Variation in Niche and Interspecific Associations across Elevations in Subtropical Forest Communities of the Wuyi Mountains, Southeastern China
Abstract
1. Introduction
2. Materials and Methods
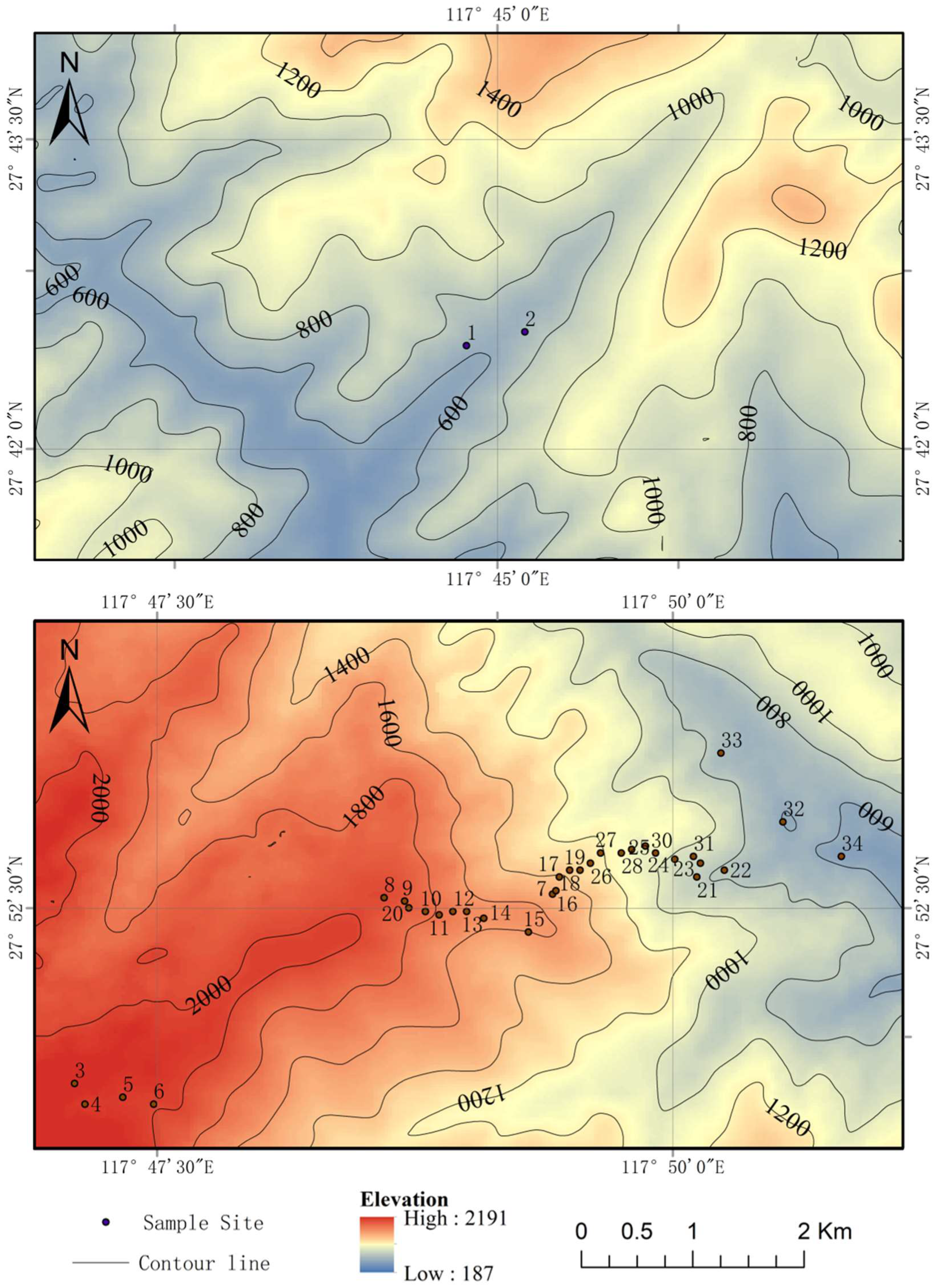
2.1. The Study Area
2.2. Plot Establishment and Data Collection
2.3. Data Analysis
2.3.1. Calculation of Importance Values
2.3.2. Determination of Interspecific Association
2.3.3. Calculation of Niche Width and Niche Overlap
3. Results
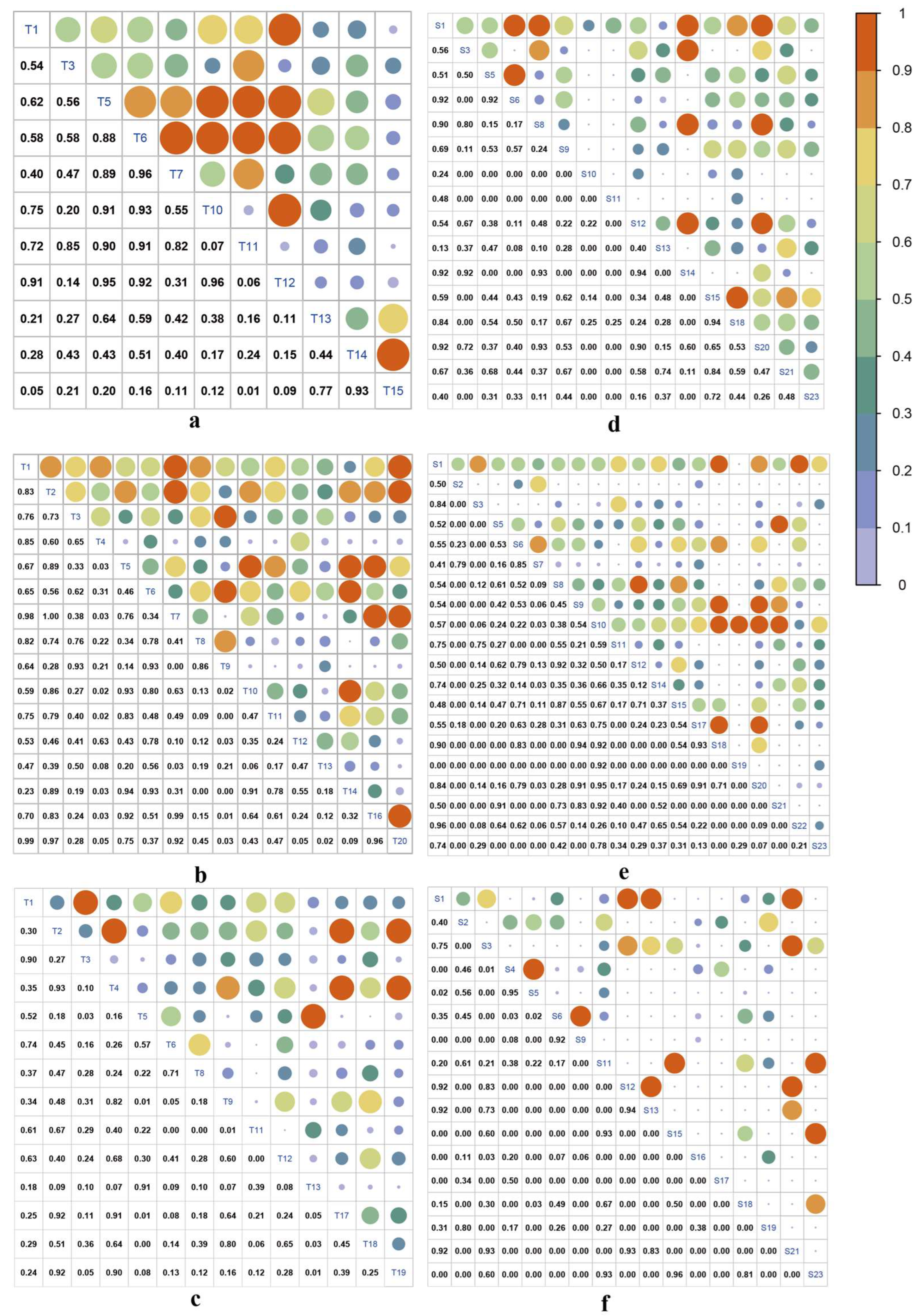
3.1. Variations in Interspecific Associations with the Elevation Gradient
3.2. Niche Width Analysis
4. Discussion
4.1. Regulation Mechanisms of Interspecific Associations Along Environmental Ranges
4.2. Response of Niche Overlap to Environmental Ranges
4.3. Relationship between Niche Overlap and Interspecific Associations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | No. | H1 | H2 | H3 |
|---|---|---|---|---|
| Rhododendron latoucheae Franch. | T1 | 2.45 | 7.56 | 4.43 |
| Pinus taiwanensis Hayata | T2 | 0 | 7.33 | 6.12 |
| Schima superba Gardner and Champ. | T3 | 3.12 | 5.99 | 2.56 |
| Enkianthus quinqueflorus Lour. | T4 | 0 | 2.14 | 5.27 |
| Rhododendron ovatum (Lindl.) Planch. ex Maxim. | T5 | 4.21 | 4.5 | 1.98 |
| Vaccinium sprengelii (G. Don) Sleumer | T6 | 4.84 | 6.44 | 1.89 |
| Adinandra millettii (Hook. and Arn.) Benth. and Hook. f. ex Hance | T7 | 3.29 | 3.32 | 0 |
| Rhododendron taronense Hutch. | T8 | 0 | 3.87 | 2.24 |
| Rhododendron basilicum Balf. f. and W. W. Sm. | T9 | 0 | 2.2 | 2.83 |
| Rhododendron farrerae Sweet | T10 | 1.98 | 2.25 | 0 |
| Eurya muricata Dunn | T11 | 1.87 | 3.43 | 2.27 |
| Quercus multinervis (W. C. Cheng and T. Hong) J. Q. Li | T12 | 1.35 | 2.87 | 3.52 |
| Quercus glauca Thunb. | T13 | 5.08 | 2.86 | 1.54 |
| Castanopsis eyrei (Champ. ex Benth.) Tutcher | T14 | 6.62 | 1.58 | 0 |
| Engelhardia roxburghiana Wall. | T15 | 2.78 | 0 | 0 |
| Acer rubrum L. | T16 | 0 | 2.56 | 0 |
| Tsuga chinensis (Franch.) E. Pritz. | T17 | 0 | 0 | 3.02 |
| Rhododendron fortunei Lindl. | T18 | 0 | 0 | 3.51 |
| Pyrus calleryana Decne. | T19 | 0 | 0 | 3.34 |
| Clethra barbinervis Siebold and Zucc. | T20 | 0 | 2.28 | 0 |
| Begonia acetosella Craib | S1 | 3.38 | 6.52 | 2.46 |
| Enkianthus quinqueflorus Lour. | S2 | 0 | 1.01 | 3.86 |
| Mahonia bealei (Fortune) Carr. | S3 | 2.6 | 1.01 | 2.16 |
| Vaccinium japonicum Miq. | S4 | 0 | 0 | 3.11 |
| Eurya muricata Dunn | S5 | 2.46 | 2.91 | 1.10 |
| Rhododendron simsii Planch. | S6 | 1.06 | 3.60 | 2.92 |
| Symplocos glomerata King ex Gamble | S7 | 0 | 1.56 | 0 |
| Ilex wilsonii Loes. | S8 | 2.13 | 3.87 | 0 |
| Prunus mume Siebold and Zucc. | S9 | 2.33 | 3.59 | 1.02 |
| Vaccinium sprengelii (G. Don) Sleumer | S10 | 2.14 | 7.04 | 0 |
| Litsea cubeba (Lour.) Pers. | S11 | 1.04 | 1.64 | 3.77 |
| Itea omeiensis C. K. Schneid. | S12 | 4.26 | 2.33 | 1.01 |
| Maesa japonica Maesa japonica | S13 | 4.83 | 0 | 1.00 |
| Rhododendron tsoi Merr. | S14 | 1.00 | 4.12 | 0 |
| Rhododendron ovatum (Lindl.) Planch. ex Maxim. | S15 | 3.76 | 3.77 | 1.03 |
| Stranvaesia davidiana Decne. | S16 | 0 | 0 | 3.46 |
| Rhododendron simiarum Hance | S17 | 0 | 4.84 | 1.00 |
| Rhododendron latoucheae Franch. | S18 | 2.67 | 1.12 | 2.02 |
| Rubus corchorifolius L. f. | S19 | 0 | 1.17 | 2.89 |
