Complete Chloroplast Genome Sequences of Endangered Tropical Fosbergia Species (Family: Rubiaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Sampling and DNA Sequencing
2.2. Chloroplast Genome Assembly and Annotation
2.3. SSRs and Identification of Repeats
2.4. Comparative Chloroplast Genome and Codon Preference Analysis
2.5. Phylogenetic Analysis
3. Results
3.1. Characteristics of the Chloroplast Genomes of Fosbergia
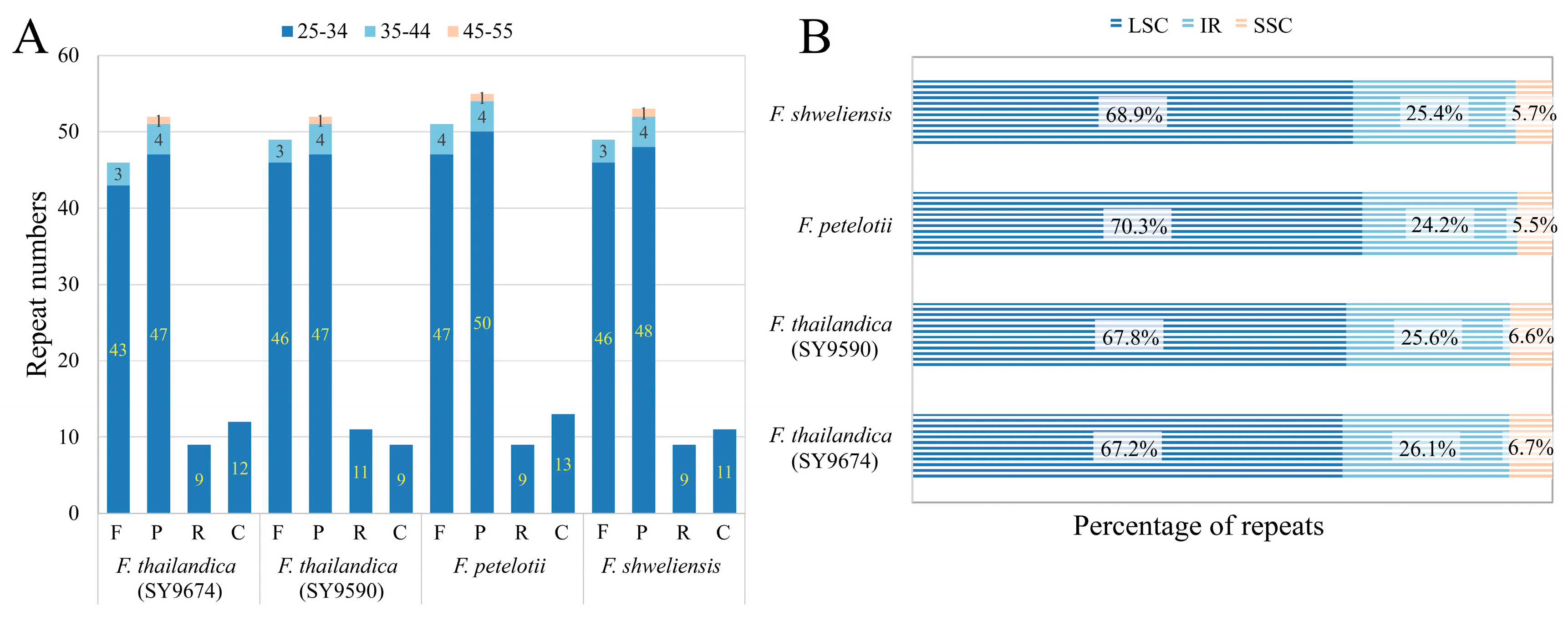
3.2. Repeat Sequence Analysis
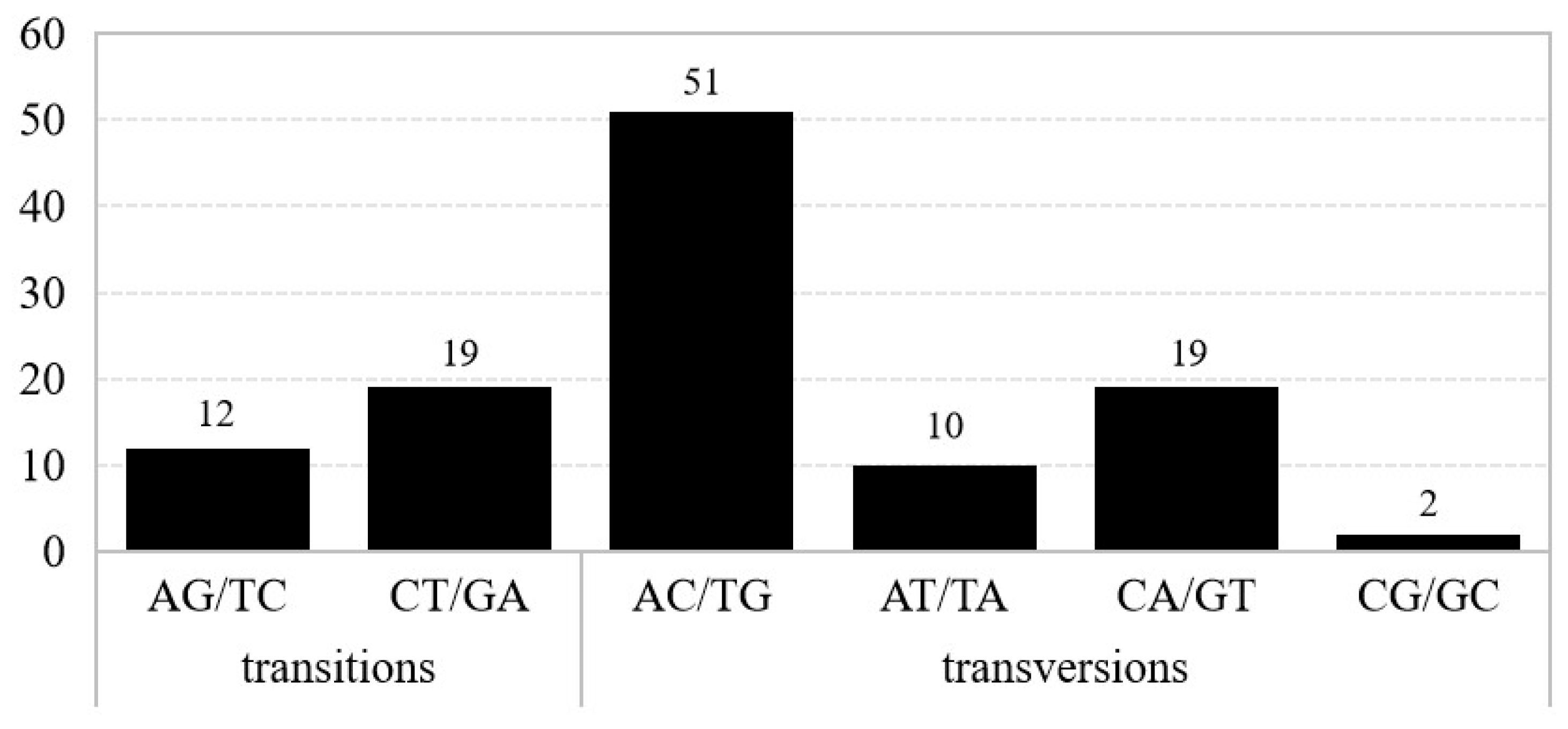
3.3. Number and Forms of Microstructural Mutations
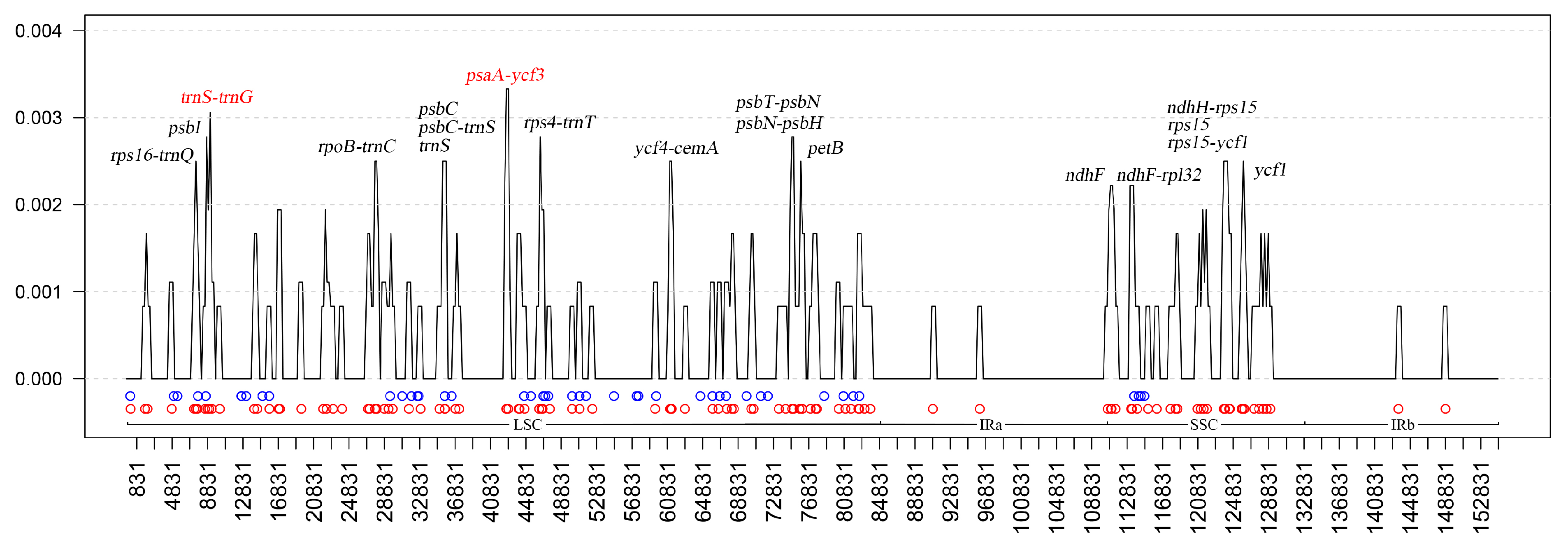
3.4. Comparative Analysis of the Four Fosbergia Chloroplast Sequences
3.5. Codon Bias Analysis
3.6. Phylogenetic Analysis
4. Discussion
4.1. Characteristics of the Chloroplast Genomes of Fosbergia
4.2. Repeats and SSRs
4.3. Indels and SNPs
4.4. Comparative Analysis
4.5. Codon Usage Bias
4.6. Phylogenetic Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.D.; Chen, W.H.; Shui, Y.M. Miscellaneous notes on the tribe Gardenieae (Rubiaceae) from China and Vietnam. Acta Phytotaxon. Sin. 2007, 45, 90–93. [Google Scholar]
- Li, H.; Dao, Z.L.; Li, R. Reappraisal of Fosbergia shweliensis (Rubiaceae), a species endemic to the Gaoligong Mountains, Western Yunnan, China. Acta Phytotaxon. Sin. 2006, 44, 707–711. [Google Scholar] [CrossRef]
- Dao, Z.L.; Ji, Y.H.; Liu, T.; Liu, K.M.; Li, H. Development of ten polymorphic microsatellite loci for Fosbergia shweliensis (Rubiaceae), a potentially crisis endangered tree. Am. J. Bot. 2011, 98, e161–e163. [Google Scholar] [CrossRef] [PubMed]
- Li, A.H.; Yang, J.; He, H.J.; Yang, X.Y. Seed desiccation tolerance and germination of a potentially threatened Chinese species, Fosbergia shweliensis. Seed Sci. Technol. 2013, 41, 479–482. [Google Scholar] [CrossRef][Green Version]
- Ly, S.N.; Garavito, A.; De Block, P.; Asselman, P.; Guyeux, C.; Charr, J.-C.; Janssens, S.; Mouly, A.; Hamon, P.; Guyot, R. Chloroplast genomes of Rubiaceae: Comparative genomics and molecular phylogeny in subfamily Ixoroideae. PLoS ONE 2020, 15, e0232295. [Google Scholar] [CrossRef]
