Temporal Variations in Enzymatic and Non-Enzymatic Antioxidant Activity in Silver Birch (Betula pendula Roth.): The Genetic Component
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sowing Material and Growth Conditions
2.2. Sampling
2.3. Total Phenol Content
2.3.1. Extract Preparation
2.3.2. Phenol Analysis
2.4. Antioxidant Enzymes
2.4.1. Extract Preparation
2.4.2. Protein Analysis
2.4.3. Superoxide Dismutase Analysis
2.4.4. Catalase Analysis
2.4.5. Ascorbate Peroxidase Analysis
2.4.6. Peroxidase Analysis
2.4.7. Glutathione Reductase Analysis
2.5. Statistical Analysis
3. Results
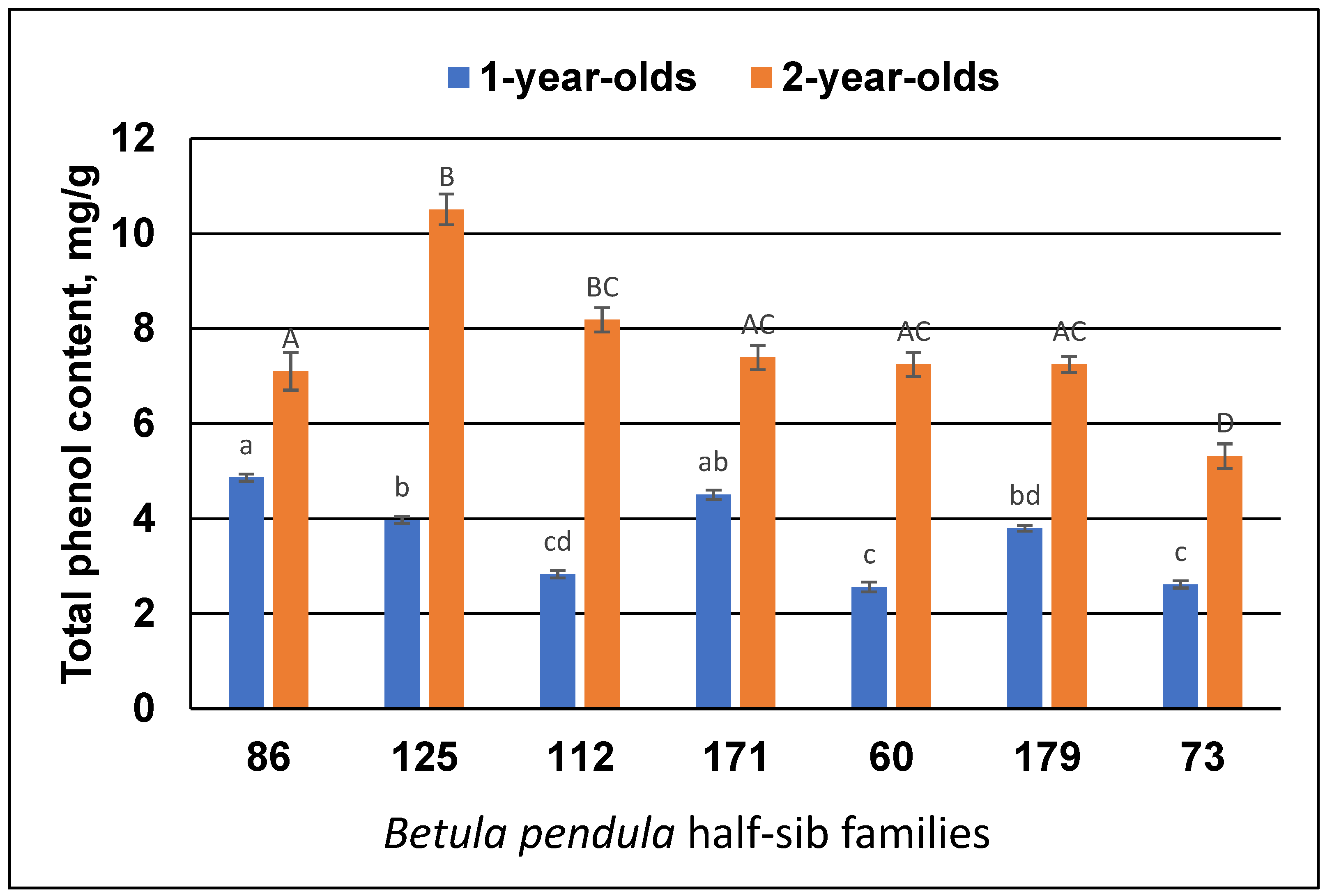
3.1. Height and TPC
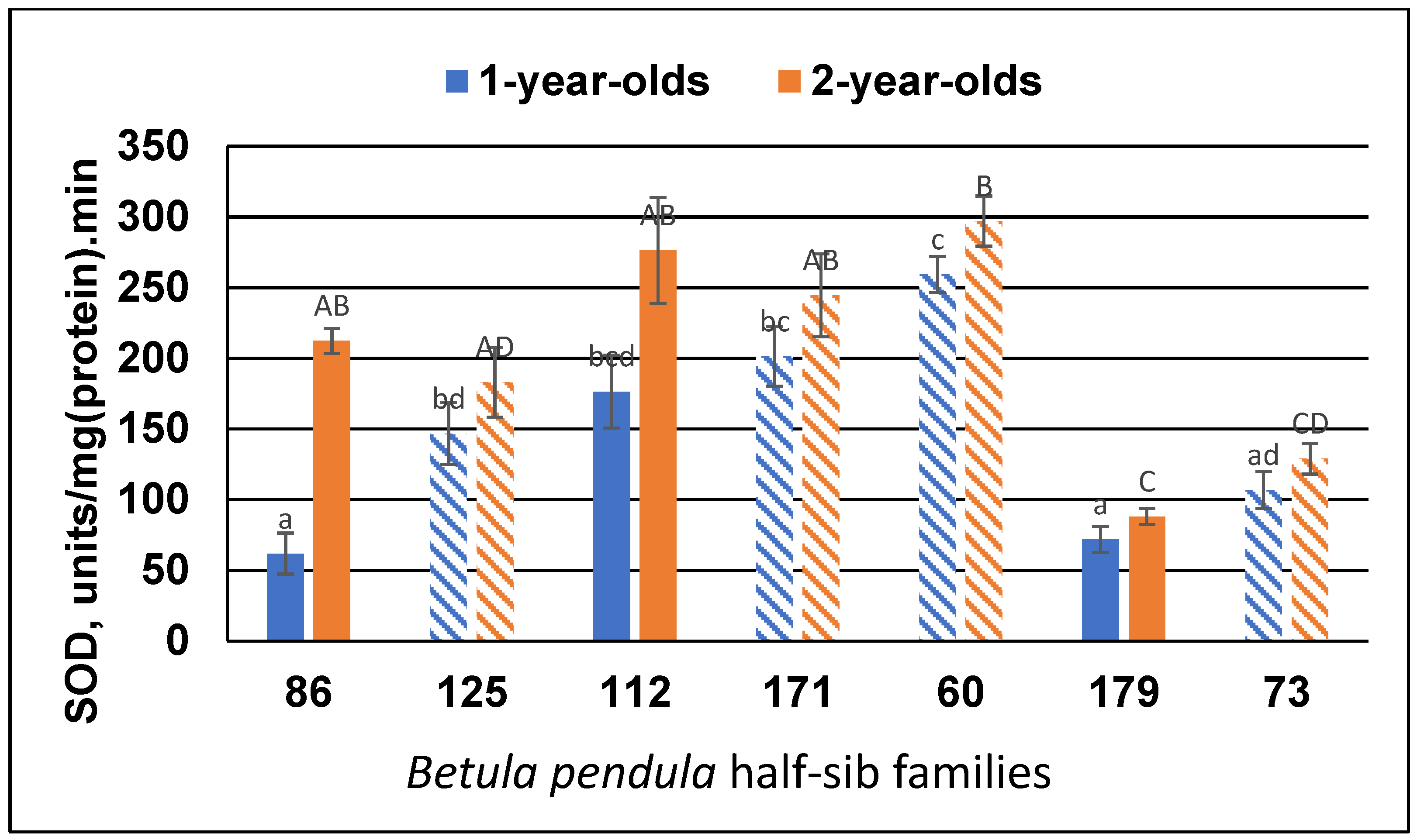
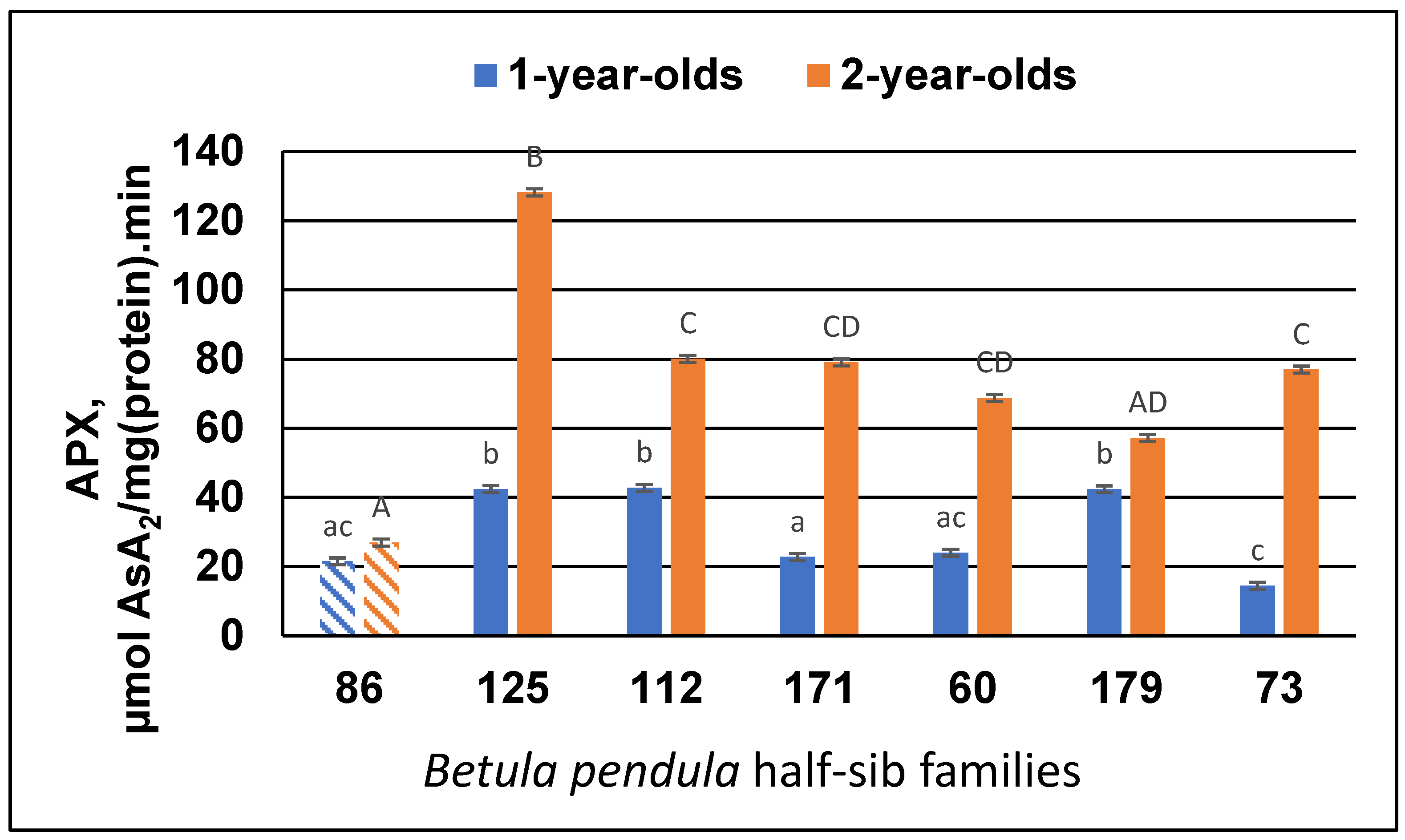
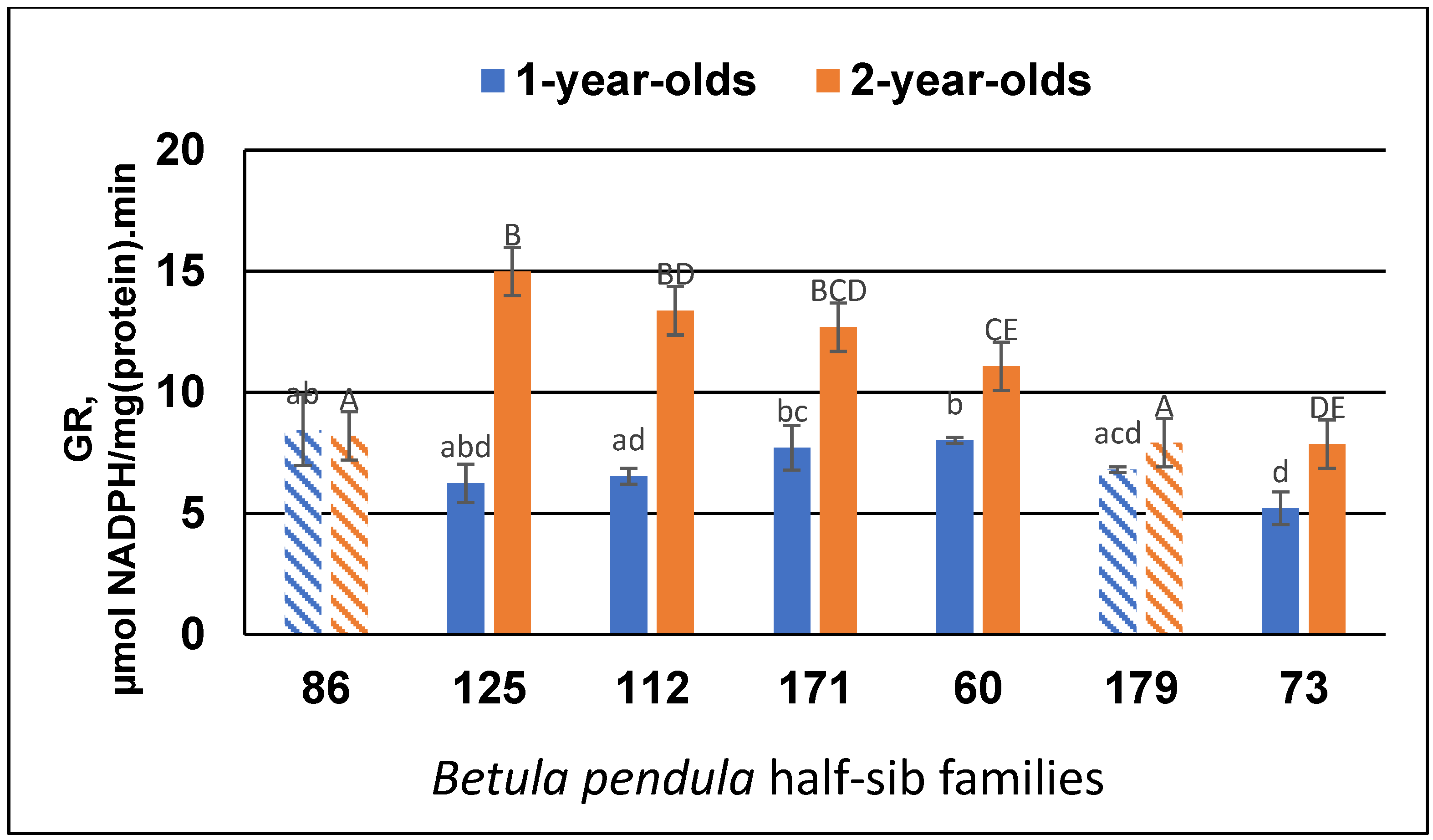
3.2. Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, J.; Niemistö, P.; Viherä-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in Northern Europe. Forestry 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Vanhellemont, M.; Van Acker, J.; Verheyen, K. Exploring life growth patterns in birch (Betula pendula). Scand. J. For. Res. 2016, 31, 561–567. [Google Scholar] [CrossRef]
- Jonczak, J.; Jankiewicz, U.; Kondras, M.; Kruczkowska, B.; Oktaba, L.; Oktaba, J.; Olejniczak, I.; Pawłowicz, E.; Polláková, N.; Raab, T.; et al. The influence of birch trees (Betula spp.) on soil environment—A review. For. Ecol. Manag. 2020, 477, 118486. [Google Scholar] [CrossRef]
- Lewis, J.; Qvarfort, U.; Sjöström, J. Betula pendula: A promising candidate for phytoremediation of tce in northern climates. Int. J. Phytoremediation 2015, 17, 9–15. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of birch (Betula pendula Roth and B. pubescens Ehrh.) for forestry and forest-based industry sector within the changing climatic and socio-economic context of western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Nagybakay, N.E.; Sarapinaite, L.; Syrpas, M.; Venskutonis, P.R.; Kitryte-Syrpa, V. Optimization of pressurized ethanol extraction for efficient recovery of hyperoside and other valuable polar antioxidant-rich extracts from Betula pendula Roth leaves. Ind. Crop Prod. 2023, 205, 117565. [Google Scholar] [CrossRef]
- Germanò, M.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula leaves: Polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012, 83, 877–882. [Google Scholar] [CrossRef]
- Muilenburg, V.L.; Phelan, P.L.; Bonello, P. Inter- and intra-specific variation in stem phloem phenolics of paper birch (Betula papyrifera) and European white birch (Betula pendula). J. Chem. Ecol. 2011, 37, 1193–1202. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.; Gǎluşcan, A.; Cinta-Pinzaru, S.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Cent. J. 2012, 6, 137. [Google Scholar] [CrossRef]
- Kuparinen, A.; Savolainen, O.; Schurr, F.M. Increased mortality can promote evolutionary adaptation of forest trees to climate change. For. Ecol. Manag. 2010, 259, 1003–1008. [Google Scholar] [CrossRef]
- Heimonen, K.; Valtonen, A.; Kontunen-Soppela, S.; Keski-Saari, S.; Rousi, M.; Oksanen, E.; Roininen, H. Insect herbivore damage on latitudinally translocated silver birch (Betula pendula)—Predicting the effects of climate change. Clim. Change 2015, 131, 245–257. [Google Scholar] [CrossRef]
- Tegelberg, R.; Julkunen-Tiitto, R.; Aphalo, P.J. The effects of long-term elevated UV-B on the growth and phenolics of field-grown silver birch (Betula pendula). Glob. Chang. 2001, 7, 839–848. [Google Scholar] [CrossRef]
