Insect Herbivores, Plant Sex, and Elevated Nitrogen Influence Willow Litter Decomposition and Detritivore Colonization in Early Successional Streams
Abstract
:1. Introduction
2. Materials and Methods
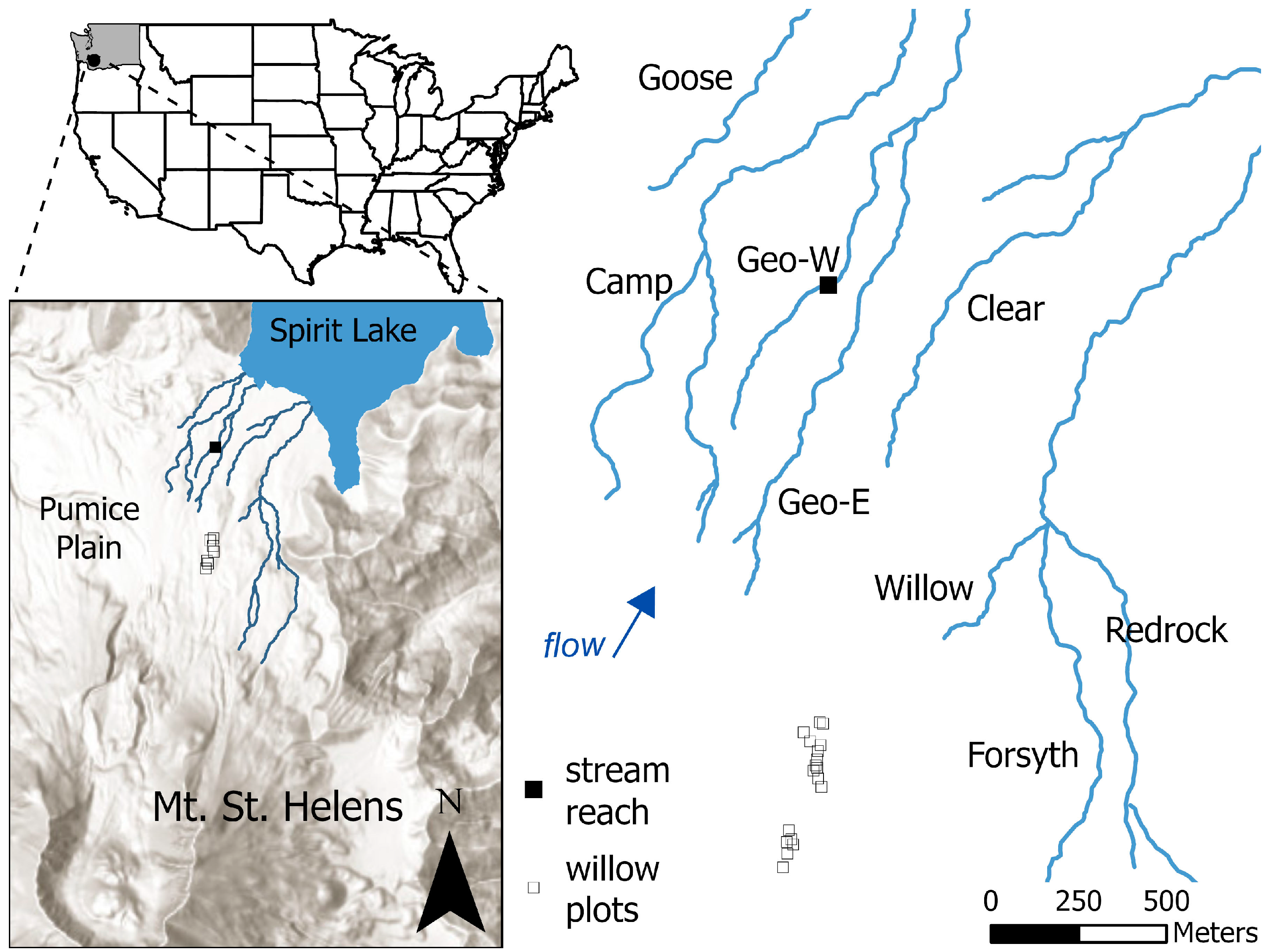
2.1. Site Description
2.2. Stream Conditions
2.3. Terrestrial Herbivory and Nitrogen Treatments
2.4. Leaf Litter Collection
2.5. Leaf Litter Chemistry
2.6. Leaf Litter Decomposition Experiment
2.7. Microbial Communities
2.8. Macroinvertebrate Communities
2.9. Statistical Analysis
3. Results
3.1. Litter Chemistry
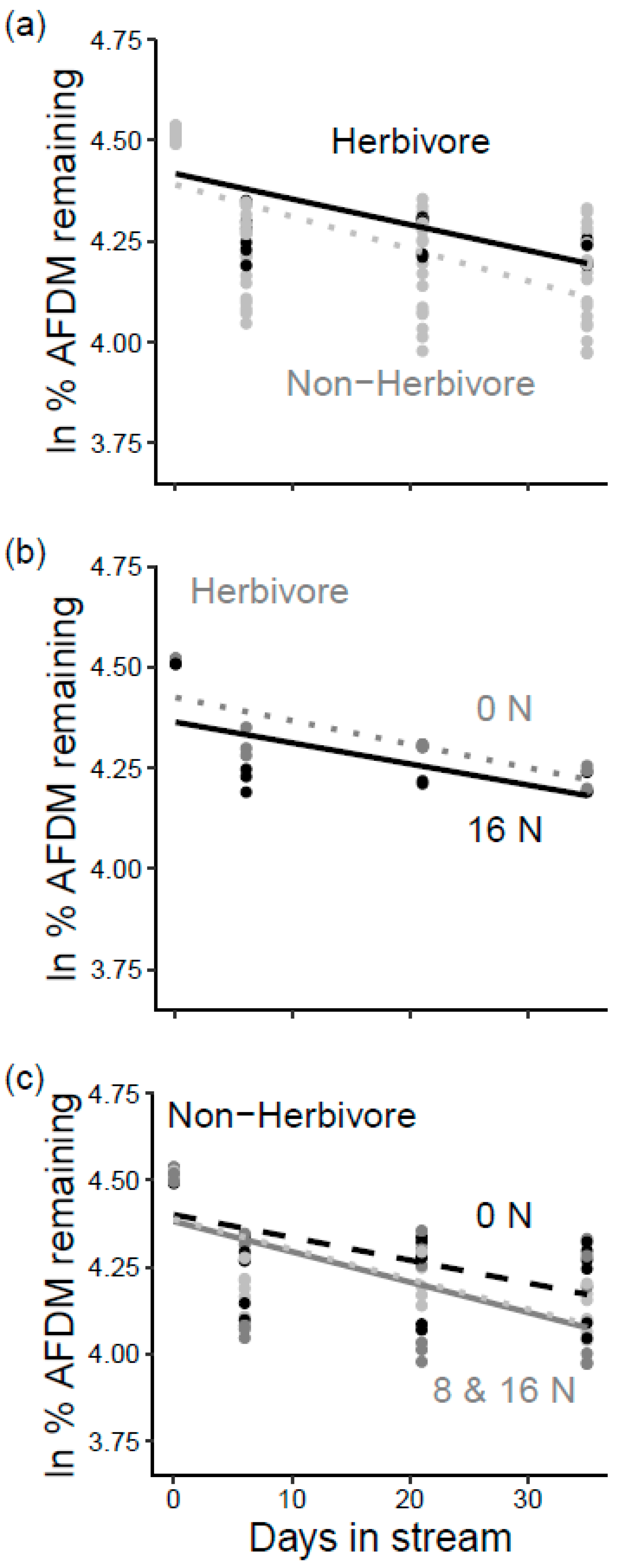
3.2. Litter Decomposition
3.3. Microbial Communities
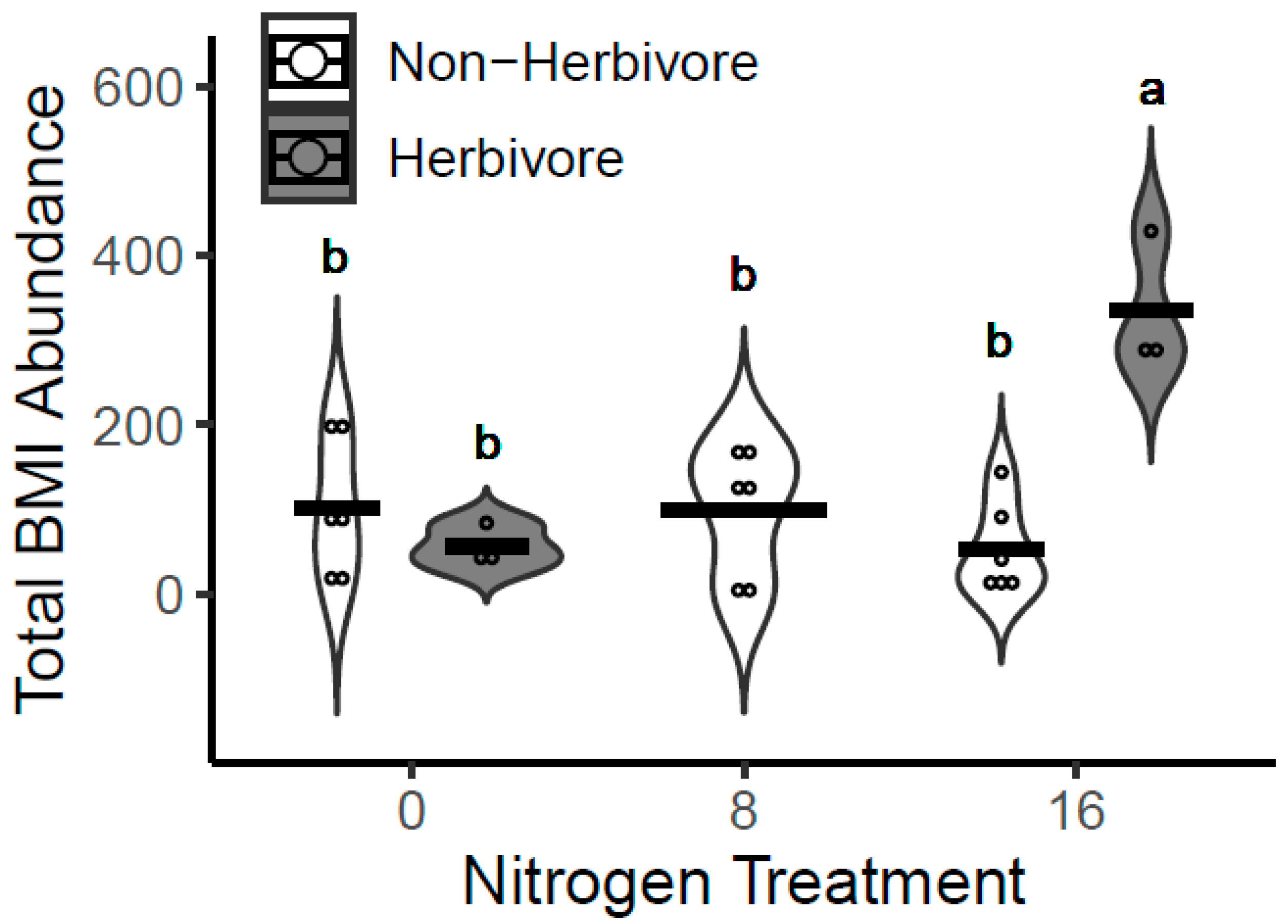
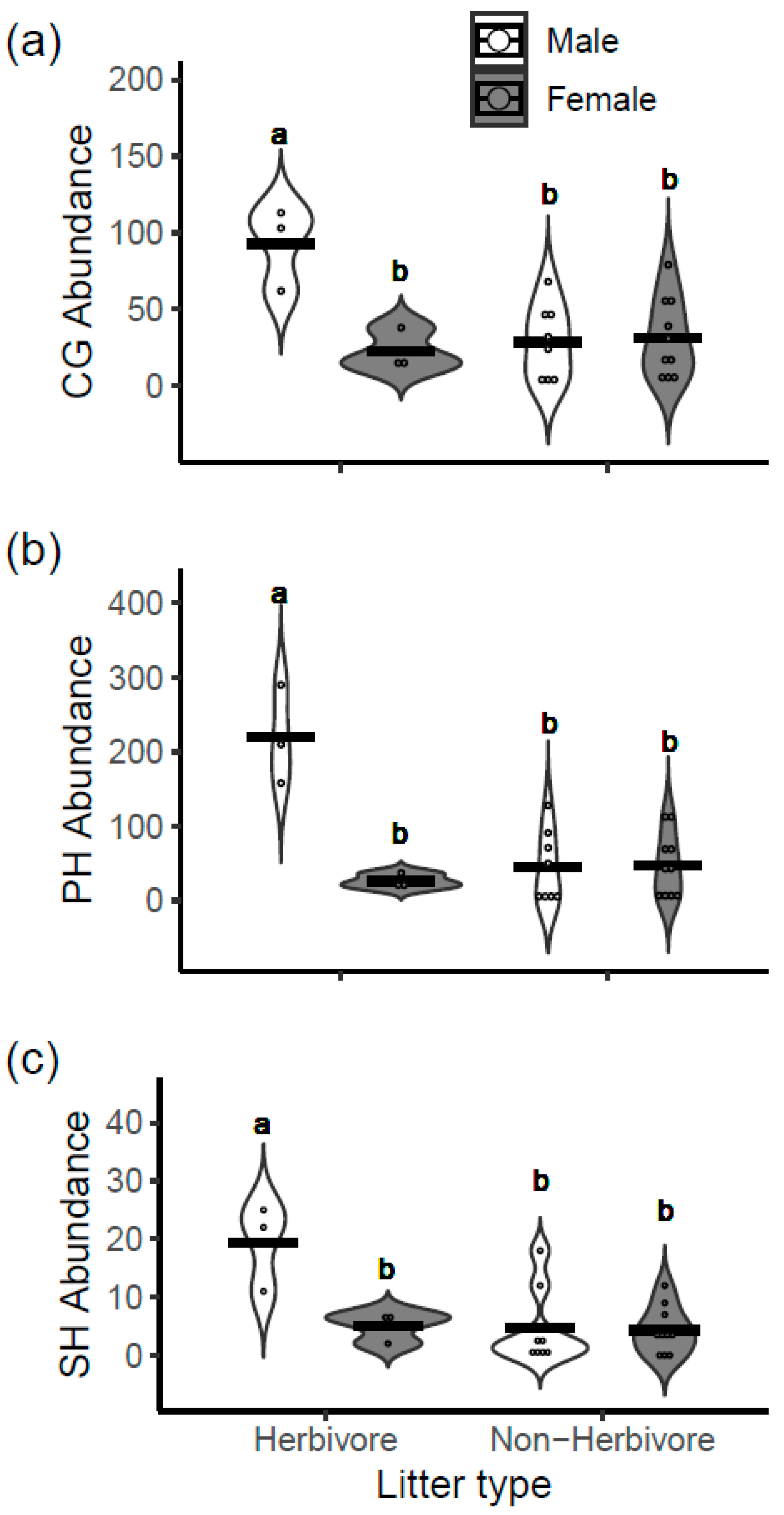
3.4. Benthic Macroinvertebrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplemental Methods
Appendix A.1. Stream Conditions
Appendix A.2. Leaf Litter Chemistry
Appendix A.3. Microbial Communities
References
- Milner, A.M.; Robertson, A.L.; Monaghan, K.A.; Veal, A.J.; Flory, E.A. Colonization and Development of an Alaskan Stream Community over 28 Years. Front. Ecol. Environ. 2008, 6, 413–419. [Google Scholar] [CrossRef]
- Brown, L.E.; Milner, A.M. Rapid Loss of Glacial Ice Reveals Stream Community Assembly Processes. Glob. Chang. Biol. 2012, 18, 2195–2204. [Google Scholar] [CrossRef]
- Claeson, S.M.; LeRoy, C.J.; Finn, D.S.; Stancheva, R.H.; Wolfe, E.R. Variation in Riparian and Stream Assemblages across the Primary Succession Landscape of Mount St. Helens, U.S.A. Freshw. Biol. 2021, 66, 1002–1017. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Claeson, S.M.; Garthwaite, I.J.; Thompson, M.A.; Thompson, L.J.; Kamakawiwo’ole, B.K.; Froedin-Morgensen, A.M.; McConathy, V.; Ramstack Hobbs, J.M.; Stancheva, R.; et al. Canopy Development Influences Early Successional Stream Ecosystem Function but Not Biotic Assemblages. Aquat. Sci. 2023, 85, 77. [Google Scholar] [CrossRef]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Multiple Trophic Levels of a Forest Stream Linked to Terrestrial Litter Inputs. Science 1997, 277, 102–104. [Google Scholar] [CrossRef]
- Graça, M.A.S. The Role of Invertebrates on Leaf Litter Decomposition in Streams—A Review. Int. Rev. Hydrobiol. 2001, 86, 383–393. [Google Scholar] [CrossRef]
