Dynamic Changes in Endogenous Substances in Flowering Organs of Camellia drupifera during the Flowering Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Selection of Sample Trees and Sample Collection
2.3. Measurement of Tannase Activity in Floral Organs
2.4. Hormone Content Determination in Floral Organs
2.5. ROS, H2O2 Content, and Antioxidant Enzyme Activity Determination in Floral Organs
2.6. Soluble Sugar (SS) and Soluble Protein (SP) Content Determination in Floral Organs
2.7. Data Analysis
3. Results
3.1. Dynamic Changes of Endogenous Substances Content during the Flowering Stage of C. drupifera
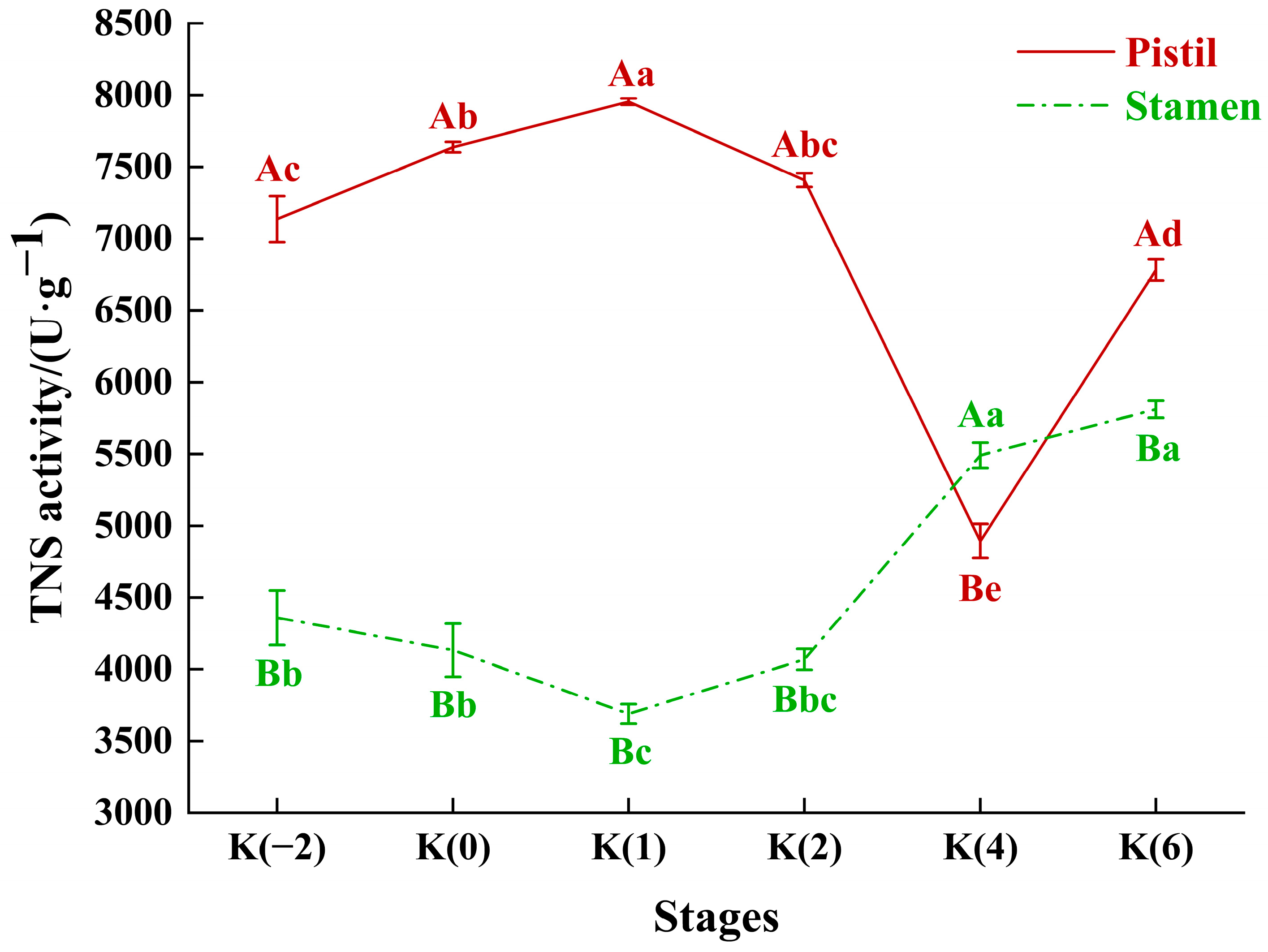
3.2. Dynamic Changes in Tannase Activity of Floral Organs during the Flowering Stage of C. drupifera
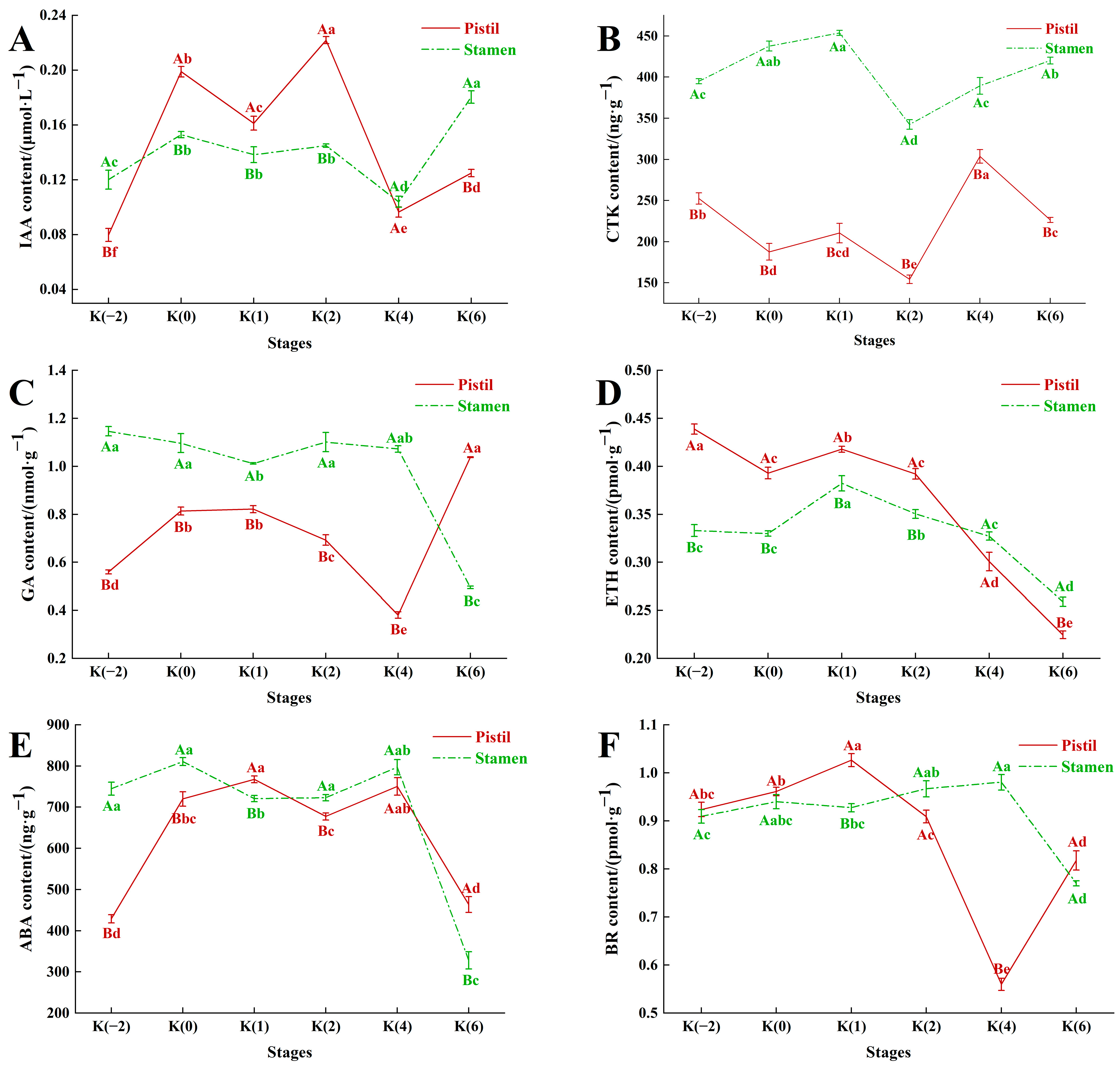
3.3. Dynamic Changes in Hormone Content of Floral Organs during Flowering Stage of C. drupifera
3.3.1. Dynamic Changes in IAA Content
3.3.2. Dynamic Changes in CTK Content
3.3.3. Dynamic Changes in GA Content
3.3.4. Dynamic Changes in TEH Content
3.3.5. Dynamic Changes in ABA Content
3.3.6. Dynamic Changes in BR Content
