Study on Associations between Root and Aboveground Growth of Mixed-Planting Seedlings of Populus tomentosa and Pinus tabuliformis under Soil Nutrient Heterogeneity
Abstract
:1. Introduction
2. Materials and Methods
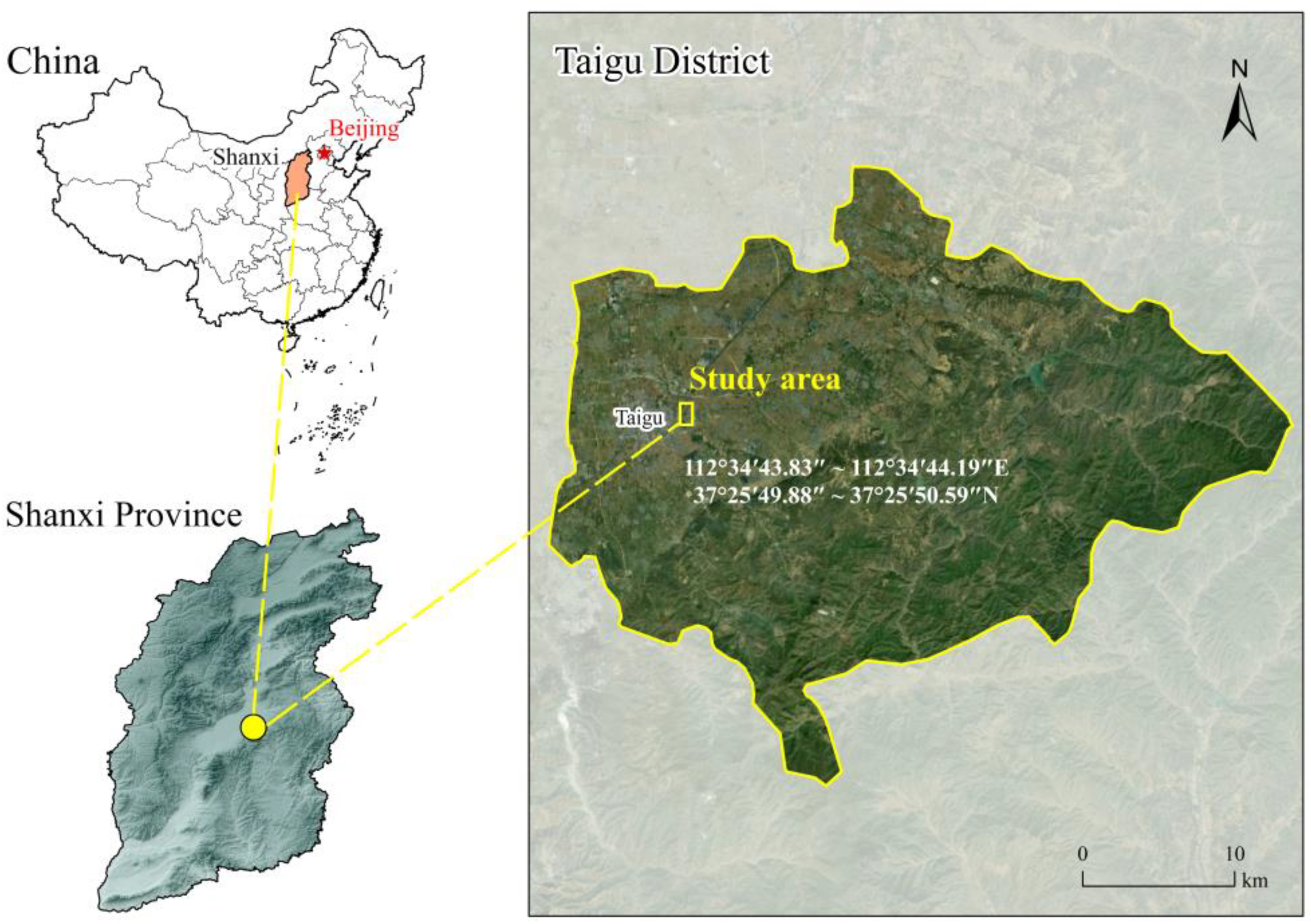
2.1. Overview of the Study Area
2.2. Research Methods
2.2.1. Test Materials
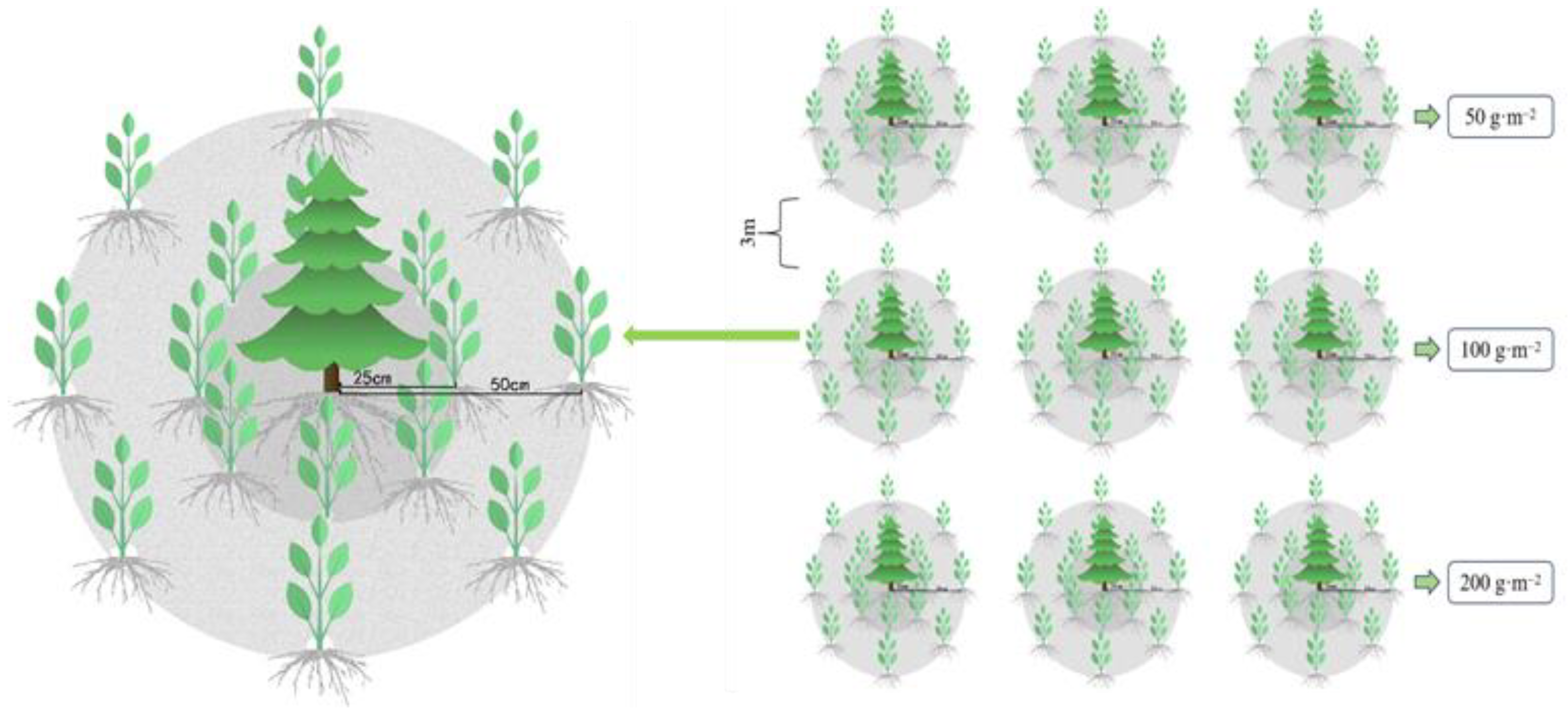
2.2.2. Experimental Design
2.2.3. Sample Collection and Processing
2.3. Data Processing
2.3.1. Vertical Center of Fine Roots
2.3.2. Allometric Growth Model
2.3.3. Correlation Analysis
2.3.4. Mixed-Effects Model
3. Result Analysis
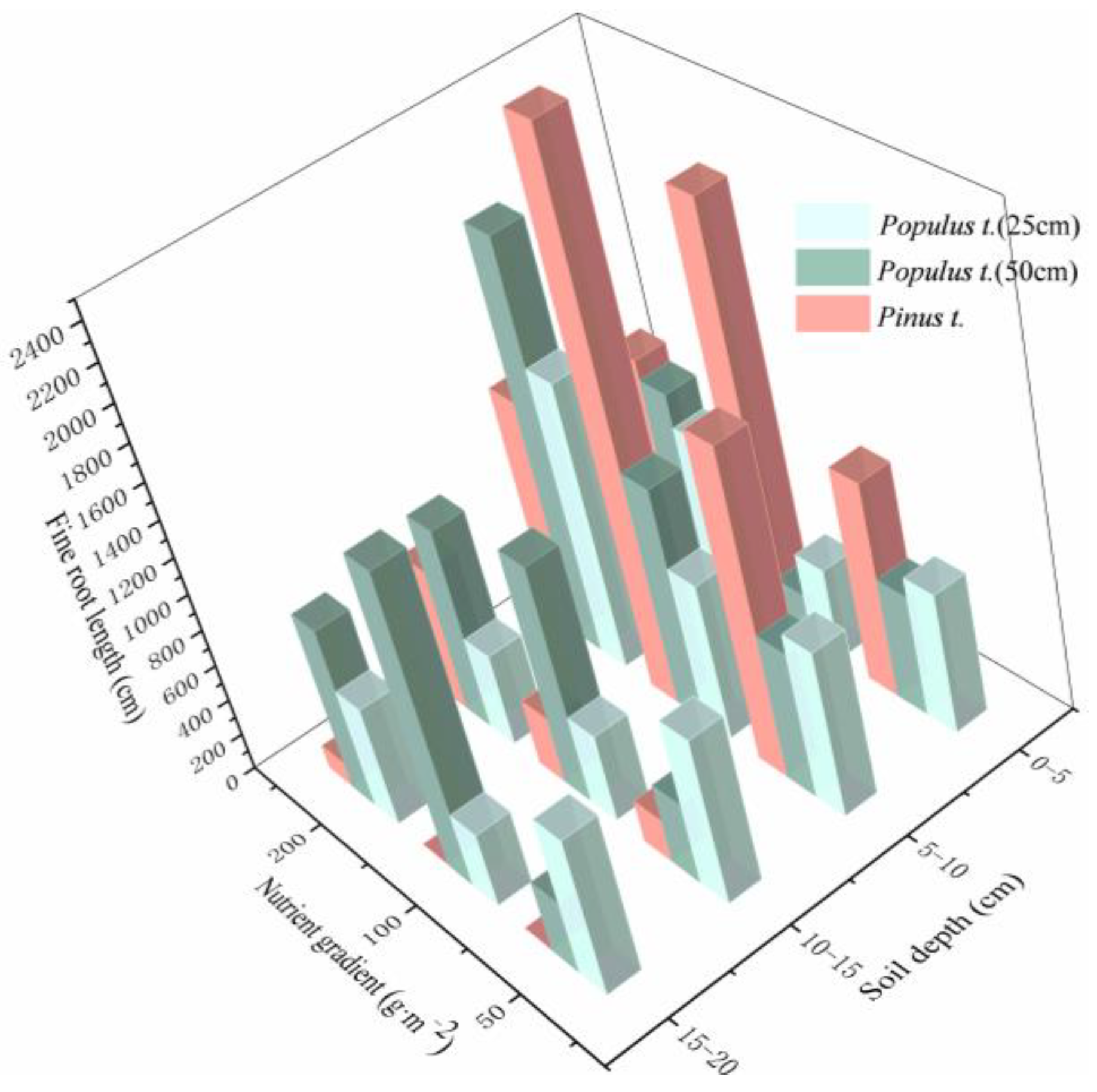
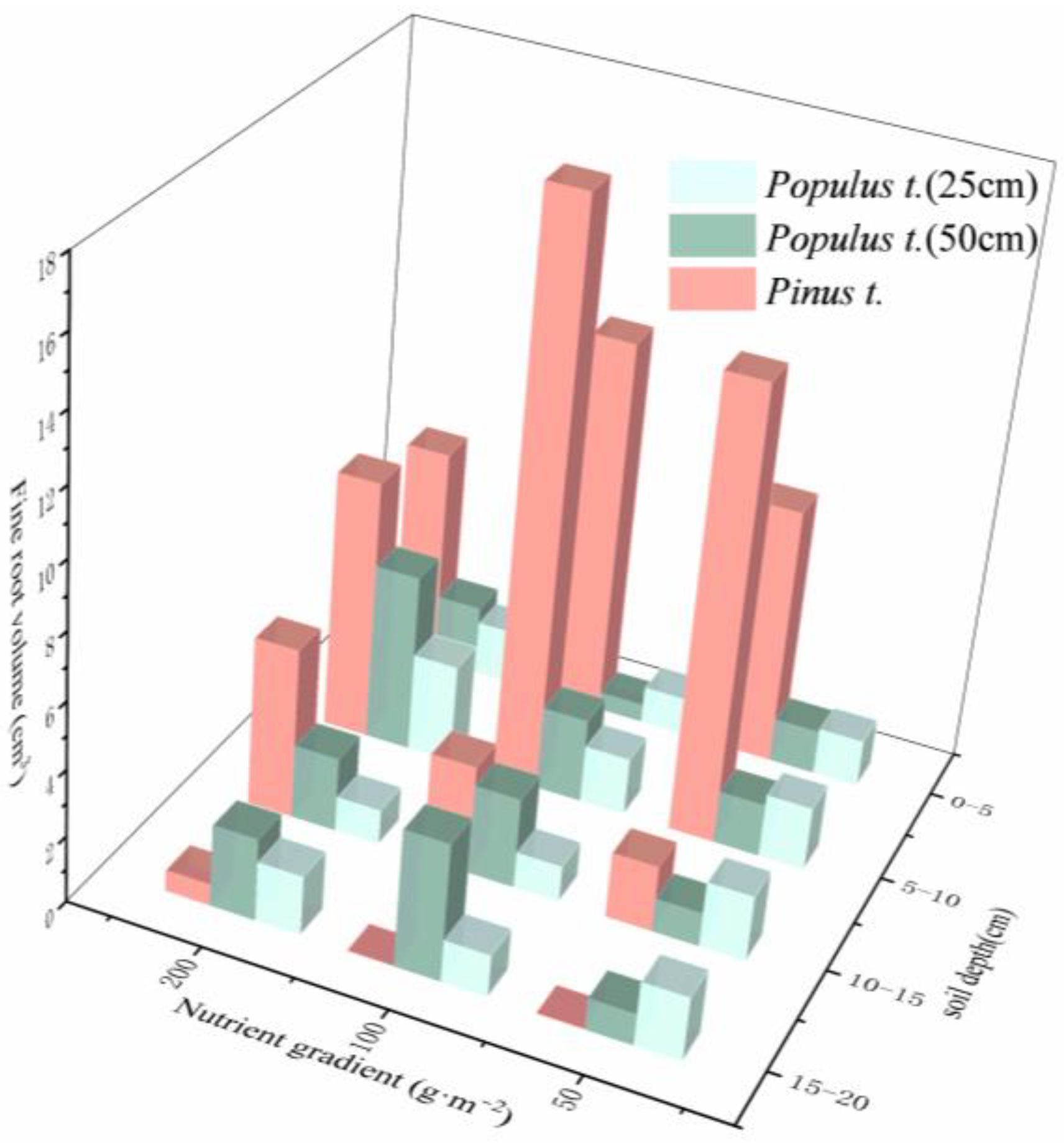
3.1. Changes in Root Traits under Heterogeneous Soil Nutrient Conditions
3.2. Biomass of Roots and Various Organs under Heterogeneous Soil Nutrient Conditions
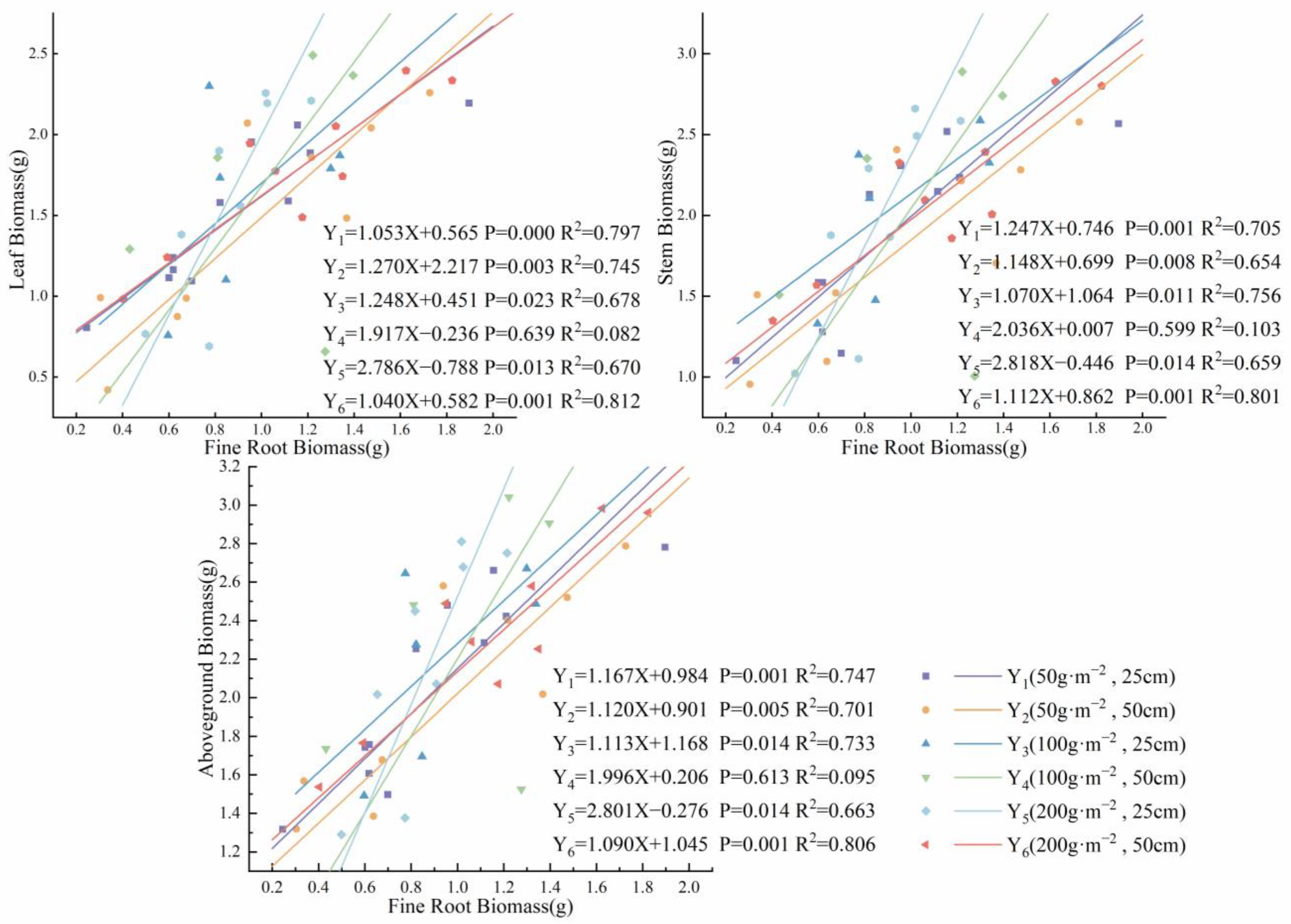
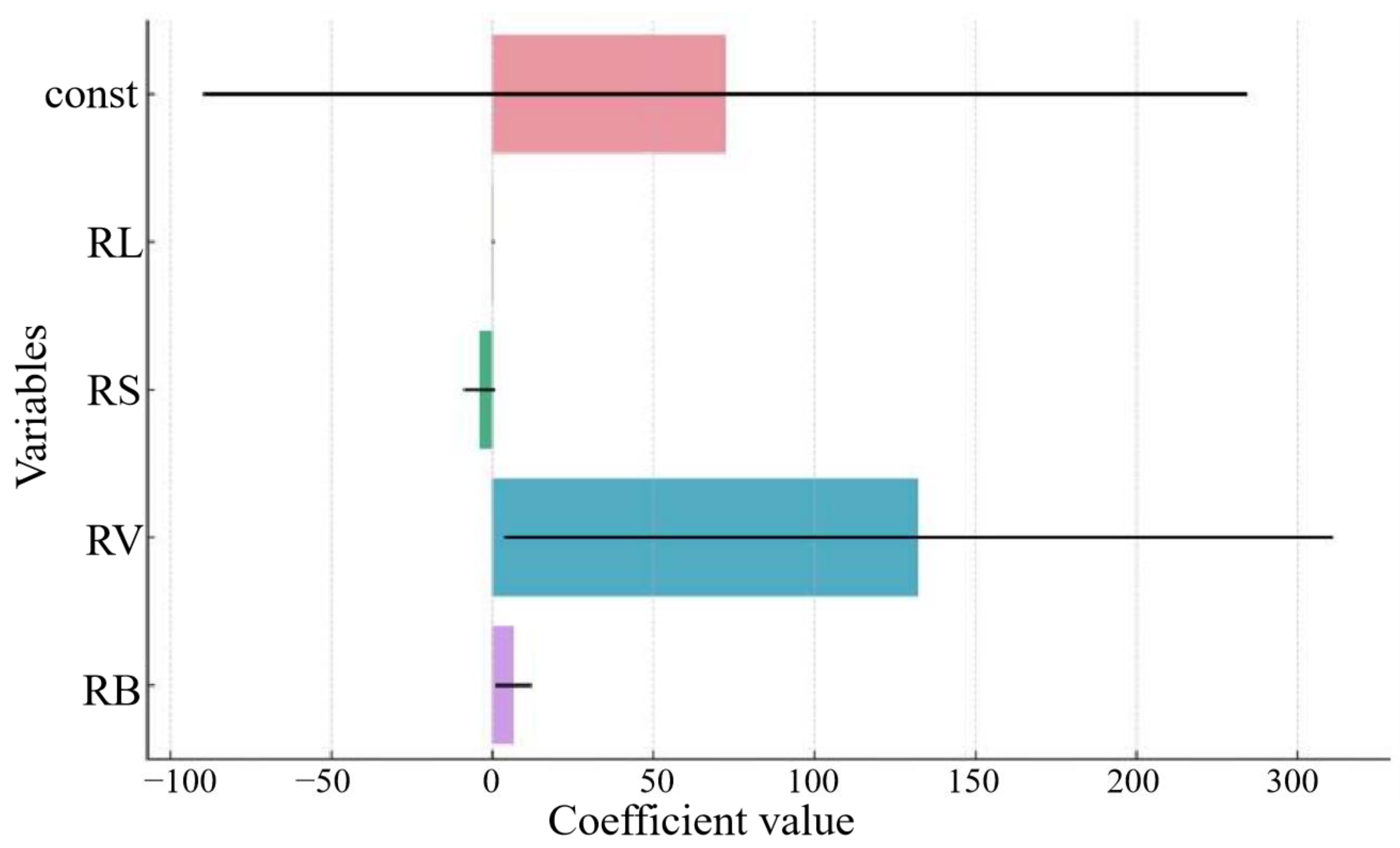
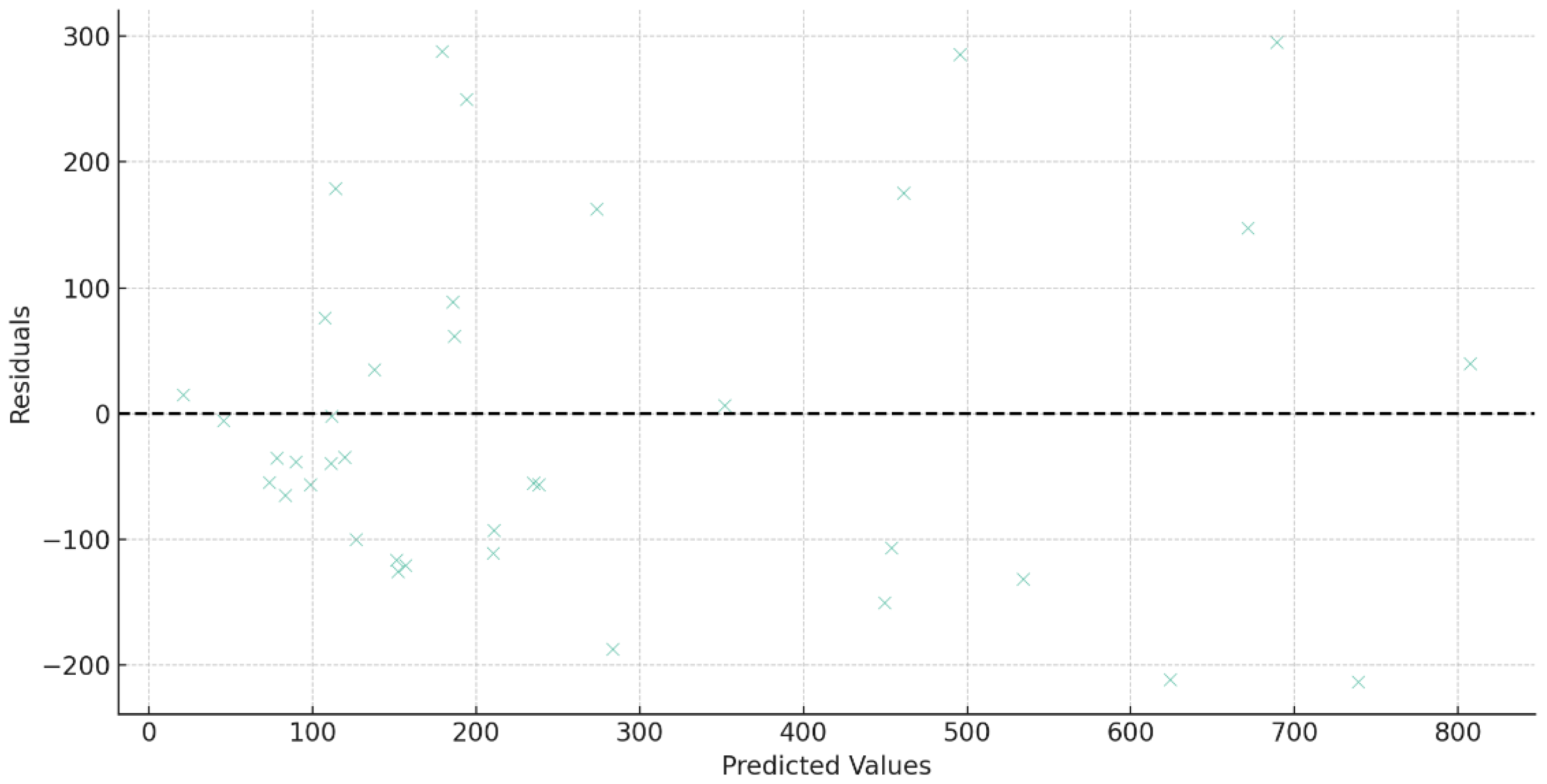
3.3. Relationship between Root Competition and Aboveground Growth under Heterogeneous Soil Nutrient Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention Forestry to Maintain Multifunctional Forests: A World Perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Li, Y.; Mei, B.; Linhares-Juvenal, T. The economic contribution of the world’s forest sector. For. Policy Econ. 2019, 100, 236–253. [Google Scholar] [CrossRef]
- Lu, Y.C.; Zhang, S.G.; Lei, X.D.; Ning, J.K.; Wang, Y.X. Theoretical Basis and Implementation Techniques on Close-to-nature Transformation of Plantations. World For. Res. 2009, 22, 20–27. [Google Scholar]
- Shirong, L.; Yujing, Y.; Hui, W. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10. [Google Scholar]
