Flame-Retardant Coating on Wood Surface by Natural Biomass Polyelectrolyte via a Layer-by-Layer Self-Assembly Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Self-Assembly Solution
2.3. Construction of Flame-Retardant Coating on the Wood Surface
2.4. Characterization and Measurements
3. Results and Discussion
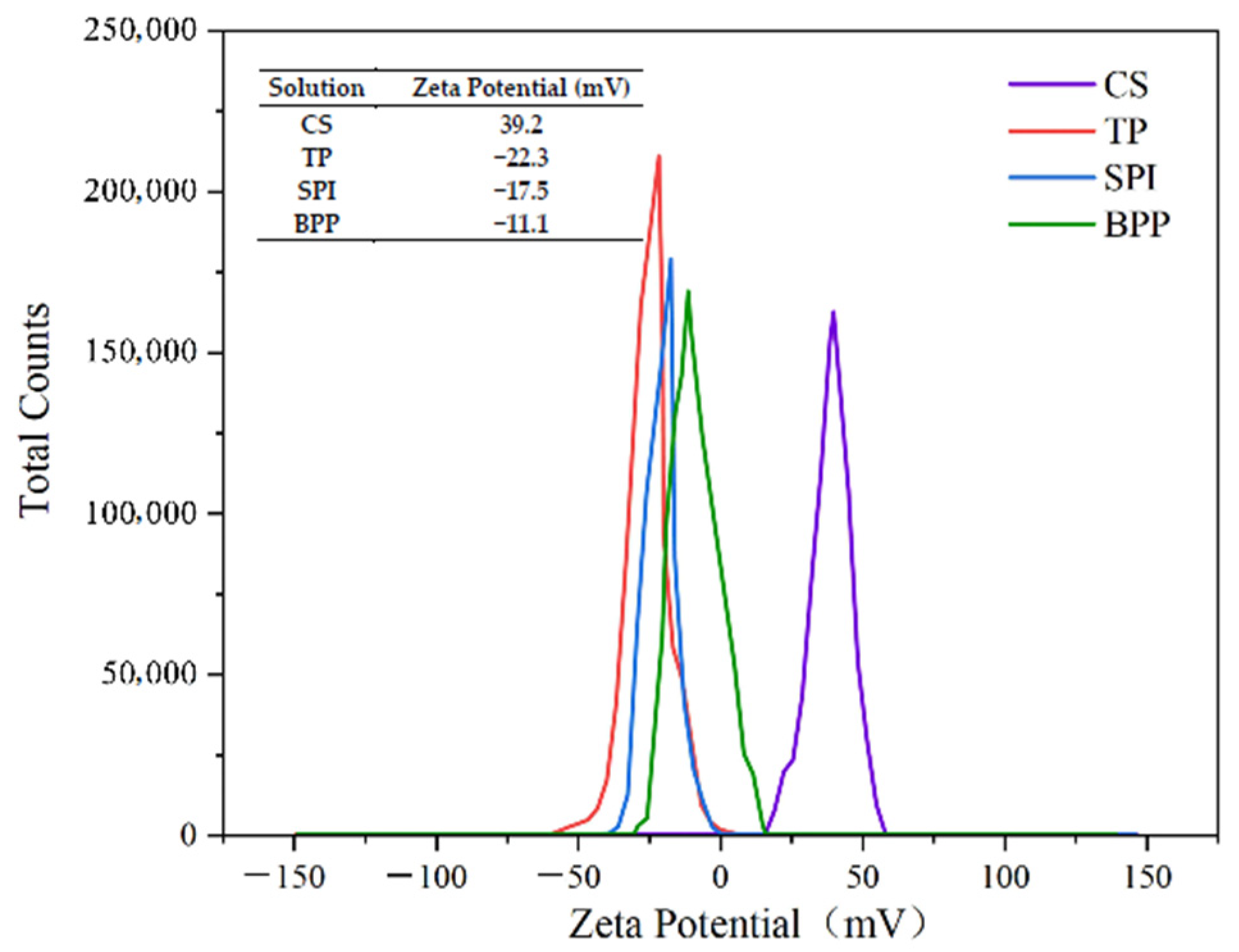
3.1. Zeta Potential Analysis
3.2. Morphology and Elemental Composition of the Flame-Retardant Coating on Wood
3.3. FT-IR Analysis
3.4. Thermal Stability
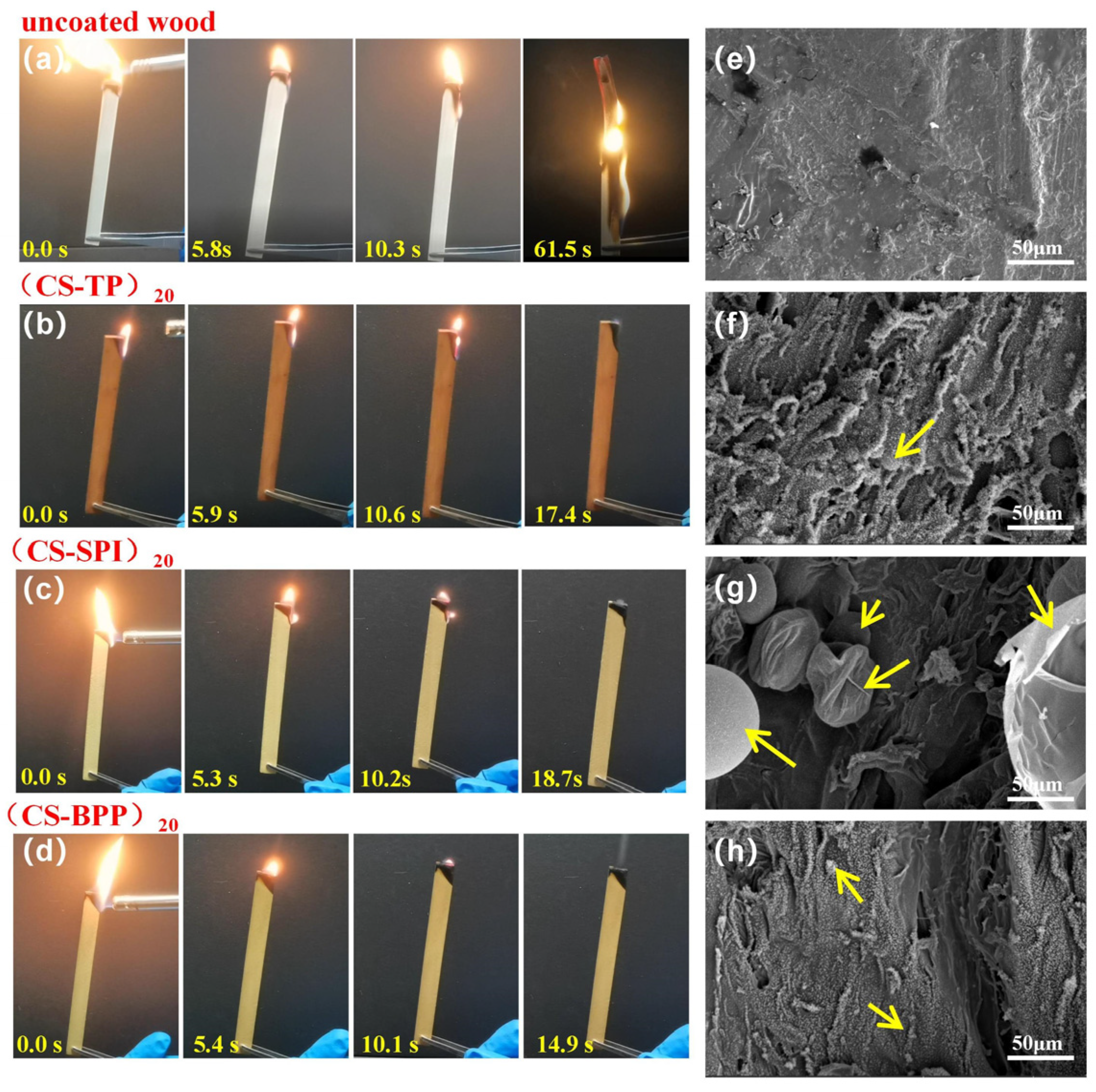
3.5. Exposure Combustion and Char Residue Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Li, L.; Xu, F. Chemical characteristics of wood cell wall with an emphasis on ultrastructure: A mini-review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, K.; Li, J.; Zhang, S.; Shi, S.Q. Environmentally benign wood modifications: A review. ACS Sustain. Chem. Eng. 2020, 8, 3532–3540. [Google Scholar] [CrossRef]
- Gan, W.; Chen, C.; Wang, Z.; Song, J.; Kuang, Y.; He, S.; Mi, R.; Sunderland, P.B.; Hu, L. Dense, Self-formed char layer enables afireretardant wood structural material. Adv. Funct. Mater. 2019, 29, 1807444. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In situ polymerization of furfuryl alcohol with ammonium dihydrogen phosphate in poplar wood for improved dimensional stability and flame retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Chen, G.; Chen, C.; Pei, Y.; He, S.; Liu, Y.; Jiang, B.; Jiao, M.; Gan, W.; Liu, D.; Yang, B.; et al. A strong, flame-retardant, and thermally insulating wood laminate. Chem. Eng. J. 2020, 383, 123109. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, D.; Hao, J.; Gao, M. One-step treated wood by using natural source phytic acid and uracil for enhanced mechanical properties and flame retardancy. Polym. Adv. Technol. 2020, 32, 1176–1186. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yu, Z.; Qi, C. Properties of fast-growing poplar wood simultaneously treated with dye and flame retardant. Eur. J. Wood Prod. 2016, 75, 325–333. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, T.; Wang, Q.; Guo, C. Synthesis of Biobased Flame-Retardant Carboxylic Acid Curing Agent and Application in Wood Surface Coating. ACS Sustain. Chem. Eng. 2019, 7, 14727–14738. [Google Scholar] [CrossRef]
