Repeated Mild Summer Drought in Crataegus monogyna Jacq. Provokes Compensation Growth in the Following Year
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
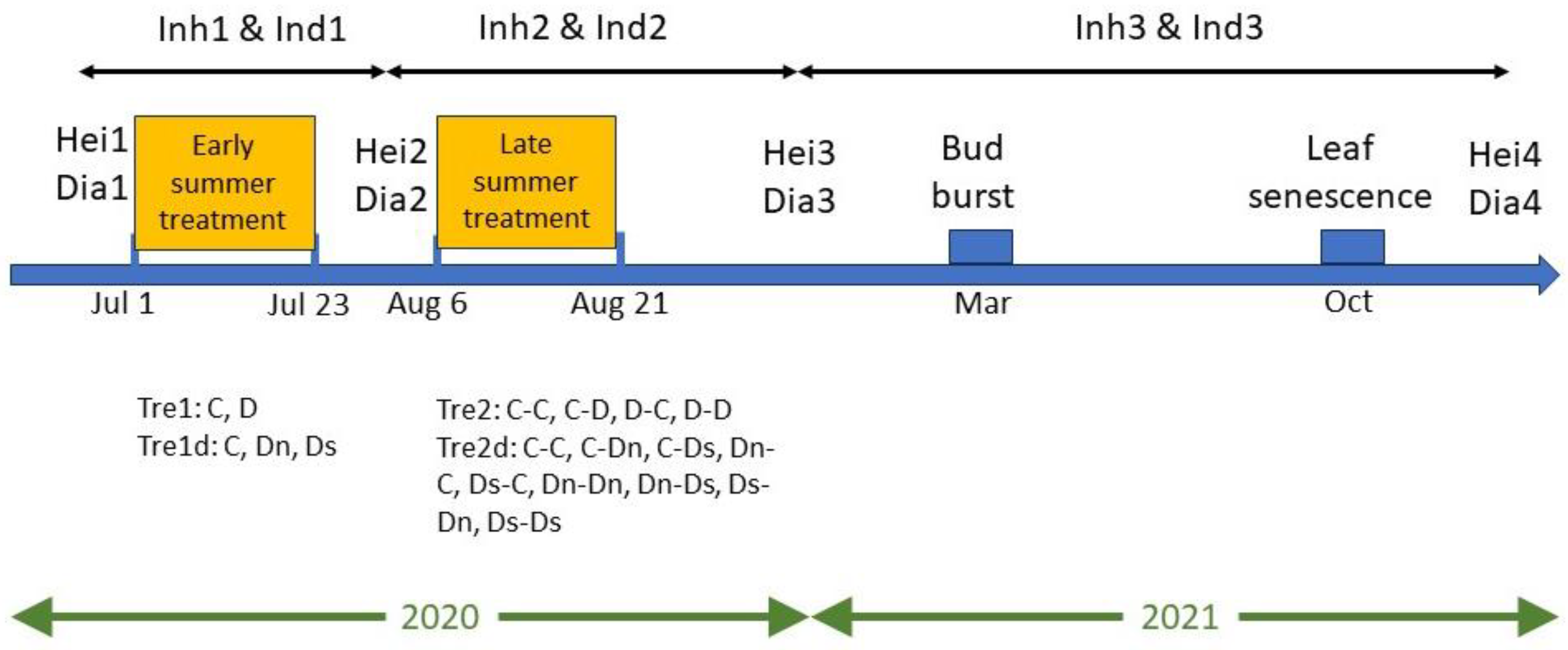
2.2. Drought Treatments
2.3. Measurements and Observations
2.4. Statistical Analysis
3. Results
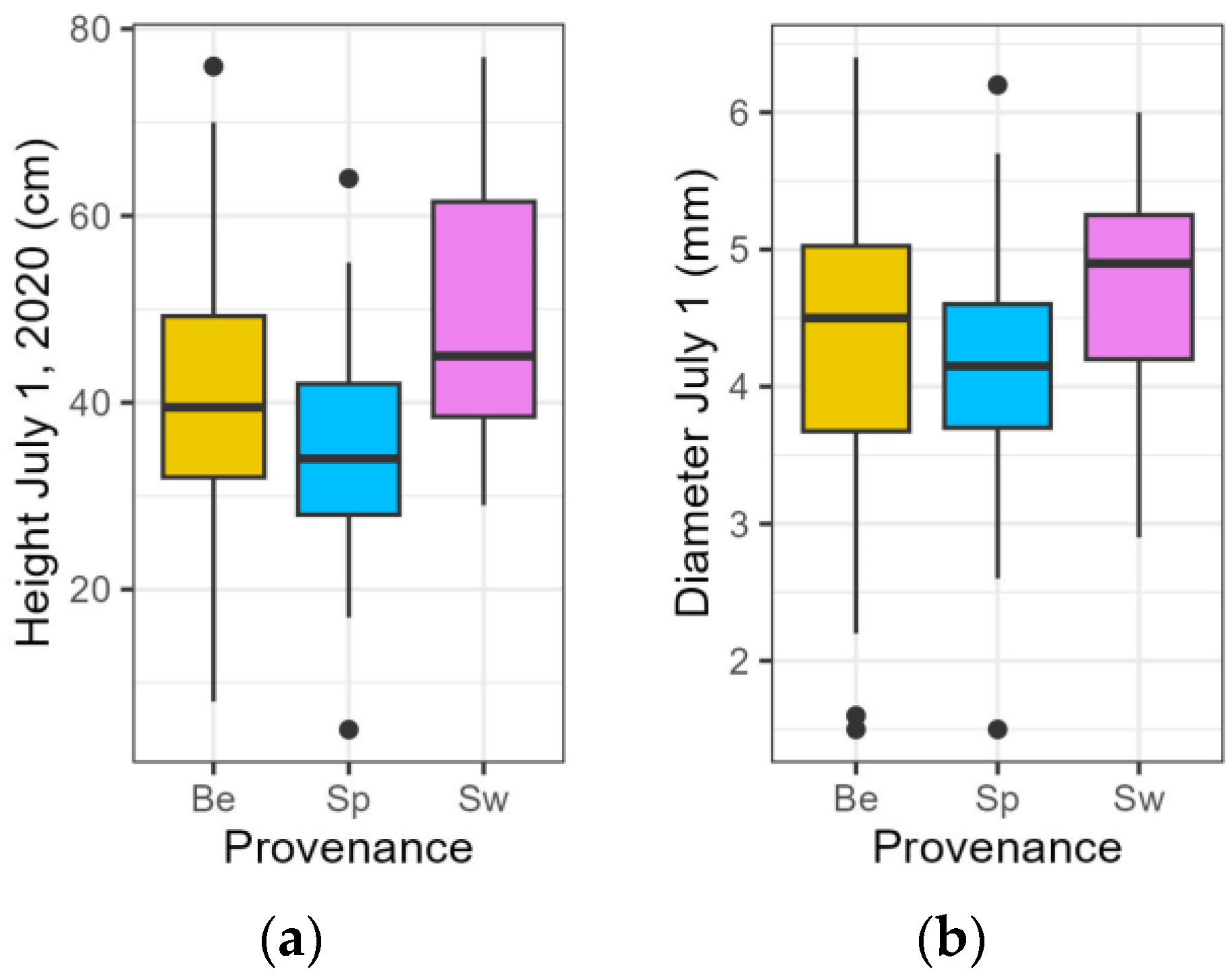
3.1. Growth Traits in the Year of the Drought Treatments
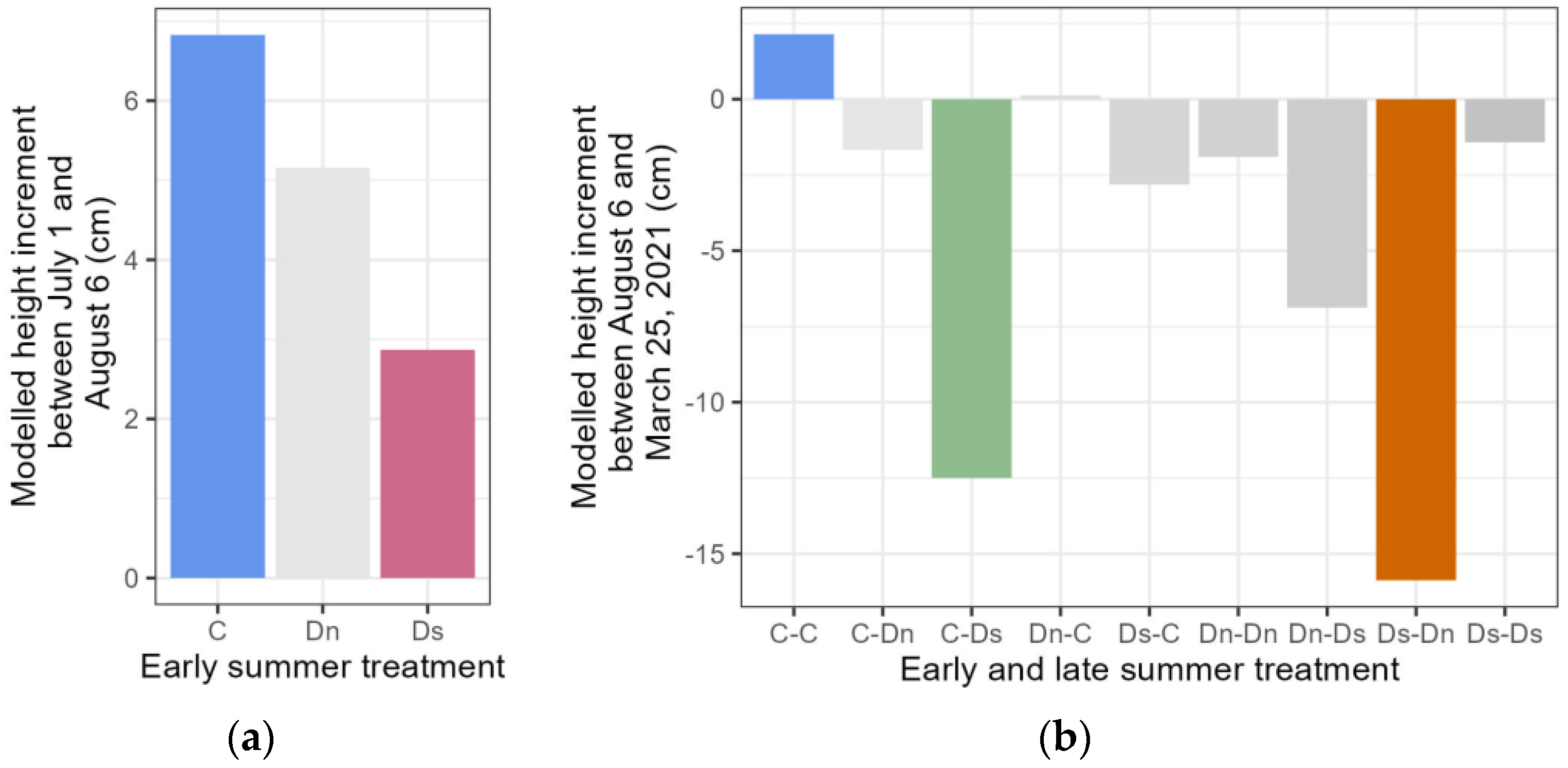
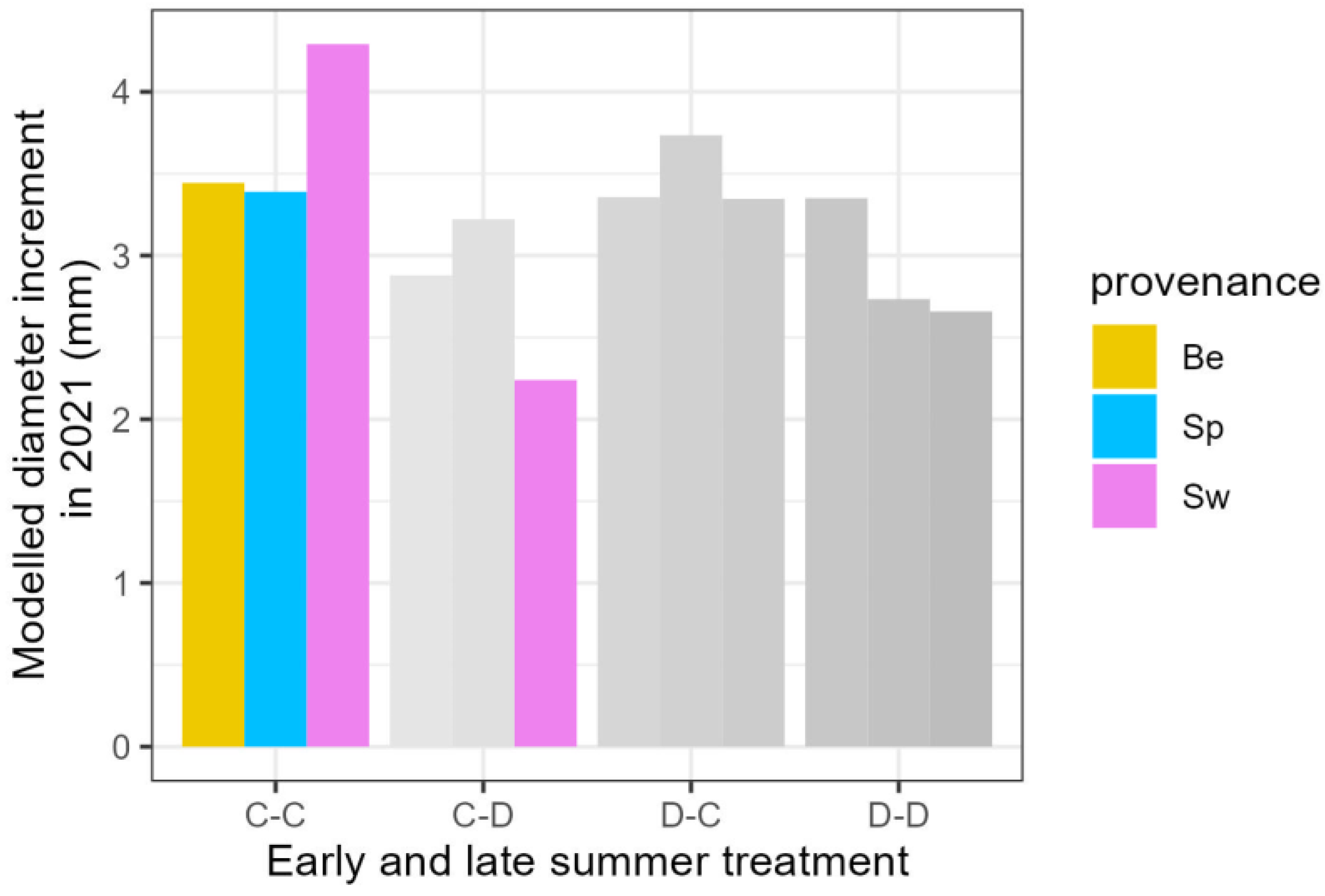
3.2. Growth Traits in the Year Following the Drought Treatments
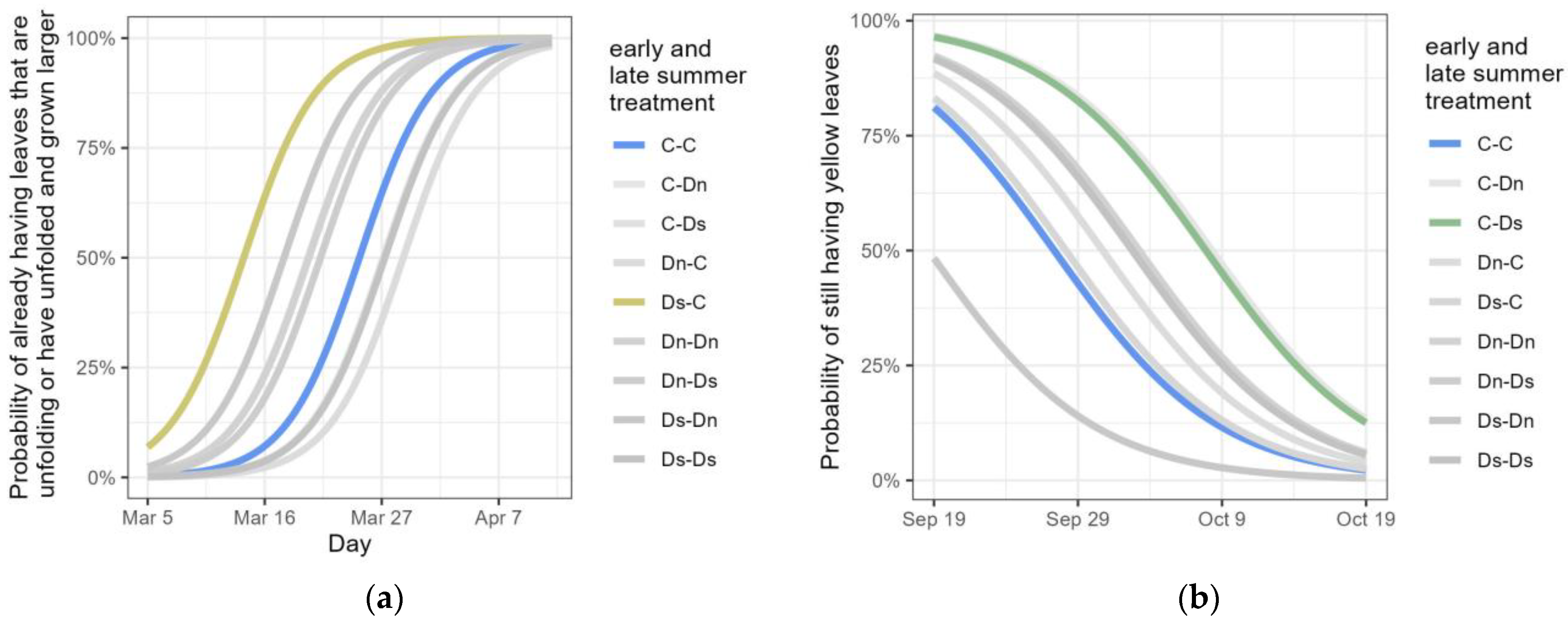
3.3. Leaf Phenological Traits in the Year Following the Drought Treatments
4. Discussion
4.1. Compensation Growth
4.2. Timing of the Droughts and Legacies in the Timing of Leaf Phenological Traits
4.3. Influence of Provenance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Monteith, J.L. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 1995, 18, 357–364. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulics and drought stress: Response, recovery and survivorship in four woody temperate plant species. Plant Cell Environ. 2009, 32, 1584–1595. [Google Scholar] [CrossRef]
- Blackman, C.J.; Creek, D.; Maier, C.; Aspinwall, M.J.; Drake, J.E.; Pfautsch, S.; O’Grady, A.; Delzon, S.; Medlyn, B.E.; Tissue, D.T.; et al. Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry-down to hydraulic failure. Tree Physiol. 