Site-Level Modelling Comparison of Carbon Capture by Mixed-Species Forest and Woodland Reforestation in Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.1.1. Validation Data
2.1.2. Calibration Data
2.2. Models
2.2.1. FastTrack
Calibration of Growth Parameters of the FastTrack Model
2.2.2. FullCAM
3. Results
3.1. Above-Ground Biomass
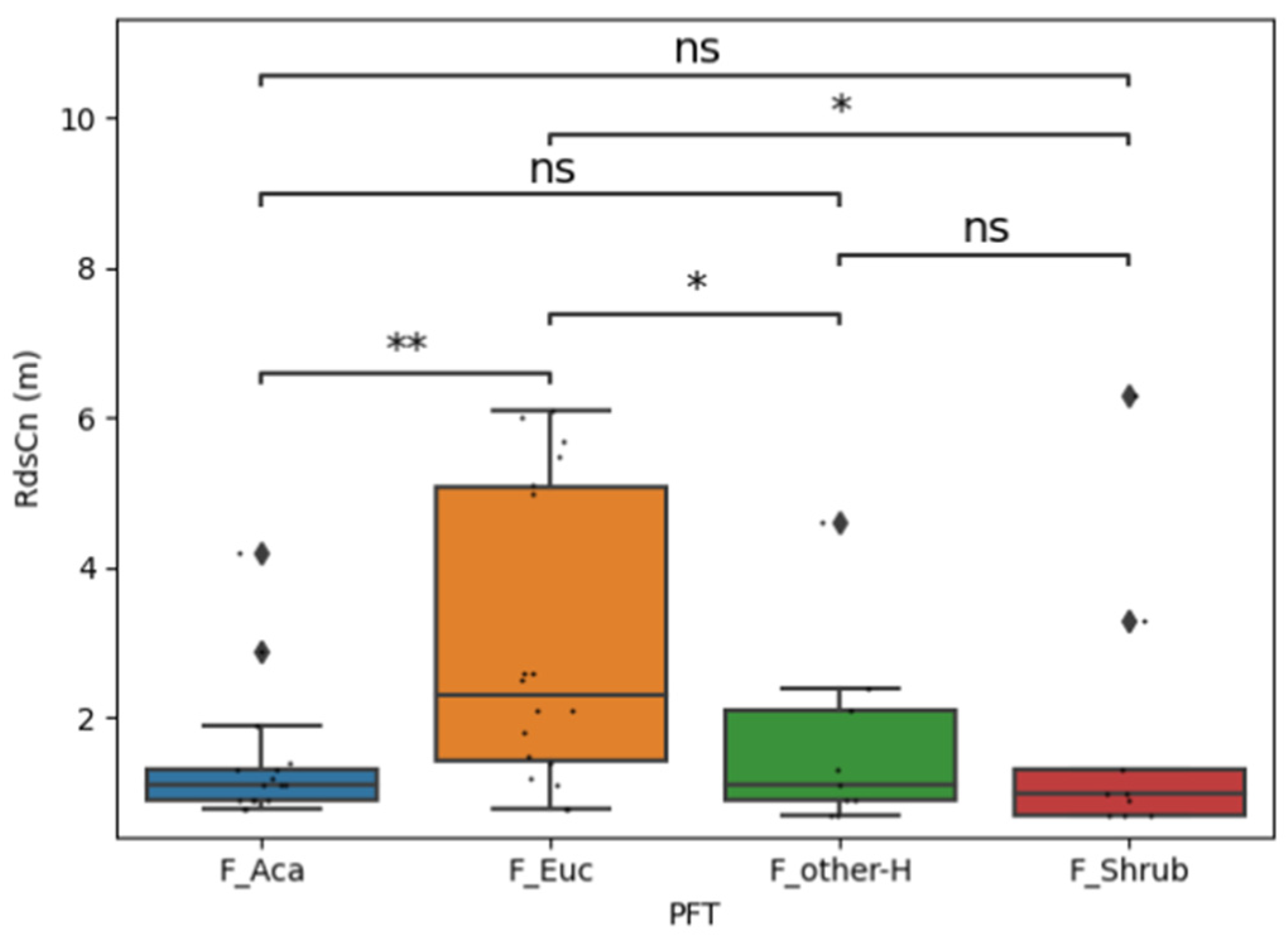
3.2. Crown Radius
3.3. Canopy Cover
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Portillo-Quintero, C.; Sanchez-Azofeifa, A.; Calvo-Alvarado, J.; Quesada, M.; do Espirito Santo, M.M. The Role of Tropical Dry Forests for Biodiversity, Carbon and Water Conservation in the Neotropics: Lessons Learned and Opportunities for Its Sustainable Management. Reg. Environ. Chang. 2015, 15, 1039–1049. [Google Scholar] [CrossRef]
- Quijas, S.; Romero-Duque, L.P.; Trilleras, J.M.; Conti, G.; Kolb, M.; Brignone, E.; Dellafiore, C. Linking Biodiversity, Ecosystem Services, and Beneficiaries of Tropical Dry Forests of Latin America: Review and New Perspectives. Ecosyst. Serv. 2019, 36, 100909. [Google Scholar] [CrossRef]
- FAO. Forests and Land Use in Drylands: The First Global Assessment—Full Report; FAO Forestry Paper No. 184; FAO: Rome, Italy, 2019; Volume 184, p. 193. [Google Scholar]
- Morton, S.; Lonsdale, M.; Sheppard, A. Biodiversity: Science and Solutions for Australia; Csiro Publishing: Clayton, VIC, Australia, 2014; ISBN 1486302076. [Google Scholar]
- Yates, C.J.; Hobbs, R.J. Temperate Eucalypt Woodlands: A Review of Their Status, Processes Threatening Their Persistence and Techniques for Restoration. Aust. J. Bot. 1997, 45, 949–973. [Google Scholar] [CrossRef]
- Lunt, I.D.; Eldridge, D.J.; Morgan, J.W.; Witt, G.B. A Framework to Predict the Effects of Livestock Grazing and Grazing Exclusion on Conservation Values in Natural Ecosystems in Australia. Aust. J. Bot. 2007, 55, 401–415. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Bennett, A.F.; Hobbs, R. An Overview of the Ecology, Management and Conservation of Australia’s Temperate Woodlands. Ecol. Manag. Restor. 2010, 11, 201–209. [Google Scholar] [CrossRef]
- Murphy, H.; van Leeuwen, S. Australia State of the Environment 2021: Biodiversity; Independent Report to the Australian Government Minister for the Environment; Canberra, ACT, Autralia, 2021. [Google Scholar]