- Lu, J.H.; Jiang, P.; Chen, Z.L.; Li, L.M.; Huang, Y.X. Flame retardancy, thermal stability, and hygroscopicity of wood materials modified with melamine and amino trimethylene phosphonic acid. Constr. Build. Mater. 2021, 267, 121042. [Google Scholar] [CrossRef]
- Fan, C.; Gao, Y.; Li, Y.; Yan, L.; Zhu, D.; Guo, S.; Ou, C.; Wang, Z. A flame-retardant densified wood as robust and fire-safe structural material. Wood Sci. Technol. 2023, 57, 111–134. [Google Scholar] [CrossRef]
- Wen, M.; Kang, C.; Park, H.J. Impregnation and mechanical properties of three softwoods treated with a new fire retardant chemical. J. Wood Sci. 2014, 60, 367–375. [Google Scholar] [CrossRef]
- Sethurajaperumal, A.R.; Manohar, A.; Banerjee, A.; Varrla, E.; Wang, H.; Ostrikov, K. Thermally-insulating vermiculite nanosheetsepoxy nanocomposite paint as fire-resistant wood coating. Nanoscale Adv. 2021, 3, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Xu, M.; Liu, L.; Wang, W.; Gao, S.; Li, B. Effect of a biomass based waterborne fire retardant coating on the flame retardancy for wood. Polym. Adv. Technol. 2021, 32, 4805–4814. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Wang, Y. Improvement of flame-retardant and antistatic property of wood using a spray-assisted layer-by-layer self-assembly technique. Wood Mater. Sci. Eng. 2023, 18, 1553–1561. [Google Scholar] [CrossRef]
- Mahr, M.S.; Hübert, T.; Sabel, M.; Schartel, B.; Bahr, H.; Militz, H. Fire retardancy of sol–gel derived titania wood-inorganic composites. J. Mater. Sci. 2012, 47, 6849–6861. [Google Scholar] [CrossRef]
- Yang, T.; Xia, M.; Chen, S.; Mu, M.; Yuan, G. Enhancing the thermal stability of silica-mineralized wood via layer-by-layer self-assembly. J. Therm. Anal. Calorim. 2021, 145, 309–318. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Y. Construction of a Chitosan/ZnO-Based Light-Resistant Coating System to Protect Dyed Wood from Ultraviolet Irradiation via Layer-by-Layer Self-Assembly. Int. J. Mol. Sci. 2022, 23, 15735. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Renneckar, S.; Zhou, Y. Nanoscale coatings on wood: Polyelectrolyte adsorption and layer-by-layer assembled film formation. ACS Appl. Mater. Interfaces 2009, 1, 559–566. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, Y.; Guan, J.; Tang, R.; Chen, G. Preparation of a flame retardant, antibacterial, and colored silkfabric with chitosan and vitamin B2 sodium phosphate by electrostatic layer by layerassembly. Mater. Lett. 2019, 241, 136–139. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Z.; Ma, Z.; Zheng, M. Natural silk fibroin based flame retardant LbL-coating for Dongba paper. Cellulose 2022, 29, 9393–9406. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Du, X.; Song, P.; Fang, Z. Green and Scalable Fabrication of Core-Shell Biobased Flame Retardants for Reducing Flammability of Polylactic Acid. ACS Sustain. Chem. Eng. 2019, 7, 8954–8963. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Y. Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 296. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, T.; Hou, S.; Fu, Y. Preparation and Synergistic Effect of Chitosan/Sodium Phytate/MgO Nanoparticle Fire-Retardant Coatings on Wood Substrate through Layer-By-Layer Self-Assembly. Coatings 2020, 10, 848. [Google Scholar] [CrossRef]
- Yan, Y.; Dong, S.; Jiang, H.; Hou, B.; Wang, Z.; Jin, C. Efficient and Durable Flame-Retardant Coatings on Wood Fabricated by Chitosan, Graphene Oxide, and Ammonium Polyphosphate Ternary Complexes via a Layer-by-Layer Self-Assembly Approach. ACS Omega 2022, 7, 29369–29379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Shi, L.; Fan, Z.W.; Yu, Y.Y.; Liu, R.T. Bio-based coating of phytic acid, chitosan, and biochar for flame-retardant cotton fabrics. Polym. Degrad. Stab. 2022, 199, 109898. [Google Scholar] [CrossRef]
- Liang, Y.; Jian, H.; Deng, C.; Xu, J.; Liu, Y.; Park, H.; Wen, M.; Sun, Y. Research and Application of Biomass-Based Wood Flame Retardants: A Review. Polymers 2023, 15, 950. [Google Scholar] [CrossRef]
- Cheng, X.; Guan, J.; Kiekens, P.; Yang, X.; Tang, C. Preparation and evaluation of an eco-friendly, reactive, and phytic acid-based flame retardant for wool. React. Funct. Polym. 2019, 134, 58–66. [Google Scholar] [CrossRef]
- Yao, F.; Zhai, C.; Wang, H.; Tao, J. Characterization of tea polyphenols as potential environment-friendly fire retardants. IOP Conf. Ser. Earth Environ. Sci. 2018, 121, 022016. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Essawy, H.; Pizzi, A.; Fredon, E.; Gerardin, C.; Du, G.; Zhou, X. Flame-retardant and thermally-insulating tannin and soybean protein isolate (SPI) based foams for potential applications in building materials. Constr. Build. Mater. 2022, 315, 125711. [Google Scholar] [CrossRef]