2019, 39, 910–924. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Batz, T.A.; McAdam, S.A.M. Xylem Embolism Resistance Determines Leaf Mortality during Drought in Persea americana. Plant Physiol. 2020, 182, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.T.; Sperry, J.S.; Kursar, T.A. Does leaf shedding protect stems from cavitation during seasonal droughts? A test of the hydraulic fuse hypothesis. New Phytol. 2016, 212, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Windt, C.W.; Ponomarenko, A.; Zhang, Y.-J.; Gersony, J.; Rockwell, F.E.; Holbrook, N.M. Stomatal Closure, Basal Leaf Embolism, and Shedding Protect the Hydraulic Integrity of Grape Stems. Plant Physiol. 2017, 174, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Vilagrosa, A.; Bellot, J.; Vallejo, V.R.; Gil-Pelegrin, E. Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. J. Exp. Bot. 2003, 54, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Marchin, R.; Zeng, H.; Hoffmann, W. Drought-deciduous behavior reduces nutrient losses from temperate deciduous trees under severe drought. Oecologia 2010, 163, 845–854. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Grote, R.; Mayr, S.; Arneth, A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol 2019, 39, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The way back: Recovery of trees from drought and its implication for acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Ingrisch, J.; Bahn, M. Towards a Comparable Quantification of Resilience. Trends Ecol. Evol. 2018, 33, 251–259. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyn, B.E.; Palmer, A.; et al. Drought and resprouting plants. New Phytol. 2015, 206, 583–589. [Google Scholar] [CrossRef]
- Bryant, K.; Fredericksen, B.; Hudiburg, T.; Rosenthal, D. Physiological strategies for handling summer water stress differ among co-existing species and between juvenile and mature trees. Front. For. Glob. Chang. 2023, 5, 1018789. [Google Scholar] [CrossRef]
- Weemstra, M.; Eilmann, B.; Sass-Klaassen, U.G.W.; Sterck, F.J. Summer droughts limit tree growth across 10 temperate species on a productive forest site. For. Ecol. Manag. 2013, 306, 142–149. [Google Scholar] [CrossRef]
- Wolf, S.; Paul-Limoges, E. Drought and heat reduce forest carbon uptake. Nat. Commun. 2023, 14, 6217. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Vander Mijnsbrugge, K.; Onkelinx, T.; De Cuyper, B. Variation in bud burst and flower opening responses of local versus non-local provenances of hawthorn (Crataegus monogyna Jacq.) in Belgium. Plant Syst. Evol. 2014, 301, 1171–1179. [Google Scholar] [CrossRef]
- Urban, J.; Matoušková, M.; Robb, W.; Jelínek, B.; Úradníček, L. Effect of Drought on Photosynthesis of Trees and Shrubs in Habitat Corridors. Forests 2023, 14, 1521. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C. Antioxidant Capacity of Polyphenolic Extracts from Leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) Subjected to Drought and Cold Stress. J. Agric. Food Chem. 2003, 51, 3973–3976. [Google Scholar] [CrossRef]
- Fichtner, A.; Wissemann, V. Biological Flora of the British Isles: Crataegus monogyna. J. Ecol. 2021, 109, 541–571. [Google Scholar] [CrossRef]
- Uyttenbroeck, R.; De Vos, B.; Vander Mijnsbrugge, K. Verspreiding en Standplaats van Inheemse Bomen en Struiken in Vlaanderen. INBO.R.2014.1388322; Research Institute of Nature and Forest: Brussels, Belgium, 2014. [Google Scholar]
- Vander Mijnsbrugge, K.; Malanguis, J.M.; Moreels, S.; Turcsán, A.; Van der Schueren, N.; Notivol Mijnsbrugge, E. Direct Phenological Responses but Later Growth Stimulation upon Spring and Summer/Autumn Warming of Prunus spinosa L. in a Common Garden Environment. Forests 2022, 13, 23. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Janssens, A. Differentiation and Non-Linear Responses in Temporal Phenotypic Plasticity of Seasonal Phenophases in a Common Garden of Crataegus monogyna Jacq. Forests 2019, 10, 293. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, version 4.3.0; R_Core_Team: Vienna, Austria, 2023. [Google Scholar]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal data. R Package Version 2015.6-28. Available online: http://www.cran.r-project.org/package=ordinal/ (accessed on 11 July 2024).