- Leishman, M.R.; Gallagher, R.V.; Catford, J.A.; Morgan, J.W.; Grice, A.C.; Setterfield, S.A. Invasive Plants and Pathogens in Australia. In Australian Vegetation; Cambridge University Press: Cambridge, UK, 2017; pp. 207–229. [Google Scholar]
- Gill, A.M. Fire and the Australian Flora: A Review. Aust. For. 1975, 38, 4–25. [Google Scholar] [CrossRef]
- DEECA Services. Bioregions and EVC Benchmarks. Available online: https://www.environment.vic.gov.au/biodiversity/bioregions-and-evc-benchmarks (accessed on 1 February 2024).
- Bryan, B.A.; Nolan, M.; Harwood, T.D.; Connor, J.D.; Navarro-Garcia, J.; King, D.; Summers, D.M.; Newth, D.; Cai, Y.; Grigg, N. Supply of Carbon Sequestration and Biodiversity Services from Australia’s Agricultural Land under Global Change. Glob. Environ. Chang. 2014, 28, 166–181. [Google Scholar] [CrossRef]
- Bekessy, S.A.; Wintle, B.A. Using Carbon Investment to Grow the Biodiversity Bank. Conserv. Biol. 2008, 22, 510–513. [Google Scholar] [CrossRef]
- Paul, K.I.; Roxburgh, S.H.; England, J.R.; de Ligt, R.; Larmour, J.S.; Brooksbank, K.; Murphy, S.; Ritson, P.; Hobbs, T.; Lewis, T.; et al. Improved Models for Estimating Temporal Changes in Carbon Sequestration in Above-Ground Biomass of Mixed-Species Environmental Plantings. For. Ecol. Manag. 2015, 338, 208–218. [Google Scholar] [CrossRef]
- Environmental Defender Office. Nature Repair. Market Bill. Submission. 2023. Available online: https://www.edo.org.au/wp-content/uploads/2023/03/230303-Nature-Repair-Market-Bill-EDO-submission.pdf (accessed on 1 February 2024).
- CBD. Kunming-Montreal Global Biodiversity Framework, Montreal. 2022. Available online: https://unctad.org/topic/trade-and-environment/biotrade/kunming-montreal-global-biodiversity-framework (accessed on 1 February 2024).
- Paul, K.I.; Cunningham, S.C.; England, J.R.; Roxburgh, S.H.; Preece, N.D.; Lewis, T.; Brooksbank, K.; Crawford, D.F.; Polglase, P.J. Managing Reforestation to Sequester Carbon, Increase Biodiversity Potential and Minimize Loss of Agricultural Land. Land. Use Policy 2016, 51, 135–149. [Google Scholar] [CrossRef]
- Chubb, I.; Bennett, A.; Gorring, A.; Hatfield-Dodds, S. Independent Review of Australian Carbon Credit Units. 2023. Available online: https://apo.org.au/node/321347 (accessed on 1 February 2024).