- Swan, C.; Palmer, M. Leaf Diversity Alters Litter Breakdown in Piedmont Stream. J. N. Am. Benthol. Soc. 2004, 23, 15–28. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Marks, J.C. Litter Quality, Stream Characteristics and Litter Diversity Influence Decomposition Rates and Macroinvertebrates. Freshw. Biol. 2006, 51, 605–617. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Whitham, T.G.; Wooley, S.C.; Marks, J.C. Within-Species Variation in Foliar Chemistry Influences Leaf-Litter Decomposition in a Utah River. J. N. Am. Benthol. Soc. 2007, 26, 426–438. [Google Scholar] [CrossRef]
- Jackrel, S.L.; Morton, T.C.; Wootton, J.T. Intraspecific Leaf Chemistry Drives Locally Accelerated Ecosystem Function in Aquatic and Terrestrial Communities. Ecology 2016, 97, 2125–2135. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Ramstack Hobbs, J.M.; Claeson, S.M.; Moffett, J.; Garthwaite, I.; Criss, N.; Walker, L. Plant Sex Influences Aquatic–Terrestrial Interactions. Ecosphere 2020, 11, e02994. [Google Scholar] [CrossRef]
- Scheuerell, R.P.; LeRoy, C.J. Plant Sex Influences on Riparian Communities and Ecosystems. Ecol. Evol. 2023, 13, e10308. [Google Scholar] [CrossRef]
- Findlay, S.; Jones, C.G. Exposure of Cottonwood Plants to Ozone Alters Subsequent Leaf Decomposition. Oecologia 1990, 82, 248–250. [Google Scholar] [CrossRef]
- Aneja, M.K.; Sharma, S.; Fleischmann, F.; Stich, S.; Heller, W.; Bahnweg, G.; Munch, J.C.; Schloter, M. Influence of Ozone on Litter Quality and Its Subsequent Effects on the Initial Structure of Colonizing Microbial Communities. Microb. Ecol. 2007, 54, 151–160. [Google Scholar] [CrossRef]
- Tuchman, N.C.; Wahtera, K.A.; Wetzel, R.G.; Teeri, J.A. Elevated Atmospheric CO2 Alters Leaf Litter Quality for Stream Ecosystems: An in Situ Leaf Decomposition Study. Hydrobiologia 2003, 495, 203–211. [Google Scholar] [CrossRef]
- Amani, M.; Graça, M.A.S.; Ferreira, V. Effects of Elevated Atmospheric CO2 Concentration and Temperature on Litter Decomposition in Streams: A Meta-Analysis. Int. Rev. Hydrobiol. 2019, 104, 14–25. [Google Scholar] [CrossRef]