- Sun, D.J.; Wen, Y.; Luo, Y.H.; Li, X.Q.; Zhang, W.X.; Ming, A.G. Effect of close-to-nature management on species diversity in a Cunninghamia lanceolata plantation. For. Res. 2015, 28, 202–208. [Google Scholar]
- Ratcliffe, S.; Holzwarth, F.; Nadrowski, K.; Levick, S.; Wirth, C. Tree neighbourhood matters-Tree species composition drives diversity-productivity patterns in a near-natural beech forest. For. Ecol. Manag. 2015, 335, 225–234. [Google Scholar] [CrossRef]
- Wen, L.; Lei, P.F.; Xiang, W.H.; Yan, W.D.; Liu, S.G. Soil microbial biomass carbon and nitrogen in pure and mixed stands of Pinus massoniana and Cinnamomum camphora differing in stand age. For. Ecol. Manag. 2014, 328, 150–158. [Google Scholar] [CrossRef]
- Huang, X.M.; Liu, S.R.; Wang, H.; Hu, Z.D.; Li, Z.G.; You, Y.M. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Curtis, E.J.; Gorrod, E.J.; Ellis, M.V.; Chisholm, L.A. A spatio-temporal analysis of canopy dynamics and intra-stand competition in a riparian forest, south-eastern Australia. For. Ecol. Manag. 2019, 432, 189–199. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Li, W.L.; Huang, P.; Zong, J.W.; Li, Z.Z. Effect of potassium fertilizer and plastic film mulching on growth and size inequality in Lilium davidii var. unicolor. Acta Prataculturae Sin. 2011, 20, 217–222. [Google Scholar]
- Deng, J.-M.; Wang, G.-X.; Morris, E.; Wei, X.-P.; Li, D.-X.; Chen, B.-M.; Zhao, C.-M.; Liu, J.; Wang, Y.U.N. Plant mass-density relationship along a moisture gradient in north-west China. J. Ecol. 2006, 94, 953–958. [Google Scholar] [CrossRef]
- Lin, Y.; Berger, U.; Grimm, V.; Ji, Q.U. Differences between symmetric and asymmetric facilitation matter: Exploring the interplay between modes of positive and negative plant interactions. J. Ecol. 2012, 100, 1482–1491. [Google Scholar] [CrossRef]