| Vaccinium trichocladum Merr. and F. P. Metcalf | S20 | 1.92 | 1.81 | 0 |
| Prunus spinulosa Siebold and Zucc. | S21 | 3.24 | 1.00 | 1.01 |
| Smilax polycolea Warb. | S22 | 0 | 2.88 | 0 |
| Stauntonia obovatifoliola subsp. urophylla (Hand.-Mazz.) H. N. Qin | S23 | 3.03 | 3.77 | 1.00 |
References
- Spehn, E.M.; Joshi, J.; Schmid, B.; Diemer, M.; Körner, C. Above-ground resource use increases with plant species richness in experimental grassland ecosystems. Funct. Ecol. 2000, 14, 326–337. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Wang, J.; Ma, W.; Meng, J. Species association of the dominant tree species in an old-growth forest and implications for enrichment planting for the restoration of natural degraded forest in subtropical China. Forests 2019, 10, 957. [Google Scholar] [CrossRef]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar] [CrossRef]
- Peralta, G.; Perry, G.L.; Vázquez, D.P.; Dehling, D.M.; Tylianakis, J.M. Strength of niche processes for species interactions is lower for generalists and exotic species. J. Anim. Ecol. 2020, 89, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Falster, D.S.; Brännström, Å.; Westoby, M.; Dieckmann, U. Multitrait successional forest dynamics enable diverse competitive coexistence. Proc. Natl. Acad. Sci. USA 2017, 114, E2719–E2728. [Google Scholar] [CrossRef]
- Fox Jeremy, W.; Morin Peter, J. Effects of intra-and interspecific interactions on species responses to environmental change. J. Anim. Ecol. 2001, 70, 80–90. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K.; Riggle, R.; Brannen, P.; Madigan, B.; Beard, S. Spatial variability of soil properties in the shortgrass steppe: The relative importance of topography, grazing, microsite, and plant species in controlling spatial patterns. Ecosystems 1999, 2, 422–438. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, H.; Shu, X.; Xie, F.; Zhang, K.; Zhang, Q.; Dang, H. Spatial distribution and interspecific associations in a deciduous broad-leaved forest in north-central China. J. Veg. Sci. 2019, 30, 1153–1163. [Google Scholar] [CrossRef]
- Vleminckx, J.; Barrantes, O.V.; Fortunel, C.; Paine, C.T.; Bauman, D.; Engel, J.; Petronelli, P.; Dávila, N.; Rios, M.; Valderrama Sandoval, E.H. Niche breadth of Amazonian trees increases with niche optimum across broad edaphic gradients. Ecology 2023, 104, e4053. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.E.; Brown, P.; Tan, S.; Davies, S.J. Interspecific demographic trade-offs and soil-related habitat associations of tree species along resource gradients. J. Ecol. 2008, 96, 192–203. [Google Scholar] [CrossRef]
- Marpaung, B.A.; Budiadi, B.; Pertiwiningrum, A.; Lestari, L.D.; Nurjanto, H.H.; Widiyatno, W. Interspecific associations of mangrove species and their preferences for edaphic factors and water quality. Biodiversitas J. Biol. Divers. 2022, 23, 4626–4635. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef] [PubMed]
- Kerfahi, D.; Dong, K.; Yang, Y.; Kim, H.; Takahashi, K.; Adams, J. Elevation trend in bacterial functional gene diversity decouples from taxonomic diversity. Catena 2021, 199, 105099. [Google Scholar] [CrossRef]