- Kuokkanen, K.; Julkunen-Tiitto, R.; Keinänen, M.; Niemelä, P.; Tahvanainen, J. The effect of elevated CO2 and temperature on the secondary chemistry of Betula pendula seedlings. Trees 2001, 15, 378–384. [Google Scholar] [CrossRef]
- Ostapiuk, A.; Kurach, Ł.; Strzemski, M.; Kurzepa, J.; Hordyjewska, A. Evaluation of antioxidative mechanisms in vitro and triterpenes composition of extracts from silver birch (Betula pendula Roth) and black birch (Betula obscura Kotula) barks by FT-IR and HPLC-PDA. Molecules 2021, 26, 4633. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.; Julkunen-Tiitto, R. Variation in birch (Betula pendula) Shoot secondary chemistry due to genotype, environment, and ontogeny. J. Chem. Ecol. 2005, 31, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J. Phenolic compounds of the inner bark of Betula pendula: Seasonal and genetic variation and induction by wounding. J. Chem. Ecol. 2012, 38, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Likhanov, A.F.; Vasylyshyn, R.D.; Marchuk, Y.M.; Kurdyuk, O.M.; Honchar, H.Y. Consistency of phenolic profiles with taxonomic distribution and adaptation of birch species (Betula L.) to environmental conditions. Botany 2023, 101, 400–413. [Google Scholar] [CrossRef]
- Nurzynska-Wierdak, R. Phenolic compounds from new natural sources—Plant genotype and ontogenetic variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef]