- Børja, I.; De Wit, H.A.; Steffenrem, A.; Majdi, H. Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol. 2008, 28, 773–784. [Google Scholar] [CrossRef] [PubMed]
- de Kroon, H.; Huber, H.; Stuefer, J.F.; van Groenendael, J.M. A modular concept of phenotypic plasticity in plants. New Phytol. 2005, 166, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, Y. Response of fine roots to soil nutrient spatial heterogeneity. Chin. J. Appl. Ecol. 2004, 15, 1063–1068. [Google Scholar]
- Day, K.J.; Hutchings, M.J.; John, E.A. The Effects of Spatial Pattern of Nutrient Supply on Yield, Structure and Mortality in Plant Populations. J. Ecol. 2003, 91, 541–553. [Google Scholar] [CrossRef]
- Hodge, A. Root decisions. Plant Cell Environ. 2009, 32, 628–640. [Google Scholar] [CrossRef]
- Jiali, S.; Hongbo, L.; Aiping, Z. Effects of nutrient heterogeneity on shoot and root growth of Zea mays and intraspecific competition. J. China Agric. Univ. 2022, 27, 11. [Google Scholar]
- Grime, J.P. The Scale–Precision Trade-off in Spacial Resource Foraging by Plants: Restoring Perspective. Ann. Bot. 2007, 99, 1017–1021. [Google Scholar] [CrossRef]
- Cahill, J.F.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; St Clair, C.C. Plants integrate information about nutrients and neighbors. Science 2010, 328, 1657. [Google Scholar] [CrossRef]
- McNickle, G.G.; Cahill, J.F.; Karban, R. Plant Root Growth and the Marginal Value Theorem. Proc. Natl. Acad. Sci. USA 2009, 106, 4747–4751. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure. (MPB-17), Volume 17; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Schenk, H.J. Root Competition: Beyond Resource Depletion. J. Ecol. 2006, 94, 725–739. [Google Scholar] [CrossRef]
- Blair, B. Effect of soil nutrient heterogeneity on the symmetry of belowground competition. Plant Ecol. 2001, 156, 199–203. [Google Scholar] [CrossRef]