- Standish, R.J.; Hulvey, K.B. Co-benefits of Planting Species Mixes in Carbon Projects. Ecol. Manag. Restor. 2014, 15, 26–29. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive Biodiversity-Productivity Relationship Predominant in Global Forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Marron, N.; Epron, D. Are Mixed-Tree Plantations Including a Nitrogen-Fixing Species More Productive than Monocultures? For. Ecol. Manag. 2019, 441, 242–252. [Google Scholar] [CrossRef]
- Warner, E.; Cook-Patton, S.C.; Lewis, O.T.; Brown, N.; Koricheva, J.; Eisenhauer, N.; Ferlian, O.; Gravel, D.; Hall, J.S.; Jactel, H. Young Mixed Planted Forests Store More Carbon than Monocultures—A Meta-Analysis. Front. For. Glob. Chang. 2023, 6, 1226514. [Google Scholar] [CrossRef]
- Lada, H.; Yen, J.D.L.; Cunningham, S.C.; Selwood, K.E.; Falcke, P.; Hodgson, J.C.; Mac Nally, R. Influence of Climate on Individual Tree Growth and Carbon Sequestration in Native-tree Plantings. Austral Ecol. 2019, 44, 859–867. [Google Scholar] [CrossRef]
- Bauhus, J.; Khanna, P.K.; Menden, N. Aboveground and Belowground Interactions in Mixed Plantations of Eucalyptus Globulus and Acacia Mearnsii. Can. J. For. Res. 2000, 30, 1886–1894. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L. Carbon Allocation in a Mixed-Species Plantation of Eucalyptus Globulus and Acacia Mearnsii. For. Ecol. Manag. 2006, 233, 275–284. [Google Scholar] [CrossRef]
- Levins, R. The Strategy of Model Building in Population Biology. Am. Sci. 1966, 54, 421–431. [Google Scholar]
- Sharpe, P.J.H. Forest Modeling Approaches: Compromises between Generality and Precision. Process Modeling of Forest Growth Responses to Environmental Stress; Timber Press: Portland, OR, USA, 1990; pp. 180–190. [Google Scholar]
- Mahnken, M.; Cailleret, M.; Collalti, A.; Trotta, C.; Biondo, C.; d’Andrea, E.; Dalmonech, D.; Marano, G.; Mäkelä, A.; Minunno, F. Accuracy, Realism and General Applicability of European Forest Models. Glob. Chang. Biol. 2022, 28, 6921–6943. [Google Scholar] [CrossRef]
- Fontes, L.; Bontemps, J.-D.; Bugmann, H.; van Oijen, M.; Gracia, C.; Kramer, K.; Lindner, M.; Rötzer, T.; Skovsgaard, J.P. Models for Supporting Forest Management in a Changing. For. Syst. 2010, 19, 8–29. [Google Scholar] [CrossRef]
- Pretzsch, H.; Forrester, D.I.; Rötzer, T. Representation of Species Mixing in Forest Growth Models. A Review and Perspective. Ecol. Modell. 2015, 313, 276–292. [Google Scholar] [CrossRef]
- Battaglia, M.; Sands, P.J. Process-Based Forest Productivity Models and Their Application in Forest Management. For. Ecol. Manage 1998, 102, 13–32. [Google Scholar] [CrossRef]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. IPCC Guidelines for National Greenhouse Gas Inventories. Volume 4: Agriculture, Forestry and Other Land Use; Institute for Global Environmental Strategies: Hayama, Japan, 2006; Volume 4. [Google Scholar]
- Requena Suarez, D.; Rozendaal, D.M.A.; De Sy, V.; Phillips, O.L.; Alvarez-Dávila, E.; Anderson-Teixeira, K.; Araujo-Murakami, A.; Arroyo, L.; Baker, T.R.; Bongers, F. Estimating Aboveground Net Biomass Change for Tropical and Subtropical Forests: Refinement of IPCC Default Rates Using Forest Plot Data. Glob. Chang. Biol. 2019, 25, 3609–3624. [Google Scholar] [CrossRef]
- FullCAM. Full Carbon Accounting Model (FullCAM). Available online: https://www.dcceew.gov.au/climate-change/publications/full-carbon-accounting-model-fullcam (accessed on 1 February 2024).
- Paul, K.I.; Roxburgh, S.H. Predicting Carbon Sequestration of Woody Biomass Following Land Restoration. For. Ecol. Manage 2020, 460, 117838. [Google Scholar] [CrossRef]
- Paul, K.I.; Roxburgh, S.; Larmour, J.; Davies, M.; England, J.; Peck, A.; Hagger, V.; Perring, M.; Lowson, C.; Read, Z.; et al. Stem Diameter Inventories within Stands of Environmental or Mallee Plantings or Natural Regeneration. Available online: https://ouci.dntb.gov.ua/en/works/7WoaOXL7/ (accessed on 1 February 2024).