- Ahmad, M.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R.; Lone, G.R.; Kaur, S.; Jaryan, V.; Kohli, R.K. Niche width analyses facilitate identification of high-risk endemic species at high altitudes in western Himalayas. Ecol. Indic. 2021, 126, 107653. [Google Scholar] [CrossRef]
- Yang, S.; Feng, C.; Ma, Y.; Wang, W.; Huang, C.; Qi, C.; Fu, S.; Chen, H.Y. Transition from N to P limited soil nutrients over time since restoration in degraded subtropical broadleaved mixed forests. For. Ecol. Manag. 2021, 494, 119298. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Körner, C. Why are there global gradients in species richness? Mountains might hold the answer. Trends Ecol. Evol. 2000, 15, 513–514. [Google Scholar] [CrossRef]
- Kröber, W.; Böhnke, M.; Welk, E.; Wirth, C.; Bruelheide, H. Leaf trait-environment relationships in a subtropical broadleaved forest in South-East China. PLoS ONE 2012, 7, e35742. [Google Scholar] [CrossRef]
- Pei, N.; Lian, J.-Y.; Erickson, D.L.; Swenson, N.G.; Kress, W.J.; Ye, W.-H.; Ge, X.-J. Exploring tree-habitat associations in a Chinese subtropical forest plot using a molecular phylogeny generated from DNA barcode loci. PLoS ONE 2011, 6, e21273. [Google Scholar] [CrossRef]
- Umaña, M.N.; Swenson, N.G. Intraspecific variation in traits and tree growth along an elevational gradient in a subtropical forest. Oecologia 2019, 191, 153–164. [Google Scholar] [CrossRef]
- Xu, P.; Li, Y.; Zhang, S.; Liu, B. Features of Rodent Species Diversity in Wuyi Mountain National Nature Reserve, Jiangxi Province, China. Pak. J. Zool. 2023, 55, 1501–2000. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Ruan, H.; Luo, Y.; Wang, J. Temperature sensitivity increases with soil organic carbon recalcitrance along an elevational gradient in the Wuyi Mountains, China. Soil Biol. Biochem. 2010, 42, 1811–1815. [Google Scholar] [CrossRef]
- Jin, X.; Lin, S.; Zhu, J.; Tan, F.; Zhang, H.; Chen, Q.; Hong, Y.; Liu, J.; Xu, D.; He, Z. Dominant Tree Species and Their Age Groups Drive Forest Carbon Storage in Wuyi Mountain National Park, China. Forests 2024, 15, 546. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Liu, C.; Ding, X.; Wang, Y. Species niche and interspecific associations alter flora structure along a fertilization gradient in an alpine meadow of Tianshan Mountain, Xinjiang. Ecol. Indic. 2023, 147, 109953. [Google Scholar] [CrossRef]
- Lu, S.; Xu, Y.; Fu, X.; Xiao, H.; Ding, W.; Zhang, Y. Patterns and drivers of soil respiration and vegetation at different altitudes in Southern China. Appl. Ecol. Environ. Res. 2019, 17, 3097–3106. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Li, M.; Sun, J.; Lyu, M.; Zhong, Q.; Cheng, D. Convergent nitrogen–phosphorus scaling relationships in different plant organs along an elevational gradient. AoB Plants 2020, 12, plaa021. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, Y.; Jiang, Y.; Chen, M.; Zhang, H.; Wu, P.; Ma, X. Dynamics of non-structural carbohydrates release in Chinese fir topsoil and canopy litter at different altitudes. Plants 2023, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, Y.; Peng, S.; Hua, W.; Luo, B.; Hui, D. Impacts of altitude on plant green leaf, fresh litter, and soil stoichiometry in subtropical forests. Front. For. Glob. Chang. 2024, 7, 1331623. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Liu, B.; Zhang, J.; Hua, J.; Liu, D.; Bhople, P.; Zhang, Y.; Zhang, H.; Zhang, C. Exploration of soil microbial diversity and community structure along mid-subtropical elevation gradients in southeast China. Forests 2023, 14, 769. [Google Scholar] [CrossRef]