- Wolfe, E.R.; Younginger, B.S.; LeRoy, C.J. Fungal Endophyte-Infected Leaf Litter Alters in-Stream Microbial Communities and Negatively Influences Aquatic Fungal Sporulation. Oikos 2019, 128, 405–415. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Fischer, D.; Schweitzer, J.A.; Bailey, J.K. Aphid Gall Interactions with Forest Tree Genotypes Influence Leaf Litter Decomposition in Streams. Forests 2020, 11, 182. [Google Scholar] [CrossRef]
- Risley, L.S.; Crossley, D.A. Contribution of Herbivore-Caused Greenfall to Litterfall Nitrogen Flux in Several Southern Appalachian Forested Watersheds. Am. Midl. Nat. 1993, 129, 67–74. [Google Scholar] [CrossRef]
- Gulis, V.; Suberkropp, K. Leaf Litter Decomposition and Microbial Activity in Nutrient-Enriched and Unaltered Reaches of a Headwater Stream. Freshw. Biol. 2003, 48, 123–134. [Google Scholar] [CrossRef]
- Ferreira, V.; Castagneyrol, B.; Koricheva, J.; Gulis, V.; Chauvet, E.; Graça, M.A.S. A Meta-Analysis of the Effects of Nutrient Enrichment on Litter Decomposition in Streams. Biol. Rev. 2015, 90, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D. Herbivore Induced Changes in Leaf-Litter Resource Quality: A Neglected Aspect of Herbivory in Ecosystem Nutrient Dynamics. Oikos 1988, 51, 389–393. [Google Scholar] [CrossRef]
- Grime, J.P.; Cornelissen, J.H.C.; Thompson, K.; Hodgson, J.G. Evidence of a Causal Connection between Anti-Herbivore Defence and the Decomposition Rate of Leaves. Oikos 1996, 77, 489–494. [Google Scholar] [CrossRef]
- Findlay, S.; Carreiro, M.; Krischik, V.; Jones, C.G. Effects of Damage to Living Plants on Leaf Litter Quality. Ecol. Appl. 1996, 6, 269–275. [Google Scholar] [CrossRef]
- Faeth, S.H.; Connor, E.F.; Simberloff, D. Early Leaf Abscission: A Neglected Source of Mortality for Folivores. Am. Nat. 1981, 117, 409–415. [Google Scholar] [CrossRef]
- Risley, L.S. The Influence of Herbivores on Seasonal Leaf-Fall: Premature Leaf Abscission and Petiole Clipping. J. Agric. Entomol. 1986, 3, 152–162. [Google Scholar]
- Broberg, C.L.; Borden, J.H.; Humble, L.M. Distribution and Abundance of Cryptorhynchus lapathi on Salix Spp. in British Columbia. Can. J. For. Res. 2002, 32, 561–568. [Google Scholar] [CrossRef]