- England, J.R.; Theiveyanathan, S.; Paul, K.I.; Polglase, P.; Koul, V.; Larmour, J.; Smith, J.; Stewart, L. Water Use, Growth, and Carbon Sequestration of Environmental Plantings; Final Report, Commercial Environmental Forestry Project; CSIRO: Canberra, ACT, Australia, 2006. [Google Scholar]
- Paul, K.; Roxburgh, S.; Raison, J.; Larmour, J.; England, J.; Murphy, S.; Norris, J.; Ritson, P.; Brooksbank, K.; Hobbs, M. Improved Estimation of Biomass Accumulation by Environmental Plantings and Mallee Plantings Using FullCAM; CSIRO; Department of the Environment: Canberra, ACT, Australia, 2013. [Google Scholar]
- England, J.R.; Paul, K.I.; Cunningham, S.C.; Madhavan, D.B.; Baker, T.G.; Read, Z.; Wilson, B.R.; Cavagnaro, T.R.; Lewis, T.; Perring, M.P. Previous Land Use and Climate Influence Differences in Soil Organic Carbon Following Reforestation of Agricultural Land with Mixed-Species Plantings. Agric. Ecosyst. Environ. 2016, 227, 61–72. [Google Scholar] [CrossRef]
- Paul, K.I.; England, J.R.; Baker, T.G.; Cunningham, S.C.; Perring, M.P.; Polglase, P.J.; Wilson, B.; Cavagnaro, T.R.; Lewis, T.; Read, Z. Using Measured Stocks of Biomass and Litter Carbon to Constrain Modelled Estimates of Sequestration of Soil Organic Carbon under Contrasting Mixed-Species Environmental Plantings. Sci. Total Environ. 2018, 615, 348–359. [Google Scholar] [CrossRef]
- Verschuuren, J. Towards a Regulatory Design for Reducing Emissions from Agriculture: Lessons from Australia’s Carbon Farming Initiative. Climate Law. 2017, 7, 1–51. [Google Scholar] [CrossRef]
- UNFCCC. A/R Methodological Tool “Calculation of the Number of Sample Plots for Measurements within A/R CDM Project Activities”; (EB 58 Report, Version 02.1.0, Annex 19); UNFCCC: Bonn, Germany, 2010. [Google Scholar]
- Paul, K.I.; Roxburgh, S.H.; England, J.R.; Ritson, P.; Hobbs, T.; Brooksbank, K.; Raison, R.J.; Larmour, J.S.; Murphy, S.; Norris, J. Development and Testing of Allometric Equations for Estimating Above-Ground Biomass of Mixed-Species Environmental Plantings. For. Ecol. Manag. 2013, 310, 483–494. [Google Scholar] [CrossRef]
- Paul, K.I.; Roxburgh, S.H.; Chave, J.; England, J.R.; Zerihun, A.; Specht, A.; Lewis, T.; Bennett, L.T.; Baker, T.G.; Adams, M.A.; et al. Testing the Generality of Above-Ground Biomass Allometry across Plant Functional Types at the Continent Scale. Glob. Chang. Biol. 2016, 22, 2106–2124. [Google Scholar] [CrossRef]
- Paul, K.I.; Roxburgh, S.H. A National Accounting Framework for Fire and Carbon Dynamics in Australian Savannas. Int. J. Wildland Fire 2024, 33, WF23104. [Google Scholar] [CrossRef]
- Gifford, R.M. Carbon Contents of Above-Ground Tissues of Forest and Woodland Trees; Australian Greenhouse Office: Canberra, ACT, Australia, 2000. [Google Scholar]
- Paul, K.I.; Larmour, J.; Specht, A.; Zerihun, A.; Ritson, P.; Roxburgh, S.H.; Sochacki, S.; Lewis, T.; Barton, C.V.M.; England, J.R. Testing the Generality of Below-Ground Biomass Allometry across Plant Functional Types. For. Ecol. Manag. 2019, 432, 102–114. [Google Scholar] [CrossRef]
- Falster, D.S.; Duursma, R.A.; Ishihara, M.I.; Barneche, D.R.; FitzJohn, R.G.; Vårhammar, A.; Aiba, M.; Ando, M.; Anten, N.; Aspinwall, M.J.; et al. BAAD: A Biomass and Allometry Database for Woody Plants. Ecology 2015, 96, 1445. [Google Scholar] [CrossRef]
- Martin, A.R.; Doraisami, M.; Thomas, S.C. Global Patterns in Wood Carbon Concentration across the World’s Trees and Forests. Nat. Geosci. 2018, 11, 915–920. [Google Scholar] [CrossRef]
- Zanne, A.E. Global Wood Density Database. Dryad 2009. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1998611 (accessed on 1 February 2024).
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M. TRY Plant Trait Database–Enhanced Coverage and Open Access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Gauch, H.G.; Hwang, J.T.G.; Fick, G.W. Model Evaluation by Comparison of Model-based Predictions and Measured Values. Agron. J. 2003, 95, 1442–1446. [Google Scholar] [CrossRef]
- Willmott, C.J. On the Validation of Models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Willmott, C.J. Some Comments on the Evaluation of Model Performance. Bull. Am. Meteorol. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Robeson, S.M.; Willmott, C.J. Decomposition of the Mean Absolute Error (MAE) into Systematic and Unsystematic Components. PLoS ONE 2023, 18, e0279774. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Powell, M.J.D. An Efficient Method for Finding the Minimum of a Function of Several Variables without Calculating Derivatives. Comput. J. 1964, 7, 155–162. [Google Scholar] [CrossRef]
- Pfeiffer, K.; Kheddar, A. Sequential Hierarchical Least-Squares Programming for Prioritized Non-Linear Optimal Control. Optim. Methods Softw. 2024, 1–39. [Google Scholar] [CrossRef]
- Waterworth, R.M.; Richards, G.P.; Brack, C.L.; Evans, D.M.W. A Generalised Hybrid Process-Empirical Model for Predicting Plantation Forest Growth. For. Ecol. Manag. 2007, 238, 231–243. [Google Scholar] [CrossRef]
- Kesteven, J.L.; Landsberg, J.J. Developing a National Forest Productivity Model; Australian Greenhouse Office: Canberra, ACT, Australia, 2004. [Google Scholar]
- Paul, K.I.; Roxburgh, S.H.; Larmour, J.; Davies, M.; England, J.; Peck, A.; Hagger, V.; Perring, M.; Lowson, C.; Read, Z.; et al. Secondment with DoEE NISIR. Available online: https://data.csiro.au/categories/pt/Secondment%20with%20DoEE%20NISIR (accessed on 1 February 2024).