- Kong, F.; Nie, B.; Han, C.; Zhao, D.; Hou, Y.; Xu, Y. Flame retardancy and thermal property of environment-friendly poly(lactic acid) composites based on banana peel powder. Materials 2022, 15, 5977. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, H.; Lu, Z.; Meng, G.; Liu, R.; Wang, H.; Liu, W.; Li, G. Improved stability and anticancer activity of curcumin via pH-driven self-assembly with soy protein isolate. Process Biochem. 2024, 137, 217–228. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Li, Y.; Wu, J. Synergistic flame retardancy of wood treated with zirconium phosphate/ammonium polyphosphate. J. For. Eng. 2020, 5, 79–86. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, S.; Mei, C.; Pan, M. Polyethyleneimine/ammonium polyphosphate polyelectrolyte complex effects on the curing behavior of urea-formaldehyde resin and its flame retardant wood particle board. Acta Mater. Compos. Sin. 2023, 40, 6583–6595. [Google Scholar] [CrossRef]





| Element Samples | C (%) | O (%) | N (%) |
|---|---|---|---|
| Uncoated wood | 39.5 | 60.5 | 0 |
| CS-coated wood | 40.2 | 59.8 | 0 |
| (CS-TP)20-coated wood | 38.2 | 59.2 | 2.6 |
| (CS-SPI)20-coated wood | 42.7 | 49.4 | 7.9 |
| (CS-BPP)20-coated wood | 44.3 | 51.8 | 3.9 |
| Samples | T5% (°C) | T10% (°C) | Tmax (°C) | Rmax (%/min) | Char Residue at 400 °C (100%) | Char Residue at 800 °C (100%) |
|---|---|---|---|---|---|---|
| uncoated wood | 240.29 | 270.29 | 371.14 | 12.72 | 19.97 | 11.45 |
| (CS-TP)20 | 179.91 | 255.98 | 346.54 | 7.56 | 29.91 | 19.09 |
| (CS-SPI)20 | 115.83 | 260.57 | 366.31 | 10.8 | 27.79 | 17.22 |
| (CS-BPP)20 | 77.11 | 240.28 | 365.02 | 11.52 | 27.01 | 16.52 |
| Element Samples | C (%) | O (%) | N (%) |
|---|---|---|---|
| Uncoated wood | 27.5 | 44.2 | 28.5 |
| (CS-TP)20-coated wood | 75.9 | 20.3 | 3.8 |
| (CS-SPI)20-coated wood | 56.9 | 29.5 | 13.6 |
| (CS-BPP)20-coated wood | 61.5 | 23.3 | 15.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, M.; Fu, Y.; Xu, W. Flame-Retardant Coating on Wood Surface by Natural Biomass Polyelectrolyte via a Layer-by-Layer Self-Assembly Approach. Forests 2024, 15, 1362. https://doi.org/10.3390/f15081362
Weng M, Fu Y, Xu W. Flame-Retardant Coating on Wood Surface by Natural Biomass Polyelectrolyte via a Layer-by-Layer Self-Assembly Approach. Forests. 2024; 15(8):1362. https://doi.org/10.3390/f15081362
Chicago/Turabian StyleWeng, Mengyun, Yanchun Fu, and Wei Xu. 2024. "Flame-Retardant Coating on Wood Surface by Natural Biomass Polyelectrolyte via a Layer-by-Layer Self-Assembly Approach" Forests 15, no. 8: 1362. https://doi.org/10.3390/f15081362