- Wang, W.C.; Shao, F.Q.; Deng, X.; Liu, Y.W.; Chen, S.Y.; Li, Y.Q.; Guo, W.; Jiang, Q.B.; Liang, H.; Zhang, X.Z. Genome surveying reveals the complete chloroplast genome and nuclear genomic features of the crocin-producing plant Gardenia jasminoides Ellis. Genet. Resour. Crop Evol. 2020, 68, 1165–1180. [Google Scholar] [CrossRef]
- Saldaña, C.L.; Rodriguez-Grados, P.; Chávez-Galarza, J.C.; Feijoo, S.; Guerrero-Abad, J.C.; Vásquez, H.V.; Maicelo, J.L.; Jhoncon, J.H.; Arbizu, C.I. Unlocking the complete chloroplast genome of a native tree species from the amazon basin, capirona (calycophyllum spruceanum, rubiaceae), and its comparative analysis with other Ixoroideae species. Genes 2022, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.F.; Li, Y.Q.; Yuan, X.L.; Luo, T.; Wang, Y. The complete chloroplast genome sequence of Fosbergia shweliensis, an endemic species to Yunnan of China. Mitochondrial DNA Part B 2020, 5, 1796–1797. [Google Scholar] [CrossRef]
- Gong, S.F.; Miao, B.L.; Dong, X.X. Comparative analysis of complete chloroplast genomes of Gardenia jasminoides and contribution to the phylogeny and adaptive evolution. J. Am. Soc. Hortic. Sci. 2022, 147, 260–269. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; de Pamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, L.; Zhu, Y.; Chen, H.; Zhang, J.; Lin, X.; Guan, X. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genom. 2012, 13, 715. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuclc Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.R.; Li, W.W. The codon Adaptation Index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef]
- Li, H.; Guo, Q.Q.; Xu, L.; Gao, H.D.; Liu, L.; Zhou, X.Y. CPJSdraw: Analysis and visualization of junction sites of chloroplast genomes. PeerJ 2023, 11, e15326. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; de Pamphilis, C.W.; Müller, K.F.; Quandt, D.J.P.M.B. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Qian, J.; Song, J.; Gao, H.; Zhu, Y.; Xu, J.; Pang, X.; Yao, H.; Sun, C.; Li, X.E.; Li, C.; et al. The complete chloroplast genome sequence of the medicinal plant Salvia miltiorrhiza. PLoS ONE 2013, 8, e57607. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yang, L.; Xin, Y.; Xu, W.; Li, Q.; Zhang, H.; Tu, Y.; Song, Y.; Xin, P. Comparative and phylogenetic analysis of complete chloroplast genomes from seven Neocinnamomum taxa (Lauraceae). Front. Plant Sci. 2023, 14, 1205051. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. Structure, function, and inheritance of plastid genomes. In Cell and Molecular Biology of Plastids; Bock, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 29–63. [Google Scholar]