- Zhu, G.; Niklas, K.J.; Li, M.; Sun, J.; Lyu, M.; Chen, X.; Wang, M.; Zhong, Q.; Cheng, D. “Diminishing Returns” in the scaling between leaf area and twig size in three forest communities along an elevation gradient of Wuyi Mountain, China. Forests 2019, 10, 1138. [Google Scholar] [CrossRef]
- Walker, B.H. Biodiversity and ecological redundancy. Conserv. Biol. 1992, 6, 18–23. [Google Scholar] [CrossRef]
- Chen, S.; Xie, L.; Zhou, W.; Chen, H.; Xu, X.; Jiang, S.; Zang, M.; Peng, Y.; Chen, X.; Duan, Y. Species diversity has a positive interrelationship with aboveground biomass and a mismatch with productivity in a subtropical broadleaf forest on the Wuyi Mountains, China. Diversity 2022, 14, 952. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, H.; Han, Y. Effects of microclimate, litter type, and mesh size on leaf litter decomposition along an elevation gradient in the Wuyi Mountains, China. Ecol. Res. 2010, 25, 1113–1120. [Google Scholar] [CrossRef]
- Chen, L.; Comita, L.S.; Wright, S.J.; Swenson, N.G.; Zimmerman, J.K.; Mi, X.; Hao, Z.; Ye, W.; Hubbell, S.P.; Kress, W.J. Forest tree neighborhoods are structured more by negative conspecific density dependence than by interactions among closely related species. Ecography 2018, 41, 1114–1123. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Díaz, M. The role of temporal shrub encroachment for the maintenance of Spanish holm oak Quercus ilex dehesas. For. Ecol. Manag. 2008, 255, 1976–1983. [Google Scholar] [CrossRef]
- De’Ath, G. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.C.; Hernández, C.; Romoleroux, K.; Losos, E.; Magård, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Arbainsyah; de Iongh, H.; Kustiawan, W.; De Snoo, G. Structure, composition and diversity of plant communities in FSC-certified, selectively logged forests of different ages compared to primary rain forest. Biodivers. Conserv. 2014, 23, 2445–2472. [Google Scholar] [CrossRef]
- Kikvidze, Z. Plant species associations in alpine-subnival vegetation patches in the Central Caucasus. J. Veg. Sci. 1993, 4, 297–302. [Google Scholar] [CrossRef]
- Ofomata, V.; Overholt, W.; Van Huis, A.; Egwuatu, R.; Ngi-Song, A. Niche overlap and interspecific association between Chilo partellus and Chilo orichalcociliellus on the Kenya coast. Entomol. Exp. Et Appl. 1999, 93, 141–148. [Google Scholar] [CrossRef]
- Su, S.-j.; Liu, J.-f.; He, Z.-s.; Zheng, S.-q.; Hong, W.; Xu, D.-w. Ecological species groups and interspecific association of dominant tree species in Daiyun Mountain National Nature Reserve. J. Mt. Sci. 2015, 12, 637–646. [Google Scholar] [CrossRef]
- Rousset, O.; Lepart, J. Positive and negative interactions at different life stages of a colonizing species (Quercus humilis). J. Ecol. 2000, 88, 401–412. [Google Scholar] [CrossRef]
- Turkington, R.A.; Cavers, P.B.; Aarssen, L.W. Neighbour relationships in grass–legume communities. I. Interspecific contacts in four grassland communities near London, Ontario. Can. J. Bot. 1977, 55, 2701–2711. [Google Scholar] [CrossRef]