- Ke, J.; Laskar, D.D.; Chen, S. Biodegradation of Hardwood Lignocellulosics by the Western Poplar Clearwing Borer, Paranthrene robiniae (Hy. Edwards). Biomacromolecules 2011, 12, 1610–1620. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen Additions and Litter Decomposition: A Meta-Analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Pastor, A.; Compson, Z.G.; Dijkstra, P.; Riera, J.L.; Martí, E.; Sabater, F.; Hungate, B.A.; Marks, J.C. Stream Carbon and Nitrogen Supplements during Leaf Litter Decomposition: Contrasting Patterns for Two Foundation Species. Oecologia 2014, 176, 1111–1121. [Google Scholar] [CrossRef]
- Biasi, C.; Graça, M.A.S.; Santos, S.; Ferreira, V. Nutrient Enrichment in Water More than in Leaves Affects Aquatic Microbial Litter Processing. Oecologia 2017, 184, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Swanson, F.J.; Major, J.J. Physical Events, Environments, and Geological—Ecological Interactions at Mount St. Helens: March 1980–2004. In Ecological Responses to the 1980 Eruption of Mount St. Helens; Dale, V.H., Swanson, F.J., Crisafulli, C.M., Eds.; Springer: New York, NY, USA, 2005; pp. 27–44. ISBN 978-0-387-28150-6. [Google Scholar]
- del Moral, R.; Jones, C. Vegetation Development on Pumice at Mount St. Helens, USA. Plant Ecol. 2002, 162, 9–22. [Google Scholar] [CrossRef]
- Garthwaite, I.J.; Froedin-Morgensen, A.; Hartford, S.H.; Claeson, S.M.; Ramstack Hobbs, J.M.; LeRoy, C.J. Summer Flower Pulses: Catkin Litter Processing in Headwater Streams. Fundam. Appl. Limnol. 2021, 195, 243–254. [Google Scholar] [CrossRef]
- Miller-Pierce, M. Ecological Effects of Anthropogenic Nitrogen on Ecological Systems. Doctoral Dissertation, Washington State University, Vancouver, WA, USA, 2017. [Google Scholar]
- Fisher, M.J. The Morphology and Anatomy of the Flowers of the Salicaceae I. Am. J. Bot. 1928, 15, 307–326. [Google Scholar] [CrossRef]
- Ramstack Hobbs, J.M.; Garthwaite, I.J.; Lancaster, L.; Moffett-Dobbs, J.A.; Johnson, K.; Criss, N.; McConathy, V.; James, C.A.; Gipe, A.; Claeson, S.M.; et al. The Influence of Weevil Herbivory on Leaf Litter Chemistry in Dioecious Willows. Ecol. Evol. 2022, 12, e9626. [Google Scholar] [CrossRef] [PubMed]