- Cavalier-smith, T. Chloroplast evolution: Secondary symbiogenesis and multiple losses. Curr. Biol. 2002, 12, R62–R64. [Google Scholar] [CrossRef]
- Nie, X.; Lv, S.; Zhang, Y.; Du, X.; Wang, L.; Biradar, S.S.; Tan, X.; Wan, F.; Weining, S. Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora). PLoS ONE 2012, 7, e36869. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, Y. The chloroplast genome of Gardenia jasminoides and related phylogenetic analysis (Rubiaceae). Mitochondrial DNA Part B. 2020, 5, 1743–1745. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Cai, J.; Tian, X.; Han, L.; Song, Y. Characteristics of the complete plastid genome sequences of the monotypic genus dodecadenia (family: Lauraceae) and its phylogenomic implications. Forests 2022, 13, 1240. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, R.; Shi, M.; Song, Y.; Xin, Y.; Li, F.; Ma, J.; Xin, P. The complete plastid genome of the endangered shrub Brassaiopsis angustifolia (Araliaceae): Comparative genetic and phylogenetic analysis. PLoS ONE 2022, 17, e0269819. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, M.; Vekemans, X.; Godé, C.; Frérot, H.; Castric, V.; Saumitou-Laprade, P. Nuclear and chloroplast DNA phylogeography reveals vicariance among European populations of the model species for the study of metal tolerance, Arabidopsis halleri (Brassicaceae). New Phytol. 2012, 193, 916–928. [Google Scholar] [CrossRef]
- Biju, V.C.; Shidhi, P.R.; Vijayan, S.; Rajan, V.S.; Sasi, A.; Janardhanan, A.; Nair, A.S. The complete chloroplast genome of Trichopus zeylanicus, and phylogenetic analysis with dioscoreales. Plant Genome 2019, 12, 190032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.-W.; Yang, Y.; Li, X.-N. Structural and comparative analysis of the complete chloroplast genome of a mangrove plant: Scyphiphora hydrophyllacea Gaertn. f. and related Rubiaceae species. Forests 2019, 10, 1000. [Google Scholar] [CrossRef]