- Wickham, H. ggplot2: Elegant Graphics for data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Belsky, A.J. Does Herbivory Benefit Plants? A Review of the Evidence. Am. Nat. 1986, 127, 870–892. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, L.; Lv, X.; Yang, G.; Wang, Y.; Wang, X. Compensatory growth as a response to post-drought in grassland. Front. Plant Sci. 2022, 13, 1004553. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Barclay, H.; Roitberg, B.; Lalonde, R. Ecology and Prediction of Compensatory Growth: From Theory to Application in Forestry. Front Plant Sci. 2021, 12, 655417. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Loreau, M. The Causes and Consequences of Compensatory Dynamics in Ecological Communities. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 393. [Google Scholar] [CrossRef]
- Maschinski, J.; Whitham, T.G. The Continuum of Plant Responses to Herbivory: The Influence of Plant Association, Nutrient Availability, and Timing. Am. Nat. 1989, 134, 1–19. [Google Scholar] [CrossRef]
- Aldous, S.E. Deer Browse Clipping Study in the Lake States Region. J. Wildl. Manag. 1952, 16, 401–409. [Google Scholar] [CrossRef]
- Garrison, G.A. Effects of Clipping on Some Range Shrubs. J. Range Manag. 1953, 6, 309–317. [Google Scholar] [CrossRef]
- Wilcox, K.R.; Koerner, S.E.; Hoover, D.L.; Borkenhagen, A.K.; Burkepile, D.E.; Collins, S.L.; Hoffman, A.M.; Kirkman, K.P.; Knapp, A.K.; Strydom, T.; et al. Rapid recovery of ecosystem function following extreme drought in a South African savanna grassland. Ecology 2020, 101, e02983. [Google Scholar] [CrossRef]
- Hoover, D.L.; Knapp, A.K.; Smith, M.D. Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 2014, 95, 2646–2656. [Google Scholar] [CrossRef]
- Connell, S.D.; Ghedini, G. Resisting regime-shifts: The stabilising effect of compensatory processes. Trends Ecol. Evol. 2015, 30, 513–515. [Google Scholar] [CrossRef]
- El Omari, B. Accumulation versus storage of total non-structural carbohydrates in woody plants. Trees 2022, 36, 869–881. [Google Scholar] [CrossRef]
- Turcsán, A.; Steppe, K.; Sárközi, E.; Erdélyi, É.; Missoorten, M.; Mees, G.; Vander Mijnsbrugge, K. Early summer drought stress during the first growing year stimulates extra shoot growth in oak seedlings (Quercus petraea). Front. Plant Sci. 2016, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Spiess, N.; Oufir, M.; Matusikova, I.; Stierschneider, M.; Kopecky, D.; Homolka, A.; Burg, K.; Fluch, S.; Hausman, J.F.; Wilhelm, E. Ecophysiological and transcriptomic responses of oak (Quercus robur) to long-term drought exposure and rewatering. Environ. Exp. Bot. 2012, 77, 117–126. [Google Scholar] [CrossRef]
- Kaya, Z.; Adams, W.T.; Campbell, R.K. Adaptive significance of intermittent shoot growth in Douglas-fir seedlings. Tree Physiol 1994, 14, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.; Matiu, M.; Menzel, A. Compensatory Growth of Scots Pine Seedlings Mitigates Impacts of Multiple Droughts within and Across Years. Front. Plant Sci. 2019, 10, 519. [Google Scholar] [CrossRef]