- Von Wettberg, E.J.; Weiner, J. Larger Triticum aestivum plants do not preempt nutrient-rich patches in a glasshouse experiment. Plant Ecol. 2003, 169, 85–92. [Google Scholar] [CrossRef]
- Wei, S.P.; Liang, W.J.; Wei, X.; Bu, R.Y.; Yao, J.F. Natural regeneration of Larix principis-rupprechtii plantations with different densities and its influencing factors. Chin. J. Appl. Ecol. 2022, 33, 2687–2694. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhao, H.; Cheng, Y.; Wang, W.; Jiang, Z.; Cai, J. Relationship between Hydraulic Efficiency and Gas Exchange and Growth of Six Poplar Clones. Sci. Silvae Sin. 2022, 58, 118–126. [Google Scholar] [CrossRef]
- Zhao, X.M.; Sun, X.Y.; Kang, X.Y.; Wang, H.Y. Dynamics of soil organic carbon and total nitrogen contents in short-rotation triploid Populus tomentosa plantations. Acta Ecol. Sin. 2012, 32, 4714–4721. [Google Scholar] [CrossRef]
- Kang, X.Y. Thinking and practices for strategy on a new round genetic improvement of Populus tomentosa. J. Beijing For. Univ. 2016, 38, 1–8. [Google Scholar]
- Xiao, W.J.; Ghen, Y.X. Application of Grey Correlation Analysis to the Evaluation ofHDS Sediment in Different Phytoremediation Copper Sulfide. Jiangxi Sci. 2022, 40, 1108–1113. [Google Scholar]
- Wang, H.Y.; Liu, J.G.; Yuan, H.C.; Duan, W.B.; Zhu, S.W.; Mu, M.X. Effects of Three Extracts on Physiological Indexes of 2-year-old Pinus sylvestris var. mongolica Seedlings. For. Eng. 2024, 40, 44–54. [Google Scholar]
- Huang, F.X. Effects of Different Fertilization Methods on Seedling Growth of Pinus sylvestris. Agric. Technol. Equip. 2023, 12, 129–130+133. [Google Scholar]
- Gao, Y.H. Effects of Different Thinning Methods on the Growth of Pinus Sylvestris Plantation. Sci. Technol. Qinghai Agric. For. 2023, 4, 82–85. [Google Scholar]