- de Jong, P.; Aarssen, L.W.; Turkington, R. The analysis of contact sampling data. Oecologia 1980, 45, 322–324. [Google Scholar] [CrossRef]
- Hurlbert, S.H. A coefficient of interspecific assciation. Ecology 1969, 50, 1–9. [Google Scholar] [CrossRef]
- Chai, Z.; Sun, C.; Wang, D.; Liu, W. Interspecific associations of dominant tree populations in a virgin old-growth oak forest in the Qinling Mountains, China. Bot. Stud. 2016, 57, 23. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Chen, D.; Luo, T.; Mo, J.; Luo, W.; Chen, H.; Jiang, Z. Division of ecological species groups and functional groups based on interspecific association—A case study of the tree layer in the tropical lowland rainforest of Jianfenling in Hainan Island, China. Front. For. China 2008, 3, 407–415. [Google Scholar] [CrossRef]
- Levins, R. Evolution in changing environments: Some theoretical explorations. BioScience 1969, 19, 659–660. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Yang, J.; Su, P.; Zhou, Z.; Shi, R.; Ding, X. Environmental filtering rather than dispersal limitation dominated plant community assembly in the Zoige Plateau. Ecol. Evol. 2022, 12, e9117. [Google Scholar] [CrossRef]
- Zhang, W. Interspecific associations and community structure: A local survey and analysis in a grass community. Selforganizology 2014, 1, 89–129. [Google Scholar]
- Jin, S.S.; Zhang, Y.Y.; Zhou, M.L.; Dong, X.M.; Chang, C.H.; Wang, T.; Yan, D.F. Interspecific association and community stability of tree species in natural secondary forests at different altitude gradients in the southern Taihang Mountains. Forests 2022, 13, 373. [Google Scholar] [CrossRef]
- Dai, J.; Liu, H.; Xu, C.; Qi, Y.; Zhu, X.; Zhou, M.; Liu, B.; Wu, Y. Divergent hydraulic strategies explain the interspecific associations of co-occurring trees in forest–steppe ecotone. Forests 2020, 11, 942. [Google Scholar] [CrossRef]
- Nguyen, V.; Pham, V.; Bui, T.; Nguyen, H. Niche and Interspecific Association of Dominant Tree Species in an Evergreen Broadleaved Forest in Southern Vietnam. Mosc. Univ. Biol. Sci. Bull. 2023, 78, 89–99. [Google Scholar] [CrossRef]
- Tetemke, B.A.; Birhane, E.; Rannestad, M.M.; Eid, T. Species diversity and stand structural diversity of woody plants predominantly determine aboveground carbon stock of a dry Afromontane forest in Northern Ethiopia. For. Ecol. Manag. 2021, 500, 119634. [Google Scholar] [CrossRef]
- Bruun, H.H.; Moen, J.; Virtanen, R.; Grytnes, J.A.; Oksanen, L.; Angerbjörn, A. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci. 2010, 17, 37–46. [Google Scholar] [CrossRef]
- Brown, J.H. Mammals on mountainsides: Elevational patterns of diversity. Glob. Ecol. Biogeogr. 2001, 10, 101–109. [Google Scholar] [CrossRef]
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Körner, C. Concepts in alpine plant ecology. Plants 2023, 12, 2666. [Google Scholar] [CrossRef]
- Silvertown, J.; Charlesworth, D. Introduction to Plant Population Biology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wang, G.; Zhou, G.; Yang, L.; Li, Z. Distribution, species diversity and life-form spectra of plant communities along an altitudinal gradient in the northern slopes of Qilianshan Mountains, Gansu, China. Plant Ecol. 2003, 165, 169–181. [Google Scholar] [CrossRef]