- van Kampen, R.; Fisichelli, N.; Zhang, Y.-J.; Wason, J. Drought timing and species growth phenology determine intra-annual recovery of tree height and diameter growth. AoB Plants 2022, 14, plac012. [Google Scholar] [CrossRef]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Maxwell, J.T.; Pederson, N.; D’Orangeville, L.; Ficklin, D.L.; Phillips, R.P. Drought legacies are dependent on water table depth, wood anatomy and drought timing across the eastern US. Ecol. Lett. 2019, 22, 119–127. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Anderegg, W.R.L.; Berry, J.A. Not all droughts are created equal: Translating meteorological drought into woody plant mortality. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought and Drought Release. Front. Plant Sci. 2018, 9, 187. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Vandepitte, J.; Moreels, S.; Mihaila, V.-V.; De Ligne, L.; Notivol, E.; Van Acker, J.; Van den Bulcke, J. Timing of autumnal leaf senescence in a common shrub species depends on the level of preceding summer drought symptoms. Environ. Exp. Bot. 2023, 216, 105539. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Turcsan, A.; Maes, J.; Duchene, N.; Meeus, S.; Steppe, K.; Steenackers, M. Repeated Summer Drought and Re-watering during the First Growing Year of Oak (Quercus petraea) Delay Autumn Senescence and Bud Burst in the Following Spring. Front. Plant Sci. 2016, 7, 419. [Google Scholar] [CrossRef]
- Mariën, B.; Dox, I.; De Boeck, H.J.; Willems, P.; Leys, S.; Papadimitriou, D.; Campioli, M. Does drought advance the onset of autumn leaf senescence in temperate deciduous forest trees? Biogeosciences 2021, 18, 3309–3330. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Moreels, S. Varying Levels of Genetic Control and Phenotypic Plasticity in Timing of Bud Burst, Flower Opening, Leaf Senescence and Leaf Fall in Two Common Gardens of Prunus padus L. Forests 2020, 11, 1070. [Google Scholar] [CrossRef]




| Response Variable | Variable | Estimate | St. Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Hei1 | (intercept) | 40.40 | 1.84 | 21.90 | <0.001 *** |
| Sp | −5.44 | 2.61 | −2.09 | 0.039 * | |
| Sw | 8.88 | 2.62 | 3.39 | <0.001 *** | |
| Dia1 | (intercept) | 4.32 | 0.13 | 34.40 | <0.001 *** |
| Sp | −0.19 | 0.18 | −1.08 | 0.283 | |
| Sw | 0.43 | 0.18 | 2.41 | 0.017 * |
| Response Variable | Variable | Estimate | St. Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Inh1 | (intercept) | −2.35 | 1.73 | −1.35 | 0.178 |
| Dn | −1.67 | 1.14 | −1.47 | 0.145 | |
| Ds | −3.96 | 1.49 | −2.66 | 0.009 ** | |
| Hei1 | 0.22 | 0.04 | 5.94 | <0.001 *** | |
| Ind1 | (intercept) | 0.52 | 0.20 | 2.63 | 0.010 ** |
| Dn | 0.07 | 0.08 | 0.82 | 0.411 | |
| Ds | 0.03 | 0.11 | 0.29 | 0.775 | |
| Dia1 | −0.03 | 0.04 | −0.73 | 0.465 | |
| Inh1P | (intercept) | 0.72 | 2.06 | 0.35 | 0.726 |
| Sp | −4.70 | 1.74 | −2.70 | 0.008 ** | |
| Sw | −3.78 | 1.75 | −2.16 | 0.033 * | |
| D | −3.26 | 1.72 | −1.89 | 0.060 | |
| Hei1 | 0.22 | 0.04 | 5.44 | <0.001 *** | |
| Sp:D | 2.24 | 2.43 | 0.92 | 0.358 | |
| Sw:D | 0.30 | 2.44 | 0.12 | 0.903 | |
| Ind1P | (intercept) | 0.68 | 0.20 | 3.34 | 0.001 ** |
| Sp | −0.16 | 0.12 | −1.35 | 0.179 | |
| Sw | 0.33 | 0.12 | 2.74 | 0.007 ** | |
| D | 0.09 | 0.12 | 0.75 | 0.457 | |
| Dia1 | −0.08 | 0.04 | −1.93 | 0.056 | |
| Sp:D | 0.16 | 0.17 | 0.91 | 0.365 | |
| Sw:D | −0.29 | 0.17 | −1.71 | 0.091 |
| Response Variable | Variable | Estimate | St. Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Inh2 | (intercept) | 8.18 | 4.11 | 1.99 | 0.048 * |
| C-Dn | −3.82 | 4.50 | −0.85 | 0.397 | |
| C-Ds | −14.65 | 3.59 | −4.08 | 0.000 *** | |
| Dn-C | −2.02 | 3.48 | −0.58 | 0.563 | |
| Ds-C | −4.96 | 4.58 | −1.08 | 0.280 | |