- Zhang, H.; Ye, R.H.; Wang, J. Effects of Irrigation Intensity on Seedling Growth of Pinus sylvestris var. mongolica in Mu Us Sandy Land. Prot. For. Sci. Technol. 2024, 2, 1–3. [Google Scholar] [CrossRef]
- Song, S.H.; Zhu, B.B.; Jin, L.; Chang, J.C.; Hu, L.; Song, L.P.; Chang, Q.; Song, Y.F. Effects of Forest Gaps disturbance On Regeneration Pattern of Pinus sylvestris var. mongolica Plantations. J. Northeast. For. Univ. 2022, 50, 6–10. [Google Scholar]
- Feng, D.D.; Xue, Q.Q.; Qi, J.Y.; Wang, L.J.; Li, H.; Men, L.; Zhang, Z.W. Community Structure and Occurrence Regularity of Pyralidae in Artificial Seabuckthorn Plantation. J. Shanxi Agric. Sci. 2020, 48, 793–798. [Google Scholar]
- Falster, D. User’s Guide to SMATR: Standardised Major Axis Tests and Routines Version 2.0, Copyright 2006. 2006. Available online: https://www.bio.mq.edu.au/research/groups/comparative/SMATR/ (accessed on 15 November 2023).
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. 2006, 81, 259–291. [Google Scholar] [CrossRef]
- Ding, J.; Ding, J.; Kong, D.; Zhang, Z.; Cai, Q.; Xiao, J.; Liu, Q.; Yin, H. Climate and soil nutrients differentially drive multidimensional fine root traits in ectomycorrhizal-dominated alpine coniferous forests. J. Ecol. 2020, 108, 2544–2556. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H.; et al. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef]
- Glass, N.T.; Yun, K.; Dias de Oliveira, E.A.; Zare, A.; Matamala, R.; Kim, S.H.; Gonzalez-Meler, M. Perennial grass root system specializes for multiple resource acquisitions with differential elongation and branching patterns. Front. Plant Sci. 2023, 14, 1146681. [Google Scholar] [CrossRef]