- Fu, P.-C.; Zhang, Y.-Z.; Geng, H.-M.; Chen, S.-L. The complete chloroplast genome sequence of Gentiana lawrencei var. farreri (Gentianaceae) and comparative analysis with its congeneric species. PeerJ 2016, 4, e2540. [Google Scholar] [CrossRef] [PubMed]
- Levinson, G.; Gutman, G.A. Slipped-strand mispairing: A major mechanism for DNA sequence evolution. Mol. Biol. Evol. 1987, 4, 203–221. [Google Scholar] [CrossRef]
- Shimada, H.; Sugiura, M. Pseudogenes and short repeated sequences in the rice chloroplast genome. Curr. Genet. 1989, 16, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Small, R.L. Chloroplast DNA phylogeny and phylogeography of the North American plums (Prunus subgenus Prunus section Prunocerasus, Rosaceae). Am. J. Bot. 2005, 92, 2011–2030. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, H.-L. Widespread occurrence of small inversions in the chloroplast genomes of land plants. Mol. Cells 2005, 19, 104–113. [Google Scholar] [CrossRef]
- Pombert, J.F.; Lemieux, C.; Turmel, M. The complete chloroplast DNA sequence of the green alga Oltmannsiellopsis viridis reveals a distinctive quadripartite architecture in the chloroplast genome of early diverging ulvophytes. BMC Biol. 2006, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. Plastid chromosomes: Structure and evolution. In The Molecular Biology of Plastids: Cell Culture and Somatic Cell Genetics of Plants; Bogorad, L., Vasil, I.K., Eds.; Academic Press: San Diego, CA, USA, 1991; Volume 7A, pp. 5–53. [Google Scholar]
- Song, Y.; Yao, X.; Tan, Y.; Gan, Y.; Corlett, R.T. Complete chloroplast genome sequence of the avocado: Gene organization, comparative analysis, and phylogenetic relationships with other Lauraceae. Can. J. For. Res. 2016, 46, 1293–1301. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, L.; Yang, Y.; Wang, Y.; Yang, L.; Wei, C.; Guo, J.; Yan, K.; Chen, H.; Yang, Z.; et al. Complete chloroplast genome sequences of Phlomis fruticosa and Phlomoides strigosa and comparative analysis of the genus Phlomis sensu lato (Lamiaceae). Front. Plant Sci. 2022, 13, 1022273. [Google Scholar] [CrossRef]
