dry River Basin Habitat in Southwest China. Forests. 2024; 15(6):982. https://doi.org/10.3390/f15060982
Chicago/Turabian StyleLi, Xian-Qin, Yu-Zhuo Wen, Chun-Hui Huang, Meng-Yun Tang, Wei-Xin Jiang, and Tian-Dao Bai. 2024. "Genetic Diversity, Mating System, and Seed Viability Reveal a Trade-Off between Outcrossing and Inbreeding in Pinus yunnanensis var. tenuifolia, an Ecologically Important Conifer Species Growing in a Hot-Dry River Basin Habitat in Southwest China" Forests 15, no. 6: 982. https://doi.org/10.3390/f15060982