- Lobet, G.; Couvreur, V.; Meunier, F.; Javaux, M.; Draye, X. Plant Water Uptake in Drying Soils. Plant Physiol. 2014, 164, 1619–1627. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, J.F.; Dong, Y.; Yan, J.; Yang, N.; Feng, Y.H.; Wei, X.; Liang, W.J. Root distribution characteristics of monoculture and mixture of Pinus tabuliformis and Robinia pseudoacacia plantation. Ying Yong Sheng Tai Xue Bao 2023, 34, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Nan, H.; Gao, S.; Wang, X.; Zhang, M.; Liu, W. Effects of Underground Competition on Leaf and Root Development of Pinus tabulaeformis and Picea asperata Seedlings. Guangxi For. Sci. 2022, 51, 307–316. [Google Scholar]
- Robinson, D. Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. In Interactions in the Root Environment: An Integrated Approach: Proceedings of the Millenium Conference on Rhizosphere Interactions, IACR-Rothamsted, United Kingdom 10–12 April 2001; Powlson, D.S., Bateman, G.L., Davies, K.G., Gaunt, J.L., Hirsch, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 41–50. [Google Scholar]
- Sun, Y.-B.; Bi, H.-X.; Duan, H.-Q.; Peng, R.-D.; Wang, J.-J. Variation of fine root distribution and belowground competition in apple-soybean intercropping system. Chin. J. Ecol. 2019, 38, 459–466. [Google Scholar]
- Chen, J.-H. Study on the Distribution of Root Density of Robinia pseudoacacia L. and Pinus tabulaeformis Carr. Arid Zone Res. 2007, 24, 647–651. [Google Scholar]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Zhang, L.; He, M.; Zhang, K. Response of biomass allocation and allometric growth of Caragana korshinskii to nitrogen and phosphorus addition. Acta Ecol. Sin. 2023, 43, 6627–6636. [Google Scholar]
- Li, D.D.; Jiang, Y.C.; Luo, C.L.; Feng, Y.; Wang, A.P.; Dong, Q. Effects of Nitrogen and Phosphorus Separate Application and Intercropping on Soil Nutrients and Enzyme Activities at Flowering Stage of Spring Wheat. J. Shanxi Agric. Sci. 2022, 50, 1414–1421. [Google Scholar]