- Jenny, H.; Gessel, S.P.; Bingham, F.T. Comparative Study of Decomposition Rates of Organic Matter in Temperate and Tropical Regions. Soil Sci. 1949, 68, 419–432. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. (Eds.) An Introduction to the Aquatic Insects of North America, 4th ed.; Kendall Hunt Publishing: Dubuque, IA, USA, 2019; ISBN 978-0-7575-6321-8. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. User’s Guide for iNEXT Online: Software for Interpolation and Extrapolation of Species Diversity; Institute of Statistics, National Tsing Hua University: Hsin-Chu, Taiwan, 2022. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; Software Design: Gleneden Beach, OR, USA, 2002; ISBN 978-0-9721290-0-8. [Google Scholar]
- Fernandes, A.D.; Macklaim, J.M.; Linn, T.G.; Reid, G.; Gloor, G.B. ANOVA-like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. PLoS ONE 2013, 8, e67019. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef]
- Che-Castaldo, C.; Crisafulli, C.M.; Bishop, J.G.; Zipkin, E.F.; Fagan, W.F. Disentangling Herbivore Impacts in Primary Succession by Refocusing the Plant Stress and Vigor Hypotheses on Phenology. Ecol. Monogr. 2019, 89, e01389. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Madritch, M.D.; Bailey, J.K.; LeRoy, C.J.; Fischer, D.G.; Rehill, B.J.; Lindroth, R.L.; Hagerman, A.E.; Wooley, S.C.; Hart, S.C.; et al. From Genes to Ecosystems: The Genetic Basis of Condensed Tannins and Their Role in Nutrient Regulation in a Populus Model System. Ecosystems 2008, 11, 1005–1020. [Google Scholar] [CrossRef]
- Hutchens, J.J.; Benfield, E.F. Effects of Forest Defoliation by the Gypsy Moth on Detritus Processing in Southern Appalachian Streams. Am. Midl. Nat. 2000, 143, 397–404. [Google Scholar] [CrossRef]
- Chapman, S.K.; Hart, S.C.; Cobb, N.S.; Whitham, T.G.; Koch, G.W. Insect Herbivory Increases Litter Quality and Decomposition: An Extension of the Acceleration Hypothesis. Ecology 2003, 84, 2867–2876. [Google Scholar] [CrossRef]
- Jackrel, S.L.; Wootton, J.T. Cascading Effects of Induced Terrestrial Plant Defences on Aquatic and Terrestrial Ecosystem Function. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142522. [Google Scholar] [CrossRef] [PubMed]