- Ge, Y.; Dong, X.; Wu, B.; Wang, N.; Chen, D.; Chen, H.; Zou, M.; Xu, Z.; Tan, L.; Zhan, R. Evolutionary analysis of six chloroplast genomes from three Persea americana ecological races: Insights into sequence divergences and phylogenetic relationships. PLoS ONE 2019, 14, e0221827. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Zhou, S. Molecular identification of species in Prunus sect. Persica (Rosaceae), with emphasis on evaluation of candidate barcodes for plants. J. Syst. Evol. 2011, 49, 138–145. [Google Scholar] [CrossRef]
- Mo, Z.; Lou, W.; Chen, Y.; Jia, X.; Zhai, M.; Guo, Z.; Xuan, J. The chloroplast genome of Carya illinoinensis: Genome structure, adaptive evolution, and phylogenetic analysis. Forests 2020, 11, 207. [Google Scholar] [CrossRef]
- Dai, J.P.; Liu, Q.Z.; Xu, X.Y.; Tan, Z.J.; Lin, Y.X.; Gao, X.X.; Zhu, S. Comparative and phylogenetic analysis of the complete chloroplast genomes of Uncaria (Rubiaceae) species. Front. Plant Sci. 2023, 14, 1271689. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, J.B.; Kudla, G. Synonymous but not the same: The causes and consequences of codon bias. Nat. Rev. Genet. 2011, 12, 32–42. [Google Scholar] [CrossRef]
- Morton, B.R. The role of context-dependent mutations in generating compositional and codon usage bias in grass chloroplast DNA. J. Mol. Evol. 2003, 56, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Park, S.; Lee, E.M.; Park, S. Evidence of mitochondrial DNA in the chloroplast genome of Convallaria keiskei and its subsequent evolution in the Asparagales. Sci. Rep. 2019, 9, 5028. [Google Scholar] [CrossRef]




| Fosbergia shweliensis | Fosbergia thailandica | Fosbergia thailandica | Fosbergia petelotii | |
|---|---|---|---|---|
| Voucher | SY6456 | SY9590 | SY9674 | SY9593 |
| Total cpDNA size (bp) | 154,717 | 154,623 | 154,628 | 154,730 |
| LSC region (bp) | 84,747 | 84,662 | 84,667 | 84,769 |
| IRs region (bp) | 25,870 | 25,870 | 25,870 | 25,870 |
| SSC region (bp) | 18,230 | 18,221 | 18,221 | 18,221 |
| Total GC content (%) | 37.6% | 37.6% | 37.6% | 37.6% |
| LSC GC content (%) | 35.5% | 35.5% | 35.5% | 35.5% |
| IR GC content (%) | 43.2% | 43.2% | 43.2% | 43.2% |
| SSC GC content (%) | 31.4% | 31.4% | 31.4% | 31.4% |
| Total number of genes | 130 | 130 | 130 | 130 |
| Total number of PCG | 85 | 85 | 85 | 85 |
| Total number of tRNA | 37 | 37 | 37 | 37 |
| Total number of rRNA | 8 | 8 | 8 | 8 |
| No. | Location | Region | Motif | No. of Repeats | |||
|---|---|---|---|---|---|---|---|