- DoEE. National Inventory Report 2017 Volume 2. 2017. Available online: https://www.dcceew.gov.au/sites/default/files/documents/national-inventory-report-2017-volume-2.pdf (accessed on 1 February 2024).
- Roxburgh, S.; England, J.R.; Paul, K.I. Recalibration of the Tree Yield Formula in FullCAM for Plantations. 2020. Available online: https://publications.csiro.au/publications/publication/PIcsiro:EP197723 (accessed on 1 February 2024).
- Roxburgh, S.H.; Karunaratne, S.B.; Paul, K.I.; Lucas, R.M.; Armston, J.D.; Sun, J. A Revised Above-Ground Maximum Biomass Layer for the Australian Continent. For. Ecol. Manag. 2019, 432, 264–275. [Google Scholar] [CrossRef]
- Prober, S.M.; Gosper, C.R.; Gilfedder, L.; Harwood, T.D.; Thiele, K.R.; Williams, K.J.; Yates, C.J.; Keith, D.A. Temperate Eucalypt Woodlands. In Australian Vegetation; Cambridge University Press: Cambridge, UK, 2017; pp. 410–437. [Google Scholar]
- Yates, C.J.; Gosper, C.R.; Hopper, S.D.; Keith, D.A.; Prober, S.M.; Tozer, M.G. Mallee Woodlands and Shrublands: The Mallee, Muruk/Muert and Maalok Vegetation of Southern Australia. In Australian Vegetation; Cambridge University Press: Cambridge, UK, 2017; pp. 570–598. [Google Scholar]
- Giere, R.N. How Models Are Used to Represent Reality. Philos. Sci. 2004, 71, 742–752. [Google Scholar] [CrossRef]
- Wartofsky, M.W. Models: Representation and the Scientific Understanding; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 48, ISBN 9400993579. [Google Scholar]
- Matthewson, J. Trade-Offs in Model-Building: A More Target-Oriented Approach. Stud. Hist. Philos. Sci. Part A 2011, 42, 324–333. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm Wood Structure: Global Patterns in Vessel Anatomy and Their Relation to Wood Density and Potential Conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Conti, G.; Gorne, L.D.; Zeballos, S.R.; Lipoma, M.L.; Gatica, G.; Kowaljow, E.; Whitworth-Hulse, J.I.; Cuchietti, A.; Poca, M.; Pestoni, S. Developing Allometric Models to Predict the Individual Aboveground Biomass of Shrubs Worldwide. Glob. Ecol. Biogeogr. 2019, 28, 961–975. [Google Scholar] [CrossRef]
- Ma, H.; Mo, L.; Crowther, T.W.; Maynard, D.S.; van den Hoogen, J.; Stocker, B.D.; Terrer, C.; Zohner, C.M. The Global Distribution and Environmental Drivers of Aboveground versus Belowground Plant Biomass. Nat. Ecol. Evol. 2021, 5, 1110–1122. [Google Scholar] [CrossRef]
- Hartig, F.; Dyke, J.; Hickler, T.; Higgins, S.I.; O’Hara, R.B.; Scheiter, S.; Huth, A. Connecting Dynamic Vegetation Models to Data–an Inverse Perspective. J. Biogeogr. 2012, 39, 2240–2252. [Google Scholar] [CrossRef]
- Hartig, F.; Calabrese, J.M.; Reineking, B.; Wiegand, T.; Huth, A. Statistical Inference for Stochastic Simulation Models–Theory and Application. Ecol. Lett. 2011, 14, 816–827. [Google Scholar] [CrossRef]