- D’Andrea, R.; Ostling, A. Challenges in linking trait patterns to niche differentiation. Oikos 2016, 125, 1369–1385. [Google Scholar] [CrossRef]
- Levin, S.A. Community equilibria and stability, and an extension of the competitive exclusion principle. Am. Nat. 1970, 104, 413–423. [Google Scholar] [CrossRef]
- Abrams, P. The theory of limiting similarity. Annu. Rev. Ecol. Syst. 1983, 14, 359–376. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Silvertown, J.; Dodd, M.E.; Gowing, D.J.; Mountford, J.O. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 1999, 400, 61–63. [Google Scholar] [CrossRef]
- Pandey, R.; Rawat, M.; Singh, V.; Yousefpour, R.; Reshi, Z.A. Large scale field-based evaluation of niche breadth, niche overlap and interspecific association of Western Himalayan temperate forest tree species. Ecol. Indic. 2023, 146, 109876. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Competition and the structure of bird communities. Evolution 1975, 29, 581–585. [Google Scholar] [CrossRef]
- Müller, R.; Nowicki, C.; Barthlott, W.; Ibisch, P.L. Biodiversity and endemism mapping as a tool for regional conservation planning–case study of the Pleurothallidinae (Orchidaceae) of the Andean rain forests in Bolivia. Biodivers. Conserv. 2003, 12, 2005–2024. [Google Scholar] [CrossRef]
- Hurlbert, S.H. The measurement of niche overlap and some relatives. Ecology 1978, 59, 67–77. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Alexander, J.M. Competition contributes to both warm and cool range edges. Nat. Commun. 2022, 13, 2502. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gao, J.; Ou, W.; Wan, J.; Li, X. Effects of the hummock–depression microhabitat on plant communities of alpine marshy meadows in the Yellow River Source Zone, China. J. Plant Ecol. 2022, 15, 111–128. [Google Scholar] [CrossRef]
- Banerji, A. Acquisition of adaptive traits via interspecific association: Ecological consequences and applications. Ecologies 2021, 2, 43–70. [Google Scholar] [CrossRef]
- Gu, L.; Gong, Z.-w.; Li, W.-z. Niches and interspecific associations of dominant populations in three changed stages of natural secondary forests on Loess Plateau, PR China. Sci. Rep. 2017, 7, 6604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Liu, Q.; Liu, Z. Spatial patterns and interspecific associations during natural regeneration in three types of secondary forest in the central part of the Greater Khingan Mountains, Heilongjiang Province, China. Forests 2020, 11, 152. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Purves, D.W.; Murrell, D.J.; Dieckmann, U. Causes and effects of small-scale spatial structure in plant populations. Anal. Biochem. 2002. Available online: https://pure.iiasa.ac.at/6745/1/IR-02-040.pdf (accessed on 10 July 2024).
 non-significant positive association; □ non-significant negative association; ○ significant negative association; ▽ extreme significant negative association. The scientific names of trees and shrubs are shown in Table 1.
non-significant positive association; □ non-significant negative association; ○ significant negative association; ▽ extreme significant negative association. The scientific names of trees and shrubs are shown in Table 1.