- Wu, F.Z.; Wang, K.Y.; Yang, W.Q.; Lu, Y.J. Effects of Fargesia denudata density on its biomass distribution pattern. Chin. J. Appl. Ecol. 2005, 16, 991–995. [Google Scholar]
- Wang, M.L.; Jiang, Y.S.; Wei, X.; Cai, S.F.; Qi, X.X.; Li, F. Effects of planting density and fertilization on growth characters and artemisinin of Artemisia annu. L. J. Plant Nutr. Fertil. Sci. 2010, 16, 185–190. [Google Scholar] [CrossRef]
- Bläsing, O.E.; Gibon, Y.; Günther, M.; Höhne, M.; Morcuende, R.; Osuna, D.; Thimm, O.; Usadel, B.; Scheible, W.R.; Stitt, M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 2005, 17, 3257–3281. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Li, C.-B.; Zheng, Z.; Peng, Y.; Nie, X.-Q.; Yang, L.-C.; Xiao, Y.; Zhou, G.-Y. Precipitation and nitrogen addition enhance biomass allocation to aboveground in an alpine steppe. Ecol. Evol. 2019, 9, 12193–12201. [Google Scholar] [CrossRef]
- Yan, Z.; Eziz, A.; Tian, D.; Li, X.; Hou, X.-H.; Peng, H.; Han, W.; Guo, Y.-L.; Fang, J. Biomass Allocation in Response to Nitrogen and Phosphorus Availability: Insight From Experimental Manipulations of Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 598. [Google Scholar] [CrossRef]
- Kim, B.-M.; Horita, J.; Suzuki, J.-I.; Tachiki, Y. Resource allocation in tragedy of the commons game in plants for belowground competition. J. Theor. Biol. 2021, 529, 110858. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.G.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Aerts, R.; Boot, R.G.A.; van der Aart, P.J.M. The relation between above- and belowground biomass allocation patterns and competitive ability. Oecologia 1991, 87, 551–559. [Google Scholar] [CrossRef]