- Berzaghi, F.; Wright, I.J.; Kramer, K.; Oddou-Muratorio, S.; Bohn, F.J.; Reyer, C.P.O.; Sabaté, S.; Sanders, T.G.M.; Hartig, F. Towards a New Generation of Trait-Flexible Vegetation Models. Trends Ecol. Evol. 2020, 35, 191–205. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Rubin, D.B. Bayesian Data Analysis, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2003; ISBN 0429258410. [Google Scholar]
- Kalton, G.; Flores-Cervantes, I. Weighting Methods. J. Off. Stat. 2003, 19, 81. [Google Scholar]
- Vehtari, A.; Gelman, A.; Gabry, J. Practical Bayesian Model Evaluation Using Leave-One-out Cross-Validation and WAIC. Stat. Comput. 2017, 27, 1413–1432. [Google Scholar] [CrossRef]

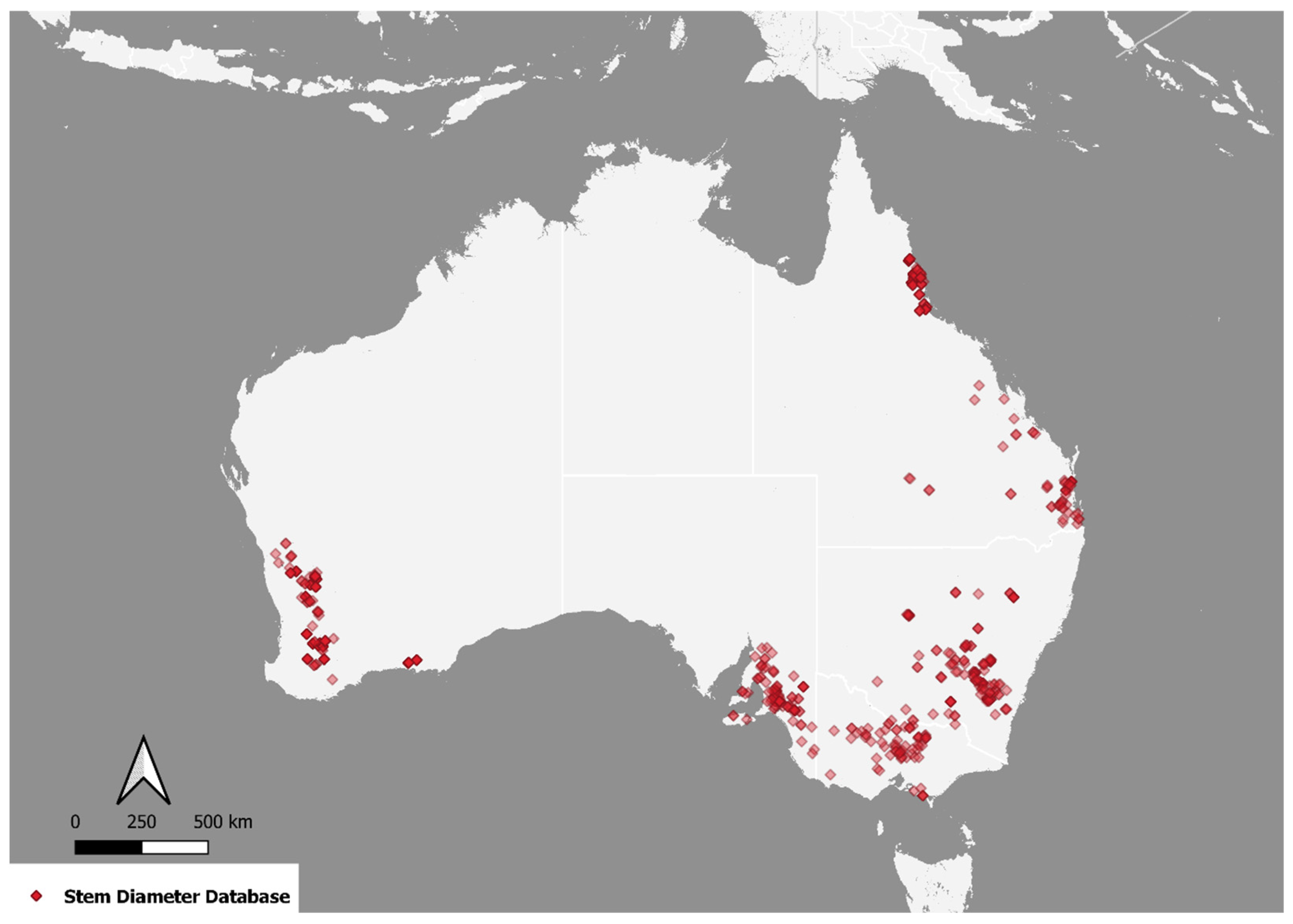


| Site ID | Age | n Trees | Planting Method | Planting Config. | Validation Type(s) | lat | lon | MAT | MAP | ASL | n Sites | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S01_DS_4 | 4 | 210 | block | DS | CR | −36.29 | 143.65 | 15.8 | 454 | 181 | ||
| S02_DS_8 | 8 | 245 | block | DS | CR | −36.77 | 143.20 | 14.5 | 505 | 263 | ||
| S03_DS-TS_9 | 9 | 184 | block | DS + TS | CR + CC | −35.92 | 141.86 | 16.3 | 349 | 81 | ||
| S04_DS_12 | 12 | 250 | block | DS | CR | −36.42 | 143.77 | 15.7 | 484 | 171 | ||
| S05_DS-TS_17 | 17 | 203 | block | DS | CR + CC | −36.15 | 141.68 | 15.8 | 373 | 131 | ||
| S06_DS-TS_21 | 21 | 702 | block | DS + TS | AGB + CR | −36.25 | 141.81 | 15.6 | 370 | 116 | 20 | 23 |
| S07_TS_23 | 23 | 425 | belt | TS | AGB + CR | −36.56 | 146.09 | 14.6 | 726 | 210 | 22 | 81 |
| S08_DS-TS_23 | 23 | 249 | block | DS + TS | CR + CC | −36.25 | 141.81 | 15.6 | 378 | 119 | ||
| S09_DS-TS_23 | 23 | 336 | block | DS + TS | AGB + CR | −36.54 | 142.61 | 15.4 | 427 | 136 | 32 | 29 |
| S10_DS_24 | 24 | 602 | belt | DS | AGB + CR | −36.82 | 145.17 | 14.8 | 583 | 132 | 25 | 68 |
| S11_DS_25 | 25 | 189 | belt | DS | AGB + CR | −36.42 | 146.09 | 14.8 | 688 | 170 | 25 | 65 |
| S12_TS_25 | 25 | 573 | belt | TS | AGB + CR | −36.53 | 146.12 | 14.3 | 759 | 218 | 37 | 215 |
| S13_TS_25 | 25 | 272 | block | TS | AGB + CR + CC | −37.25 | 144.99 | 13.0 | 605 | 277 | 50 | 94 |
| S14_TS_25 | 25 | 305 | block | TS | AGB + CR | −36.50 | 146.14 | 14.5 | 726 | 194 | 39 | 115 |
| S15_TS_26 | 26 | 165 | belt | TS | AGB + CR | −36.49 | 145.81 | 15.1 | 649 | 166 | 21 | 80 |
| S16_DS_27 | 27 | 272 | belt | DS | AGB + CR | −36.42 | 146.13 | 14.6 | 698 | 170 | 25 | 48 |
| S17_DS_27 | 27 | 515 | block | DS | AGB + CR + CC | −37.07 | 144.91 | 13.7 | 569 | 326 | 82 | 103 |
| S18_DS_31 | 31 | 160 | belt | DS | AGB + CR | −37.02 | 144.94 | 13.9 | 587 | 242 | 44 | 89 |
| S19_TS_31 | 31 | 134 | block | TS | AGB + CR + CC | −37.05 | 144.93 | 13.7 | 589 | 278 | 39 | 94 |
| S20_TS_35 | 35 | 53 | block | TS | AGB + CR + CC | −37.24 | 145.00 | 13.0 | 617 | 295 | 32 | 95 |
| Variable | Unit | Variable Description |
|---|---|---|