 non-significant positive association; □ non-significant negative association; ○ significant negative association; ▽ extreme significant negative association. The scientific names of trees and shrubs are shown in Table 1.
non-significant positive association; □ non-significant negative association; ○ significant negative association; ▽ extreme significant negative association. The scientific names of trees and shrubs are shown in Table 1.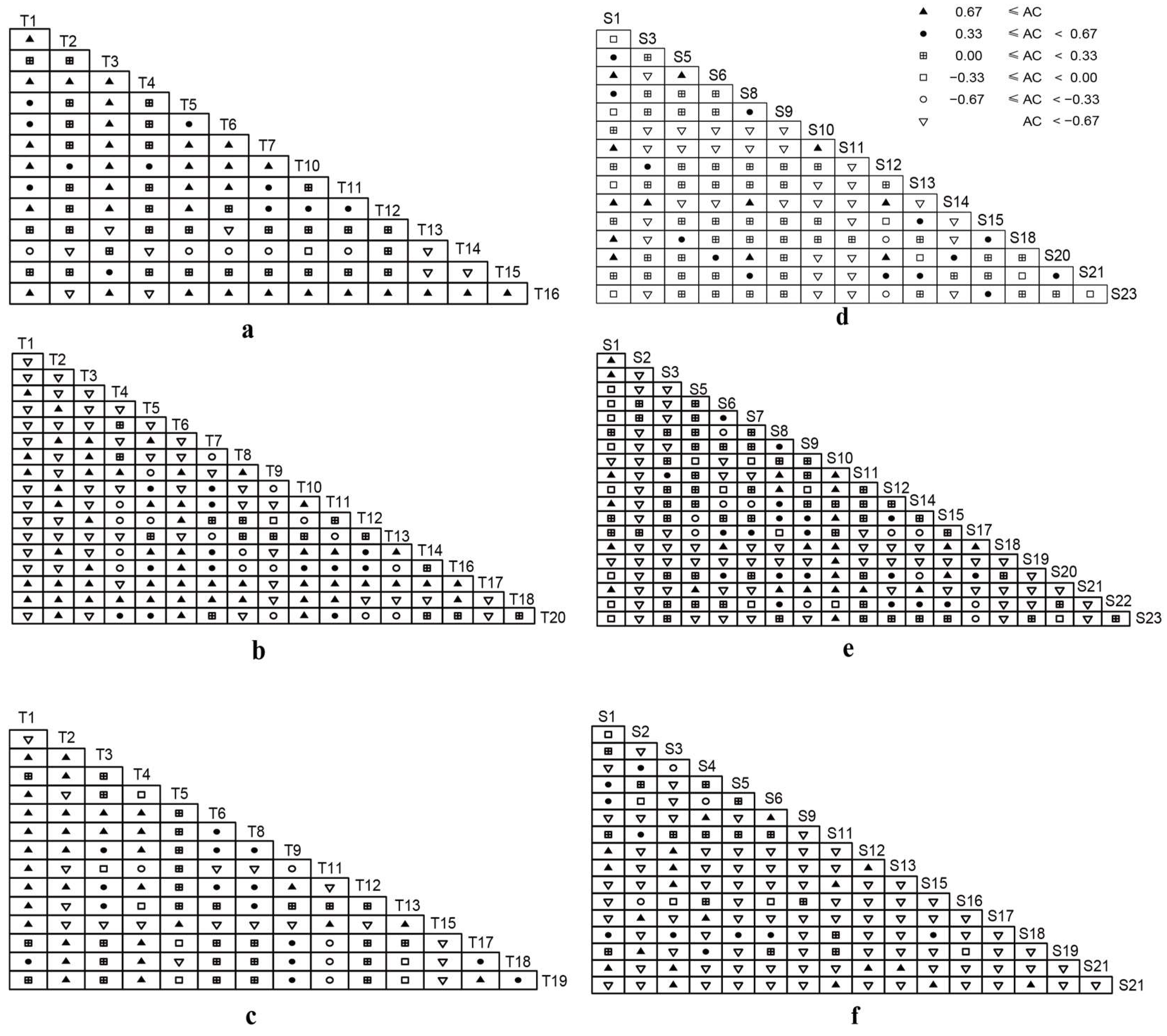

| Tree Layer | Shrub Layer | ||||||
|---|---|---|---|---|---|---|---|
| Species | Family | No. | IV/% | Species | Family | No. | IV/% |
| Rhododendron latoucheae Franch. | Ericaceae | T1 | 19.11% | Begonia acetosella Craib | Begoniaceae | S1 | 5.92% |
| Pinus taiwanensis Hayata | Pinaceae | T2 | 11.97% | Enkianthus quinqueflorus Lour. | Ericaceae | S2 | 5.34% |
| Schima superba Gardner and Champ. | Theaceae | T3 | 8.21% | Mahonia bealei (Fortune) Carr. | Berberidaceae | S3 | 3.69% |
| Enkianthus quinqueflorus Lour. | Ericaceae | T4 | 7.12% | Vaccinium japonicum Miq. | Ericaceae | S4 | 3.50% |
| Rhododendron ovatum (Lindl.) Planch. ex Maxim. | Ericaceae | T5 | 5.15% | Eurya muricata Dunn | Pentaphylacaceae | S5 | 3.50% |
| Vaccinium sprengelii (G. Don) Sleumer | Ericaceae | T6 | 4.62% | Rhododendron simsii Planch. | Ericaceae | S6 | 3.30% |
| Adinandra millettii (Hook. and Arn.) Benth. and Hook. f. ex Hance | Pentaphylacaceae | T7 | 3.33% | Symplocos glomerata King ex Gamble | Symplocaceae | S7 | 2.72% |
| Rhododendron taronense Hutch. | Ericaceae | T8 | 3.29% | Ilex wilsonii Loes. | Aquifoliaceae | S8 | 2.14% |
| Rhododendron basilicum Balf. f. and W. W. Sm. | Ericaceae | T9 | 3.14% | Prunus mume Siebold and Zucc. | Rosaceae | S9 | 2.04% |
| Rhododendron farrerae Sweet | Ericaceae | T10 | 2.09% | Vaccinium sprengelii (G. Don) Sleumer | Ericaceae | S10 | 1.94% |
| Eurya muricata Dunn | Pentaphylacaceae | T11 | 1.75% | Litsea cubeba (Lour.) Pers. | Lauraceae | S11 | 1.94% |
| Quercus multinervis (W. C. Cheng and T. Hong) J. Q. Li | Fagaceae | T12 | 1.61% | Itea omeiensis C. K. Schneid. | Iteaceae | S12 | 1.84% |
| Quercus glauca Thunb. | Fagaceae | T13 | 1.47% | Maesa japonica Maesa japonica | Primulaceae | S13 | 1.75% |
| Castanopsis eyrei (Champ. ex Benth.) Tutcher | Fagaceae | T14 | 1.35% | Rhododendron tsoi Merr. | Ericaceae | S14 | 1.75% |
| Engelhardia roxburghiana Wall. | Juglandaceae | T15 | 1.30% | Rhododendron ovatum (Lindl.) Planch. ex Maxim. | Ericaceae | S15 | 1.65% |
| Acer rubrum L. | Sapindaceae | T16 | 1.21% | Stranvaesia davidiana Decne. | Rosaceae | S16 | 1.55% |
| Tsuga chinensis (Franch.) E. Pritz. | Pinaceae | T17 | 1.20% | Rhododendron simiarum Hance | Ericaceae | S17 | 1.55% |
| Rhododendron fortunei Lindl. | Ericaceae | T18 | 1.11% | Rhododendron latoucheae Franch. | Ericaceae | S18 | 1.55% |
| Pyrus calleryana Decne. | Rosaceae | T19 | 1.05% | Rubus corchorifolius L. f. | Rosaceae | S19 | 1.46% |
| Clethra barbinervis Siebold and Zucc. | Clethraceae | T20 | 1.01% | Vaccinium trichocladum Merr. and F. P. Metcalf | Ericaceae | S20 | 1.17% |
| Prunus spinulosa Siebold and Zucc. | Rosaceae | S21 | 1.17% | ||||
| Smilax polycolea Warb. | Smilacaceae | S22 | 1.17% | ||||
| Stauntonia obovatifoliola subsp. urophylla (Hand.-Mazz.) H. N. Qin | Lardizabalaceae | S23 | 1.17% | ||||