- Hayer, M.; Wymore, A.S.; Hungate, B.A.; Schwartz, E.; Koch, B.J.; Marks, J.C. Microbes on Decomposing Litter in Streams: Entering on the Leaf or Colonizing in the Water? ISME J. 2022, 16, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.; Cortes, R.; Leão, C. Invertebrate and Microbial Colonisation in Native and Exotic Leaf Litter Species in a Mountain Stream. Int. Rev. Hydrobiol. 2001, 86, 527–540. [Google Scholar] [CrossRef]
- Melillo, J.M.; Naiman, R.J.; Aber, J.D.; Linkins, A.E. Factors Controlling Mass Loss and Nitrogen Dynamics of Plant Litter Decaying in Northern Streams. Bull. Mar. Sci. 1984, 35, 341–356. [Google Scholar]
- Kominoski, J.S.; Marczak, L.B.; Richardson, J.S. Riparian Forest Composition Affects Stream Litter Decomposition despite Similar Microbial and Invertebrate Communities. Ecology 2011, 92, 151–159. [Google Scholar] [CrossRef]
- Kaushik, N.K.K.; Hynes, H.B.N. The Fate of the Dead Leaves That Fall into Streams. Arch. Hydrobiol. 1971, 68, 465–515. [Google Scholar]
- Naiman, R.J.; Decamps, H.; Pollock, M. The Role of Riparian Corridors in Maintaining Regional Biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef]
- Gillies, C.S.; St. Clair, C.C. Riparian Corridors Enhance Movement of a Forest Specialist Bird in Fragmented Tropical Forest. Proc. Natl. Acad. Sci. USA 2008, 105, 19774–19779. [Google Scholar] [CrossRef] [PubMed]
- Irons, J.G.; Bryant, J.P.; Oswood, M.W. Effects of Moose Browsing on Decomposition Rates of Birch Leaf Litter in a Subarctic Stream. Can. J. Fish. Aquat. Sci. 1991, 48, 442–444. [Google Scholar] [CrossRef]
- Durben, R.M.; Walker, F.M.; Holeski, L.; Keith, A.R.; Kovacs, Z.; Hurteau, S.R.; Lindroth, R.L.; Shuster, S.M.; Whitham, T.G. Beavers, Bugs and Chemistry: A Mammalian Herbivore Changes Chemistry Composition and Arthropod Communities in Foundation Tree Species. Forests 2021, 12, 877. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Bailey, J.K.; Hart, S.C.; Wimp, G.M.; Chapman, S.K.; Whitham, T.G. The Interaction of Plant Genotype and Herbivory Decelerate Leaf Litter Decomposition and Alter Nutrient Dynamics. Oikos 2005, 110, 133–145. [Google Scholar] [CrossRef]
- Silfver, T.; Paaso, U.; Rasehorn, M.; Rousi, M.; Mikola, J. Genotype × Herbivore Effect on Leaf Litter Decomposition in Betula pendula Saplings: Ecological and Evolutionary Consequences and the Role of Secondary Metabolites. PLoS ONE 2015, 10, e0116806. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Ricklefs, R.E. Dioecy and Its Correlates in the Flowering Plants. Am. J. Bot. 1995, 82, 596–606. [Google Scholar] [CrossRef]