| CvrCn | f/ha | Crown cover |
| CO2 | kgCO2/tree | Carbon stock |
| DnsTr | #/ha | Tree density per hectares |
| LngHdg | m | Length of the hedge in case of direct seeding |
| RdsCn | m | Radius of the crown perpendicular to the hedge in case of direct seeding |
| RdsCn2 | m | Radius of the crown in the direction of the hedge in case of Direct seeding |
| RdsSt | cm | Radius of the stem at breast height |
| SrfCn | m2 | Crown surface area |
| VlmSt | m3/ha | Volume of the stem |
| WghRt | kg/ha | Root weight |
| WghSh | kg/ha | Shoot weight |
| WghSt | kg/ha | Stem weight |
| WghTr | kg/ha | Tree weight |
| Parameter | Unit | Parameter Description | Source |
|---|---|---|---|
| BEFD | kg/kg | Biomass expansion factor on a dry weight base, i.e., shoot-to-stem ratio. | Biomass And Allometry Database (BAAD) [50] or calibrated on AGB |
| CF | kgC/kgDM | Wood carbon concentration | [51] |
| fMrt | yr-1 | Fraction of mortality | Default mortality rates: first 3 years since planting: 5% Mortality, year 4–40: 2% mortality |
| max_dVlmSt_dt | m3/ha/yr | Maximum current annual stem volume increment | Calibrated |
| max_RdsCn | m | Maximum crown radius | Calibrated |
| max_dRdsCn_dt | yr-1 | Maximal relative increase in crown radius | Calibrated |
| R | kg/kg | Shoot-to-root ratio | Biomass And Allometry Database [50], or calibrated on BGB |
| WD | kg/m3 | Wood density | Global Wood Density Database [52], TRY database [53] |
| Above-Ground | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAca | FEuc | Fother | FShrub | ||||||||||||
| Site Id | Age | Biomass | std | n | Biomass | std | n | Biomass | std | n | Biomass | std | n | Biomass | MAI |
| S06_DS-TS_21 | 21 | 2.55 | 1.32 | 4 | 12.47 | 3.50 | 4 | 0.20 | 0.36 | 4 | 1.62 | 0.73 | 4 | 22.33 | 1.06 |
| S07_TS_23 | 23 | 0.11 | 0.09 | 3 | 63.61 | 11.84 | 3 | 2.05 | 1.61 | 3 | 0.15 | 0.07 | 3 | 86.09 | 3.74 |
| S09_DS-TS_23 | 23 | 0.02 | 0.03 | 3 | 18.07 | 5.80 | 3 | 0.86 | 0.55 | 3 | 0.37 | 0.17 | 3 | 25.71 | 1.12 |
| S10_DS_24 | 24 | 1.45 | 1.40 | 3 | 88.33 | 79.98 | 4 | 0 | 0.54 | 0.69 | 4 | 117.78 | 4.91 | ||
| S11_DS_25 | 25 | 0.41 | 0.52 | 2 | 88.81 | 23.13 | 2 | 0.05 | 1 | 0.25 | 0.15 | 2 | 115.79 | 4.63 | |
| S12_TS_25 | 25 | 0.02 | 0.02 | 4 | 49.53 | 14.10 | 4 | 1.31 | 0.42 | 3 | 0.02 | 0.03 | 3 | 66.03 | 2.64 |
| S13_TS_25 | 25 | 4.88 | 3.55 | 4 | 49.92 | 18.63 | 4 | 0.08 | 0.09 | 2 | 0.09 | 0.13 | 2 | 70.55 | 2.82 |
| S14_TS_25 | 25 | 0.10 | 0.08 | 4 | 52.10 | 0.98 | 4 | 0.00 | 0.00 | 3 | 67.87 | 2.71 | |||
| S15_TS_26 | 26 | 0.04 | 0.02 | 4 | 58.42 | 10.06 | 4 | 0.03 | 0.05 | 4 | 76.46 | 2.94 | |||
| S16_DS_27 | 27 | 0.52 | 0.12 | 2 | 73.43 | 14.35 | 3 | 0.21 | 1 | 0.20 | 0.28 | 2 | 97.02 | 3.59 | |
| S17_DS_27 | 27 | 0.07 | 0.06 | 2 | 42.82 | 9.65 | 2 | 0.23 | 0.22 | 2 | 57.73 | 2.14 | |||
| S18_DS_31 | 31 | 0.13 | 1 | 53.89 | 4.59 | 2 | 0.07 | 0.10 | 2 | 71.13 | 2.29 | ||||