- Laitinen, M.; Julkunen-Tiitto, R. Variation in phenolic compounds within a birch (Betula pendula) population. J. Chem. Ecol. 2000, 26, 1609–1622. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Mutikainen, P.; Walls, M.; Ovaska, J.; Keinanen, M.; Julkunen-Tiitto, R.; Vapaavuori, E. Herbivore resistance in Betula pendula: Effect of fertilization, defoliation, and plant genotype. Ecology 2000, 81, 49–65. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Žemaitis, P.; Jansons, Ā.; Baliuckas, V. Resistance of Scots pine half-sib families to Heterobasidion annosum in progeny field trials. Silva Fenn. 2020, 54, 10276. [Google Scholar] [CrossRef]
- Paaso, U.; Keski-Saari, S.; Keinänen, M.; Karvinen, H.; Silfver, T.; Cole, C.T. Intrapopulation genotypic variation of foliar secondary chemistry during leaf senescence and litter decomposition in silver birch (Betula pendula). Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Beniušytė, E.; Čėsnienė, I.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Genotype-dependent jasmonic acid effect on Pinus sylvestris L. growth and induced systemic resistance indicators. Plants 2023, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Čėsnienė, I.; Miškelytė, D.; Novickij, V.; Mildažienė, V.; Sirgedaitė-Šėžienė, V. Seed treatment with electromagnetic field induces different effects on emergence, growth and profiles of biochemical compounds in seven half-sib families of silver birch. Plants 2023, 12, 3048. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, H.; Wróblewska, H.; Wojtan, R.; Sajdak, M. The effect of tree age on the chemical composition of the wood of silver birch (Betula pendula Roth.) in Poland. Wood Sci. Technol. 2019, 53, 1135–1155. [Google Scholar] [CrossRef]
- Wieser, G.; Hecke, K.; Tausz, M.; Häberle, K.-H.; Grams, T.E.E.; Matyssek, R. The influence of microclimate and tree age on the defense capacity of European beech (Fagus sylvatica L.) against oxidative stress. Ann. For. Sci. 2003, 60, 131–135. [Google Scholar] [CrossRef]