- Hultine, K.R.; Bush, S.E.; West, A.G.; Ehleringer, J.R. Population Structure, Physiology and Ecohydrological Impacts of Dioecious Riparian Tree Species of Western North America. Oecologia 2007, 154, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hultine, K.R.; Bush, S.E.; West, A.G.; Ehleringer, J.R. Effect of Gender on Sap-Flux-Scaled Transpiration in a Dominant Riparian Tree Species: Box Elder (Acer negundo). J. Geophys. Res. Biogeosci. 2007, 112, G03S06. [Google Scholar] [CrossRef]
- Hultine, K.R.; Grady, K.C.; Wood, T.E.; Shuster, S.M.; Stella, J.C.; Whitham, T.G. Climate Change Perils for Dioecious Plant Species. Nat. Plants 2016, 2, 16109. [Google Scholar] [CrossRef]
- Liu, L.; Lu, L.; Li, H.; Meng, Z.; Dong, T.; Peng, C.; Xu, X. Divergence of Phyllosphere Microbial Communities between Females and Males of the Dioecious Populus cathayana. Mol. Plant. Microbe Interact. 2021, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Liu, J.; Korpelainen, H.; Li, C. Plant Sex Affects Plant-Microbiome Assemblies of Dioecious Populus cathayana Trees under Different Soil Nitrogen Conditions. Microbiome 2022, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Landis, T.D.; Dreesen, D.R.; Dumroese, R.K. Sex and the Single Salix: Considerations for Riparian Restoration. Nativ. Plants J. 2003, 4, 110–117. [Google Scholar] [CrossRef]
- Che-Castaldo, C.; Crisafulli, C.M.; Bishop, J.G.; Fagan, W.F. What Causes Female Bias in the Secondary Sex Ratios of the Dioecious Woody Shrub Salix sitchensis Colonizing a Primary Successional Landscape? Am. J. Bot. 2015, 102, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ríos, J.; Pérez, J.; Salinas, M.J.; Fenoy, E.; Boyero, L.; Casas, J.J. Climate-Induced Plasticity in Leaf Traits of Riparian Plants. Divers. Distrib. 2022, 28, 859–876. [Google Scholar] [CrossRef]