| S19_TS_31 | 31 | 0.01 | 1 | 59.03 | 15.47 | 5 | 1.35 | 2.00 | 3 | 0.00 | 1 | 78.04 | 2.52 | ||
| S20_TS_35 | 35 | 73.61 | 19.26 | 4 | 94.99 | 2.71 | |||||||||
| Model GoF | Configuration | Avg. obs | Avg. pred | RMSE | MSEu/MSE | MSEs/MSE | MAE | MAEu/MAE | MAEb/MAE | MAEp/MAE |
|---|---|---|---|---|---|---|---|---|---|---|
| tC/ha | tC/ha | tC/ha | % | % | tC/ha | % | % | % | ||
| FastTrack MAE | ||||||||||
| DS | 70.1 | 54.1 | 30.3 | 7 | 93 | 26.7 | 9 | 41 | 51 | |
| DS and TS | 18.1 | 25.6 | 11.6 | 0 | 100 | 8.8 | 0 | 46 | 54 | |
| TS | 59.4 | 73.4 | 22.6 | 57 | 43 | 16.7 | 42 | 45 | 13 | |
| total | 57.3 | 59.7 | 24.5 | 76 | 24 | 19.2 | 64 | 8 | 28 | |
| FastTrack MSE | ||||||||||
| DS | 70.1 | 68.6 | 22.4 | 27 | 73 | 19.2 | 30 | 5 | 65 | |
| DS and TS | 18.1 | 48.7 | 30.7 | 0 | 100 | 30.7 | 0 | 98 | 2 | |
| TS | 59.4 | 90.2 | 37.0 | 15 | 85 | 30.8 | 23 | 57 | 21 | |
| total | 57.3 | 76.6 | 31.6 | 52 | 48 | 26.6 | 42 | 43 | 16 | |
| FullCAM | ||||||||||
| DS | 70.1 | 31.3 | 45.4 | 2 | 98 | 38.8 | 7 | 64 | 29 | |
| DS and TS | 18.1 | 7.2 | 10.9 | 0 | 100 | 10.9 | 0 | 97 | 3 | |
| TS | 59.4 | 41 | 31.2 | 35 | 65 | 30.2 | 28 | 43 | 29 | |
| Total | 57.3 | 32.7 | 35.2 | 28 | 72 | 30.5 | 19 | 55 | 25 |
| Site Id | Age | Observed Canopy Cover (%) | Predicted Canopy Cover (%) | Year |
|---|---|---|---|---|
| S03_DS-TS_9 | 9 | 25.2 | 2021 | |
| S05_DS-TS_17 | 10 | 28.9 | 2016 | |
| S08_DS-TS_23 | 16 | 43.1 | 2016 | |
| S05_DS-TS_17 | 17 | 71.3 | 2023 | |
| S08_DS-TS_23 | 21 | 72.8 | 2021 | |
| S08_DS-TS_23 | 23 | 83.4 | 2023 | |
| S13_TS_25 | 25 | 71.1 | 78.6 ± 0.2 | 2022 |
| S17_DS_27 | 27 | 87.2 | 80.4 ± 0.7 | 2022 |
| S17_DS_27 | 29 | 84.2 | 80.5 ± 0.8 | 2024 |
| S19_TS_31 | 31 | 80.1 | 78.4 ± 0.3 | 2022 |
| S20_TS_35 | 35 | 72.5 | 78.1 ± 0.4 | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, K.; Bennett, L.T.; Borelle, R.; Byrne, P.; Dettman, P.; England, J.R.; Heida, H.; Galama, Y.; Haas, J.; van der Heijden, M.; et al. Site-Level Modelling Comparison of Carbon Capture by Mixed-Species Forest and Woodland Reforestation in Australia. Forests 2024, 15, 990. https://doi.org/10.3390/f15060990
Kramer K, Bennett LT, Borelle R, Byrne P, Dettman P, England JR, Heida H, Galama Y, Haas J, van der Heijden M, et al. Site-Level Modelling Comparison of Carbon Capture by Mixed-Species Forest and Woodland Reforestation in Australia. Forests. 2024; 15(6):990. https://doi.org/10.3390/f15060990
Chicago/Turabian StyleKramer, Koen, Lauren T. Bennett, Remi Borelle, Patrick Byrne, Paul Dettman, Jacqueline R. England, Hielke Heida, Ysbrand Galama, Josephine Haas, Marco van der Heijden, and et al. 2024. "Site-Level Modelling Comparison of Carbon Capture by Mixed-Species Forest and Woodland Reforestation in Australia" Forests 15, no. 6: 990. https://doi.org/10.3390/f15060990