- Turfan, N.; Savaci, G.; Sariyildiz, T. Variation in chemical compounds of walnut (Juglans regia L.) leaves with tree age. Artvin Çoruh Üniv. Orman. Fak. Derg. 2020, 21, 124–134. [Google Scholar] [CrossRef]
- Turfan, N.; Alay, M.; Sariyildiz, T. Effect of tree age on chemical compounds of ancient anatolian black pine (Pinus nigra subsp. pallasiana) needles in Northwest Turkey. IForest 2018, 11, 406–410. [Google Scholar] [CrossRef]
- Rosales-Castro, M.; Honorato-Salazar, J.A.; Reyes-Navarrete, M.G.; González-Laredo, R.F. Antioxidant phenolic compounds of ethanolic and aqueous extracts from pink cedar (Acrocarpus fraxinifolius Whight & Arn.) bark at two tree ages. J. Wood Chem. Technol. 2015, 35, 270–279. [Google Scholar] [CrossRef]
- Oleksyn, O.; Prus-Glowacki, W.; Giertych, M.; Reich, P.B. Relation between genetic diversity and pollution impact in a 1912 experiment with East European Pinus sylvestris provenances. Can. J. For. Res. 1994, 24, 2390–2394. [Google Scholar] [CrossRef]
- Vladimirov, M.S.; Nikolic, V.D.; Stanojevic, L.P.; Stanojevic, J.S.; Nikolic, L.B.; Danilovic, B.R.; Marinkovic, V.D. Chemical composition, antimicrobial andantioxidant activity of birch (Betula pendula Roth.) buds essential oil. J. Essent. Oil-Bear. Plants 2019, 22, 120–130. [Google Scholar] [CrossRef]
- Sirgedaitė-Šėžienė, V.; Lučinskaitė, I.; Mildažienė, V.; Ivankov, A.; Koga, K.; Shiratani, M.; Laužikė, K.; Baliuckas, V. Changes in content of bioactive compounds and antioxidant activity induced in needles of different half-sib families of norway spruce (Picea abies (L.) H. Karst) by seed treatment with cold