- Salinas, M.J.; Casas, J.J.; Rubio-Ríos, J.; López-Carrique, E.; Ramos-Miras, J.J.; Gil, C. Climate-Driven Changes of Riparian Plant Functional Types in Permanent Headwater Streams. Implications for Stream Food Webs. PLoS ONE 2018, 13, e0199898. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Choosing Appropriate Methods and Standards for Assaying Tannin. J. Chem. Ecol. 1989, 15, 1795–1810. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Stevens, J.L.; Jackson, R.L.; Olson, J.B. Slowing PCR Ramp Speed Reduces Chimera Formation from Environmental Samples. J. Microbiol. Methods 2013, 93, 203–205. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Yourstone, S.; Mieczkowski, P.; Jones, C.D.; Dangl, J.L. Practical Innovations for High-Throughput Amplicon Sequencing. Nat. Methods 2013, 10, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Sturm, M.; Schroeder, C.; Bauer, P. SeqPurge: Highly-Sensitive Adapter Trimming for Paired-End NGS Data. BMC Bioinform. 2016, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.S. Using DECIPHER v2.0 to Analyze Big Biological Sequence Data in R. R J. 2016, 8, 352–359. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome Diversity: High-Throughput Sequencing and Identification of Fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LeRoy, C.J.; Heitmann, S.J.; Thompson, M.A.; Garthwaite, I.J.; Froedin-Morgensen, A.M.; Hartford, S.; Kamakawiwo’ole, B.K.; Thompson, L.J.; Ramstack Hobbs, J.M.; Claeson, S.M.; et al. Insect Herbivores, Plant Sex, and Elevated Nitrogen Influence Willow Litter Decomposition and Detritivore Colonization in Early Successional Streams. Forests 2024, 15, 1282. https://doi.org/10.3390/f15081282
LeRoy CJ, Heitmann SJ, Thompson MA, Garthwaite IJ, Froedin-Morgensen AM, Hartford S, Kamakawiwo’ole BK, Thompson LJ, Ramstack Hobbs JM, Claeson SM, et al. Insect Herbivores, Plant Sex, and Elevated Nitrogen Influence Willow Litter Decomposition and Detritivore Colonization in Early Successional Streams. Forests. 2024; 15(8):1282. https://doi.org/10.3390/f15081282
Chicago/Turabian StyleLeRoy, Carri J., Sabrina J. Heitmann, Madeline A. Thompson, Iris J. Garthwaite, Angie M. Froedin-Morgensen, Sorrel Hartford, Brandy K. Kamakawiwo’ole, Lauren J. Thompson, Joy M. Ramstack Hobbs, Shannon M. Claeson, and et al. 2024. "Insect Herbivores, Plant Sex, and Elevated Nitrogen Influence Willow Litter Decomposition and Detritivore Colonization in Early Successional Streams" Forests 15, no. 8: 1282. https://doi.org/10.3390/f15081282