| Dn-Dn | −4.05 | 4.02 | −1.01 | 0.315 | |
| Dn-Ds | −9.02 | 5.49 | −1.64 | 0.103 | |
| Ds-Dn | −18.02 | 5.19 | −3.47 | 0.001 *** | |
| Ds-Ds | −3.56 | 8.01 | −0.45 | 0.657 | |
| Hei2 | −0.14 | 0.07 | −2.04 | 0.043 * | |
| Ind2 | (intercept) | 13.14 | 5.26 | 2.50 | 0.014 * |
| Sp | −4.68 | 5.65 | −0.83 | 0.409 | |
| Sw | 1.19 | 5.72 | 0.21 | 0.835 | |
| C-D | −11.27 | 5.66 | −1.99 | 0.048 * | |
| D-C | −4.60 | 5.63 | −0.82 | 0.416 | |
| D-D | −11.48 | 5.63 | −2.04 | 0.044 * | |
| Hei2 | −0.22 | 0.07 | −3.01 | 0.003 ** | |
| Sp: C-D | 6.37 | 8.02 | 0.80 | 0.428 | |
| Sw: C-D | −5.17 | 8.03 | −0.64 | 0.521 | |
| Sp: D-C | 5.80 | 7.96 | 0.73 | 0.467 | |
| Sw: D-C | −1.63 | 7.97 | −0.21 | 0.838 | |
| Sp: D-D | 9.32 | 7.96 | 1.17 | 0.244 | |
| Sw: D-D | −1.19 | 8.08 | −0.15 | 0.883 | |
| Inh2P | (intercept) | 0.70 | 0.24 | 2.92 | 0.004 ** |
| C-Dn | −0.13 | 0.16 | −0.80 | 0.427 | |
| C-Ds | −0.13 | 0.12 | −1.11 | 0.271 | |
| Dn-C | −0.23 | 0.12 | −1.88 | 0.062 | |
| Ds-C | −0.14 | 0.16 | −0.88 | 0.378 | |
| Dn-Dn | −0.27 | 0.14 | −1.94 | 0.055 | |
| Dn-Ds | −0.13 | 0.19 | −0.71 | 0.476 | |
| Ds-Dn | −0.06 | 0.18 | −0.34 | 0.736 | |
| Ds-Ds | 0.47 | 0.27 | 1.70 | 0.091 | |
| Dia2 | −0.07 | 0.05 | −1.50 | 0.137 | |
| Ind2P | (intercept) | 0.86 | 0.25 | 3.40 | 0.001 *** |
| Sp | −0.38 | 0.19 | −2.00 | 0.047 * | |
| Sw | −0.10 | 0.19 | −0.51 | 0.614 | |
| C-D | −0.27 | 0.19 | −1.42 | 0.157 | |
| D-C | −0.42 | 0.19 | −2.25 | 0.026 * | |
| D-D | −0.32 | 0.19 | −1.71 | 0.090 | |
| Dia2 | −0.07 | 0.04 | −1.52 | 0.131 | |
| Sp: C-D | 0.39 | 0.27 | 1.46 | 0.148 | |
| Sw: C-D | 0.01 | 0.26 | 0.05 | 0.957 | |
| Sp: D-C | 0.50 | 0.26 | 1.90 | 0.060 | |
| Sw: D-C | 0.17 | 0.27 | 0.63 | 0.533 | |
| Sp: D-D | 0.26 | 0.26 | 1.00 | 0.322 | |
| Sw: D-D | 0.32 | 0.27 | 1.21 | 0.230 |
| Response Variable | Variable | Estimate | St. Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Inh3 | (intercept) | 48.92 | 4.69 | 10.44 | <0.001 *** |
| C-Dn | 0.00 | 5.58 | 0.00 | 1.000 | |
| C-Ds | −9.48 | 4.47 | −2.12 | 0.036 * | |
| Dn-C | 5.02 | 4.36 | 1.15 | 0.252 | |
| Ds-C | −6.61 | 5.73 | −1.15 | 0.251 | |
| Dn-Dn | 10.12 | 5.03 | 2.01 | 0.046 * | |
| Dn-Ds | −5.08 | 6.88 | −0.74 | 0.462 | |
| Ds-Dn | 2.17 | 6.62 | 0.33 | 0.743 | |
| Ds-Ds | −7.81 | 10.02 | −0.78 | 0.437 | |
| Hei3 | −0.16 | 0.07 | −2.19 | 0.030 * | |
| Ind3 | (intercept) | 2.98 | 0.58 | 5.13 | <0.001 *** |
| C-Dn | −0.40 | 0.38 | −1.07 | 0.288 | |
| C-Ds | −1.17 | 0.29 | −4.00 | <0.001 *** | |
| Dn-C | −0.14 | 0.29 | −0.49 | 0.625 | |
| Ds-C | −0.25 | 0.38 | −0.67 | 0.502 | |
| Dn-Dn | −0.41 | 0.33 | −1.22 | 0.226 | |
| Dn-Ds | −1.08 | 0.45 | −2.39 | 0.018 * | |
| Ds-Dn | −0.84 | 0.45 | −1.86 | 0.066 | |
| Ds-Ds | −1.87 | 0.66 | −2.85 | 0.005 ** | |
| Dia3 | 0.14 | 0.10 | 1.34 | 0.182 | |
| Inh3P | (intercept) | 47.62 | 6.28 | 7.58 | <0.001 *** |
| Sp | 4.00 | 6.97 | 0.57 | 0.567 | |
| Sw | 6.32 | 6.99 | 0.90 | 0.368 | |
| C-D | −3.61 | 6.93 | −0.52 | 0.603 | |
| D-C | −1.39 | 6.94 | −0.20 | 0.841 | |
| D-D | 0.84 | 7.01 | 0.12 | 0.905 | |
| Hei3 | −0.20 | 0.08 | −2.60 | 0.010 * | |
| Sp: C-D | 2.57 | 9.79 | 0.26 | 0.793 | |
| Sw: C-D | −11.44 | 9.87 | −1.16 | 0.249 | |
| Sp: D-C | 0.85 | 9.80 | 0.09 | 0.931 | |
| Sw: D-C | 6.96 | 9.79 | 0.71 | 0.478 | |
| Sp: D-D | 5.12 | 9.81 | 0.52 | 0.602 | |
| Sw: D-D | 1.98 | 9.92 | 0.20 | 0.842 | |
| Ind3P | (intercept) | 3.19 | 0.62 | 5.17 | <0.001 *** |