plasma. Antioxidants 2022, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- Sirgedaitė-Šėžienė, V.; Čėsnienė, I.; Leleikaitė, G.; Baliuckas, V.; Vaitiekūnaitė, D. Phenolic and antioxidant compound accumulation of Quercus robur bark diverges based on tree genotype, phenology and extraction method. Life 2023, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Mougiou, N.; Baalbaki, B.; Doupis, G.; Kavroulakis, N.; Poulios, S.; Vlachonasios, K.E.; Koubouris, G.C. The effect of low temperature on physiological, biochemical and flowering functions of olive tree in relation to genotype. Sustainability 2020, 12, 10065. [Google Scholar] [CrossRef]
- Tegischer, K.; Tausz, M.; Wieser, G.; Grill, D. Tree- and needle-age-dependent variations in antioxidants and photoprotective pigments in Norway spruce needles at the alpine timberline. Tree Physiol. 2002, 22, 591–596. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirgedaitė-Šėžienė, V.; Čėsnienė, I.; Vaitiekūnaitė, D. Temporal Variations in Enzymatic and Non-Enzymatic Antioxidant Activity in Silver Birch (Betula pendula Roth.): The Genetic Component. Forests 2024, 15, 1262. https://doi.org/10.3390/f15071262
Sirgedaitė-Šėžienė V, Čėsnienė I, Vaitiekūnaitė D. Temporal Variations in Enzymatic and Non-Enzymatic Antioxidant Activity in Silver Birch (Betula pendula Roth.): The Genetic Component. Forests. 2024; 15(7):1262. https://doi.org/10.3390/f15071262
Chicago/Turabian StyleSirgedaitė-Šėžienė, Vaida, Ieva Čėsnienė, and Dorotėja Vaitiekūnaitė. 2024. "Temporal Variations in Enzymatic and Non-Enzymatic Antioxidant Activity in Silver Birch (Betula pendula Roth.): The Genetic Component" Forests 15, no. 7: 1262. https://doi.org/10.3390/f15071262