| F. shweliensis | F. petelotii | F. thailandica (SY9674) | F. thailandica (SY9590) | ||||
| 1 | trnH-psbA | Intergenic | T | 17 | 15 | 10 | 16 |
| 2 | trnH-psbA | Intergenic | A | 11 | 14 | 10 | 12 |
| 3 | trnK-rps16 | Intergenic | C | 10 | 8 | 9 | 9 |
| 4 | rps16 | Intron | T | 11 | 11 | 11 | 10 |
| 5 | psbK-psbI | Intergenic | T | 10 | 10 | 11 | 11 |
| 6 | atpF | Intron | TTAGAA | 3 | 2 | 3 | 3 |
| 7 | atpH-atpI | Intergenic | A | 10 | 11 | 10 | 10 |
| 8 | atpI-rps2 | Intergenic | A | 14 | 14 | 13 | 13 |
| 9 | petN-psbM | Intergenic | C | 5 | 3 | 3 | 3 |
| 10 | psbC-trnS | Intergenic | A | 9 | 8 | 9 | 8 |
| 11 | psbZ-trnG | Intergenic | C | 7 | 6 | 6 | 6 |
| 12 | ycf3 | Intron | A | 9 | 9 | 9 | 10 |
| 13 | trnT-trnL | Intergenic | TA | 4 | 4 | 5 | 5 |
| 14 | ndhK-ndhC | Intergenic | T | 9 | 10 | 9 | 10 |
| 15 | rbcL-accD | Intergenic | T | 14 | 15 | 15 | 14 |
| 16 | petA-psbJ | Intergenic | A | 9 | 10 | 8 | 9 |
| 17 | psbE-petL | Intergenic | A | 10 | 10 | 9 | 9 |
| 18 | petL-petG | Intergenic | T | 10 | 10 | 9 | 9 |
| 19 | rpl20-rps12 | Intergenic | A | 8 | 8 | 8 | 9 |
| 20 | clpP | Intron | T | 12 | 11 | 12 | 12 |
| 21 | petD-rpoA | Intergenic | A | 12 | 13 | 12 | 12 |
| 22 | infA-rps8 | Intergenic | T | 13 | 12 | 13 | 13 |
| 23 | ndhF-rpl32 | Intergenic | A | 11 | 10 | 10 | 10 |
| 24 | rpl32-trnL | Intergenic | T | 6 | 5 | 6 | 6 |
| 25 | rpl32-trnL | Intergenic | A | 8 | 9 | 8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhu, W.; Song, Y.; Zhou, Q.; Li, H. Complete Chloroplast Genome Sequences of Endangered Tropical Fosbergia Species (Family: Rubiaceae). Forests 2024, 15, 1150. https://doi.org/10.3390/f15071150
Chen L, Zhu W, Song Y, Zhou Q, Li H. Complete Chloroplast Genome Sequences of Endangered Tropical Fosbergia Species (Family: Rubiaceae). Forests. 2024; 15(7):1150. https://doi.org/10.3390/f15071150
Chicago/Turabian StyleChen, Lilin, Wen Zhu, Yu Song, Qihai Zhou, and Huimin Li. 2024. "Complete Chloroplast Genome Sequences of Endangered Tropical Fosbergia Species (Family: Rubiaceae)" Forests 15, no. 7: 1150. https://doi.org/10.3390/f15071150
APA StyleChen, L., Zhu, W., Song, Y., Zhou, Q., & Li, H. (2024). Complete Chloroplast Genome Sequences of Endangered Tropical Fosbergia Species (Family: Rubiaceae). Forests, 15(7), 1150. https://doi.org/10.3390/f15071150