- Casper, B.B.; Jackson, R.B. Plant Competition Underground. Annu. Rev. Ecol. Evol. Syst. 1997, 28, 545–570. [Google Scholar] [CrossRef]
- Lu, B.; Qian, J.; Hu, J.; Wang, P.-H.; Jin, W.; Tang, S.; He, Y.; Zhang, C. The role of fine root morphology in nitrogen uptake by riparian plants. Plant Soil 2022, 472, 527–542. [Google Scholar] [CrossRef]




| Soil Nutrient | Measured Value |
|---|---|
| Organic matter (g·kg−1) | 3.10 |
| Available nitrogen (mg·kg−1) | 60.61 |
| Total nitrogen (g·kg−1) | 0.41 |
| Available phosphorus (mg·kg−1) | 7.76 |
| Total phosphorus (g·kg−1) | 0.49 |
| Regression | t | p | |
|---|---|---|---|
| Fine root biomass–leaf biomass | 9.3 | 0.1 | 1.2 |
| Fine root biomass–stem biomass | 8.4 | 0.1 | 1.2 |
| Fine root biomass–aboveground biomass | 8.4 | 0.1 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Yao, J.; Guo, Y.; Sui, X.; Lv, X.; Liu, X.; Dong, Y.; Liang, W. Study on Associations between Root and Aboveground Growth of Mixed-Planting Seedlings of Populus tomentosa and Pinus tabuliformis under Soil Nutrient Heterogeneity. Forests 2024, 15, 1151. https://doi.org/10.3390/f15071151
Wei X, Yao J, Guo Y, Sui X, Lv X, Liu X, Dong Y, Liang W. Study on Associations between Root and Aboveground Growth of Mixed-Planting Seedlings of Populus tomentosa and Pinus tabuliformis under Soil Nutrient Heterogeneity. Forests. 2024; 15(7):1151. https://doi.org/10.3390/f15071151
Chicago/Turabian StyleWei, Xi, Jiafeng Yao, Yu Guo, Xiang Sui, Xiao Lv, Xiaoman Liu, Yuan Dong, and Wenjun Liang. 2024. "Study on Associations between Root and Aboveground Growth of Mixed-Planting Seedlings of Populus tomentosa and Pinus tabuliformis under Soil Nutrient Heterogeneity" Forests 15, no. 7: 1151. https://doi.org/10.3390/f15071151