| Elevation Range Category | Sample Plot No. | Elevation (m) | Forest Type | Main Dominant Species | |
|---|---|---|---|---|---|
| Tree Layer | Shrub Layer | ||||
| H1 | 34 | 560 | Evergreen broad-leaved forests | Castanopsis eyrei, Quercus glauca, Vaccinium sprengelii, Rhododendron ovatum | Itea omeiensis, Rhododendron ovatum, Begonia acetosella, Laurocerasus spinulosa |
| 32 | 600 | ||||
| 1 | 690 | ||||
| 33 | 710 | ||||
| 2 | 750 | ||||
| 22 | 790 | ||||
| 21 | 850 | ||||
| 23 | 900 | ||||
| 31 | 950 | ||||
| 24 | 1000 | ||||
| H2 | 25 | 1050 | Broad-leaved mixed forests | Rhododendron latoucheae, Pinus taiwanensis, Vaccinium sprengelii, Schima superba | Vaccinium sprengelii, Begonia acetosella, Rhododendron simiarum, Rhododendron tsoi |
| 30 | 1100 | ||||
| 29 | 1150 | ||||
| 28 | 1200 | ||||
| 27 | 1250 | ||||
| 26 | 1300 | ||||
| 19 | 1350 | ||||
| 18 | 1400 | ||||
| 17 | 1450 | ||||
| 16 | 1500 | ||||
| H3 | 7 | 1550 | Coniferous forests | Pinus taiwanensis, Enkianthus quinqueflorus, Rhododendron latoucheae, Quercus multinervis | Enkianthus quinqueflorus, Litsea cubeba, Stranvaesia davidiana, Vaccinium japonicum |
| 15 | 1600 | ||||
| 14 | 1650 | ||||
| 13 | 1700 | ||||
| 12 | 1750 | ||||
| 11 | 1800 | ||||
| 10 | 1850 | ||||
| 20 | 1900 | ||||
| 9 | 1950 | ||||
| 6 | 2000 | ||||
| 8 | 2000 | ||||
| 5 | 2050 | ||||
| 4 | 2100 | ||||
| 3 | 2150 | ||||
| Species A | ||||
|---|---|---|---|---|
| Y | N | |||
| Species B | Y | a | b | a + b |
| N | c | d | c + d | |
| a + c | b + d | a + b + c + d | ||
| Layer | Habitat Categories | Variance Ratio (VR) | Test Statistic (W) | Result | |
|---|---|---|---|---|---|
| Tree | H1 | 4.852 | 48.524 | 3.325, 16.918 | Significant positive association |
| H2 | 1.357 | 13.569 | 3.325, 16.918 | Non-significant positive association | |
| H3 | 4.441 | 53.293 | 4.875, 19.675 | Significant positive association | |
| Shrub | H1 | 2.570 | 25.700 | 3.325, 16.918 | Significant positive association |
| H2 | 1.847 | 18.470 | 3.325, 16.918 | Significant positive association | |
| H3 | 0.682 | 9.545 | 4.875, 19.675 | Non-significant negative association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zheng, Z.; Wen, X.; Hu, X.; Lin, Y.; Li, J.; Ni, J.; Wu, C. Variation in Niche and Interspecific Associations across Elevations in Subtropical Forest Communities of the Wuyi Mountains, Southeastern China. Forests 2024, 15, 1256. https://doi.org/10.3390/f15071256
Hu J, Zheng Z, Wen X, Hu X, Lin Y, Li J, Ni J, Wu C. Variation in Niche and Interspecific Associations across Elevations in Subtropical Forest Communities of the Wuyi Mountains, Southeastern China. Forests. 2024; 15(7):1256. https://doi.org/10.3390/f15071256
Chicago/Turabian StyleHu, Jintao, Zhaoliang Zheng, Xinyi Wen, Xisheng Hu, Yongming Lin, Jian Li, Jian Ni, and Chengzhen Wu. 2024. "Variation in Niche and Interspecific Associations across Elevations in Subtropical Forest Communities of the Wuyi Mountains, Southeastern China" Forests 15, no. 7: 1256. https://doi.org/10.3390/f15071256
APA StyleHu, J., Zheng, Z., Wen, X., Hu, X., Lin, Y., Li, J., Ni, J., & Wu, C. (2024). Variation in Niche and Interspecific Associations across Elevations in Subtropical Forest Communities of the Wuyi Mountains, Southeastern China. Forests, 15(7), 1256. https://doi.org/10.3390/f15071256