| Sp | −0.05 | 0.44 | −0.12 | 0.903 | |
| Sw | 0.85 | 0.45 | 1.90 | 0.060 | |
| C-D | −0.56 | 0.44 | −1.28 | 0.205 | |
| D-C | −0.09 | 0.45 | −0.19 | 0.846 | |
| D-D | −0.09 | 0.45 | −0.20 | 0.838 | |
| Dia3 | 0.05 | 0.10 | 0.49 | 0.623 | |
| Sp: C-D | 0.40 | 0.63 | 0.63 | 0.527 | |
| Sw: C-D | −1.49 | 0.62 | −2.38 | 0.019 * | |
| Sp: D-C | 0.43 | 0.63 | 0.69 | 0.493 | |
| Sw: D-C | −0.86 | 0.63 | −1.37 | 0.173 | |
| Sp: D-D | −0.56 | 0.63 | −0.89 | 0.376 | |
| Sw: D-D | −1.54 | 0.64 | −2.41 | 0.017 * |
| Response Variable | Variable | Estimate | St. Error | z-Value | p-Value |
|---|---|---|---|---|---|
| Bud | day | −0.29 | 0.02 | −14.66 | <0.001 *** |
| C-Dn | 0.64 | 1.18 | 0.55 | 0.585 | |
| C-Ds | −1.39 | 0.99 | −1.41 | 0.160 | |
| Dn-C | 1.19 | 0.91 | 1.31 | 0.192 | |
| Ds-C | −3.14 | 1.22 | −2.56 | 0.010 * | |
| Dn-Dn | −1.37 | 1.07 | −1.29 | 0.199 | |
| Dn-Ds | −1.05 | 1.54 | −0.69 | 0.493 | |
| Ds-Dn | −2.04 | 1.46 | −1.40 | 0.162 | |
| Ds-Ds | 0.69 | 2.10 | 0.33 | 0.743 | |
| Hei3 | 0.01 | 0.02 | 0.53 | 0.597 | |
| Sen | day | 0.17 | 0.03 | 6.80 | <0.001 *** |
| C-Dn | −1.91 | 1.03 | −1.85 | 0.064 | |
| C-Ds | −1.84 | 0.87 | −2.11 | 0.035 * | |
| Dn-C | −0.59 | 0.81 | −0.73 | 0.468 | |
| Ds-C | −0.14 | 1.07 | −0.13 | 0.895 | |
| Dn-Dn | −1.03 | 0.92 | −1.12 | 0.262 | |
| Dn-Ds | −0.96 | 1.27 | −0.75 | 0.452 | |
| Ds-Dn | 1.53 | 1.24 | 1.23 | 0.217 | |
| Ds-Ds | −0.95 | 1.84 | −0.52 | 0.606 | |
| Hei4 | 0.06 | 0.01 | 4.19 | <0.001 *** | |
| BudP | day | −0.29 | 0.02 | −14.64 | <0.001 *** |
| Sp | 5.62 | 1.27 | 4.44 | <0.001 *** | |
| Sw | 0.93 | 1.22 | 0.76 | 0.445 | |
| C-D | 0.40 | 1.23 | 0.33 | 0.743 | |
| D-C | −0.50 | 1.21 | −0.42 | 0.678 | |
| D-D | −0.92 | 1.24 | −0.74 | 0.461 | |
| Hei3 | 0.03 | 0.01 | 1.95 | 0.052 | |
| Sp: C-D | −1.38 | 1.75 | −0.79 | 0.428 | |
| Sw: C-D | −1.04 | 1.78 | −0.58 | 0.560 | |
| Sp: D-C | 0.62 | 1.71 | 0.36 | 0.716 | |
| Sw: D-C | 0.92 | 1.71 | 0.54 | 0.591 | |
| Sp: D-D | −1.73 | 1.73 | −1.00 | 0.317 | |
| Sw: D-D | 1.30 | 1.77 | 0.74 | 0.462 | |
| SenP | day | 0.17 | 0.03 | 6.95 | <0.001 *** |
| Sp | −2.79 | 1.20 | −2.32 | 0.020 * | |
| Sw | −0.60 | 1.19 | −0.51 | 0.611 | |
| C-D | −2.78 | 1.20 | −2.32 | 0.021 * | |
| D-C | −0.97 | 1.17 | −0.83 | 0.408 | |
| D-D | 0.60 | 1.17 | 0.51 | 0.611 | |
| Hei4 | 0.05 | 0.01 | 4.28 | 0.000 | |
| Sp: C-D | 1.06 | 1.63 | 0.65 | 0.515 | |
| Sw: C-D | 1.65 | 1.68 | 0.98 | 0.327 | |
| Sp: D-C | 0.90 | 1.64 | 0.55 | 0.582 | |
| Sw: D-C | 0.66 | 1.66 | 0.40 | 0.691 | |
| Sp: D-D | −1.08 | 1.65 | −0.66 | 0.511 | |
| Sw: D-D | −2.10 | 1.68 | −1.25 | 0.210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijnsbrugge, K.V.; Moreels, S.; Decorte, L.; Stessens, M.; Notivol Paino, E. Repeated Mild Summer Drought in Crataegus monogyna Jacq. Provokes Compensation Growth in the Following Year. Forests 2024, 15, 1234. https://doi.org/10.3390/f15071234
Mijnsbrugge KV, Moreels S, Decorte L, Stessens M, Notivol Paino E. Repeated Mild Summer Drought in Crataegus monogyna Jacq. Provokes Compensation Growth in the Following Year. Forests. 2024; 15(7):1234. https://doi.org/10.3390/f15071234
Chicago/Turabian StyleMijnsbrugge, Kristine Vander, Stefaan Moreels, Laura Decorte, Marie Stessens, and Eduardo Notivol Paino. 2024. "Repeated Mild Summer Drought in Crataegus monogyna Jacq. Provokes Compensation Growth in the Following Year" Forests 15, no. 7: 1234. https://doi.org/10.3390/f15071234