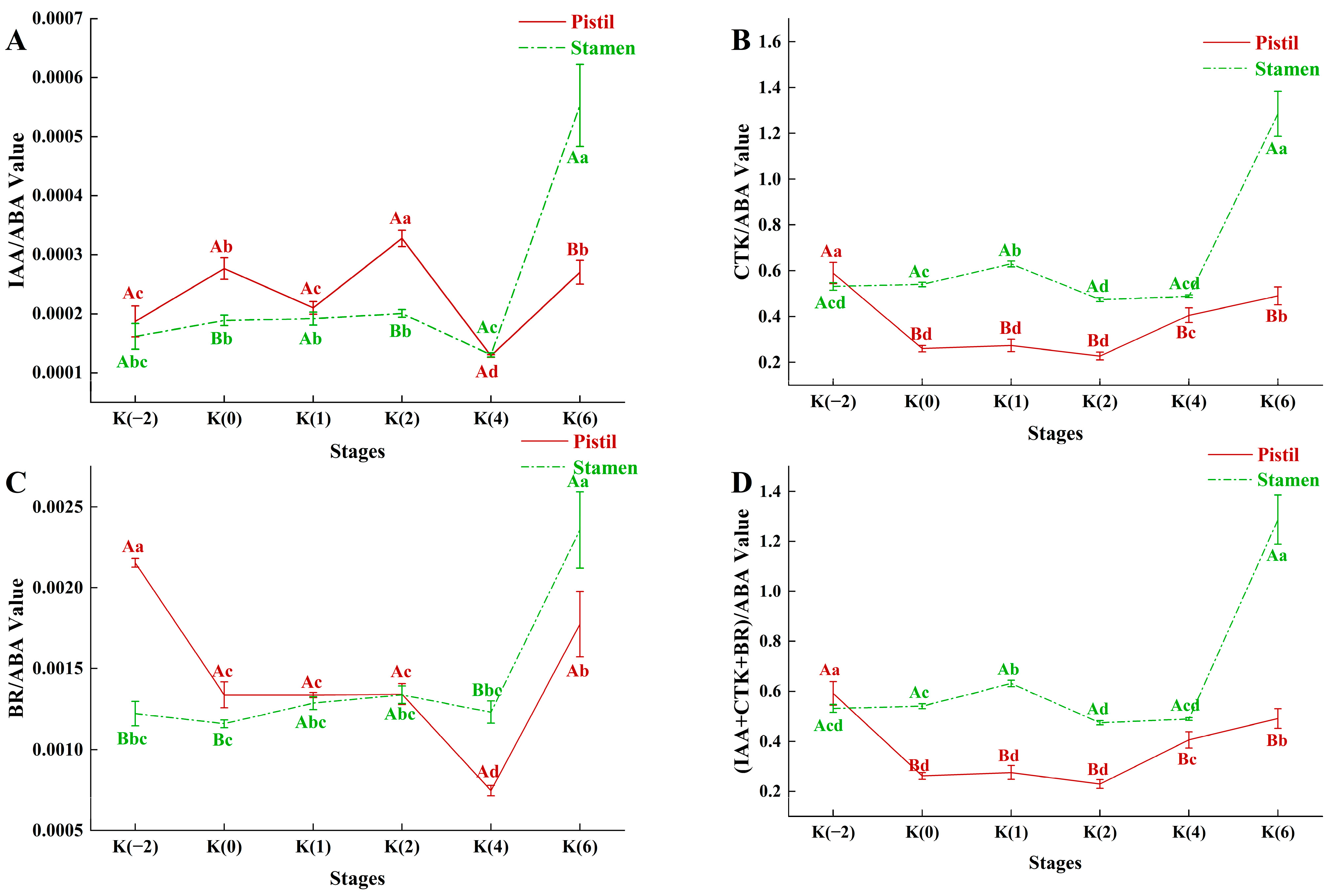
3.3.7. Balance of Endogenous Hormones during Flowering Stage of C. drupifera
3.4. Dynamic Changes in ROS, H2O2 Content, and Antioxidant Enzyme Activity in Floral Organs during the Flowering Stage of C. drupifera
3.4.1. Dynamic Changes in ROS and H2O2 Content
3.4.2. Dynamic Changes in Antioxidant Enzyme Activity
3.5. Dynamic Changes in Soluble Proteins (SP) and Soluble Sugars (SS) in Floral Organs during the Flowering Stage of C. drupifera
3.5.1. Dynamic Changes in SP Content
3.5.2. Dynamic Changes in SS Content
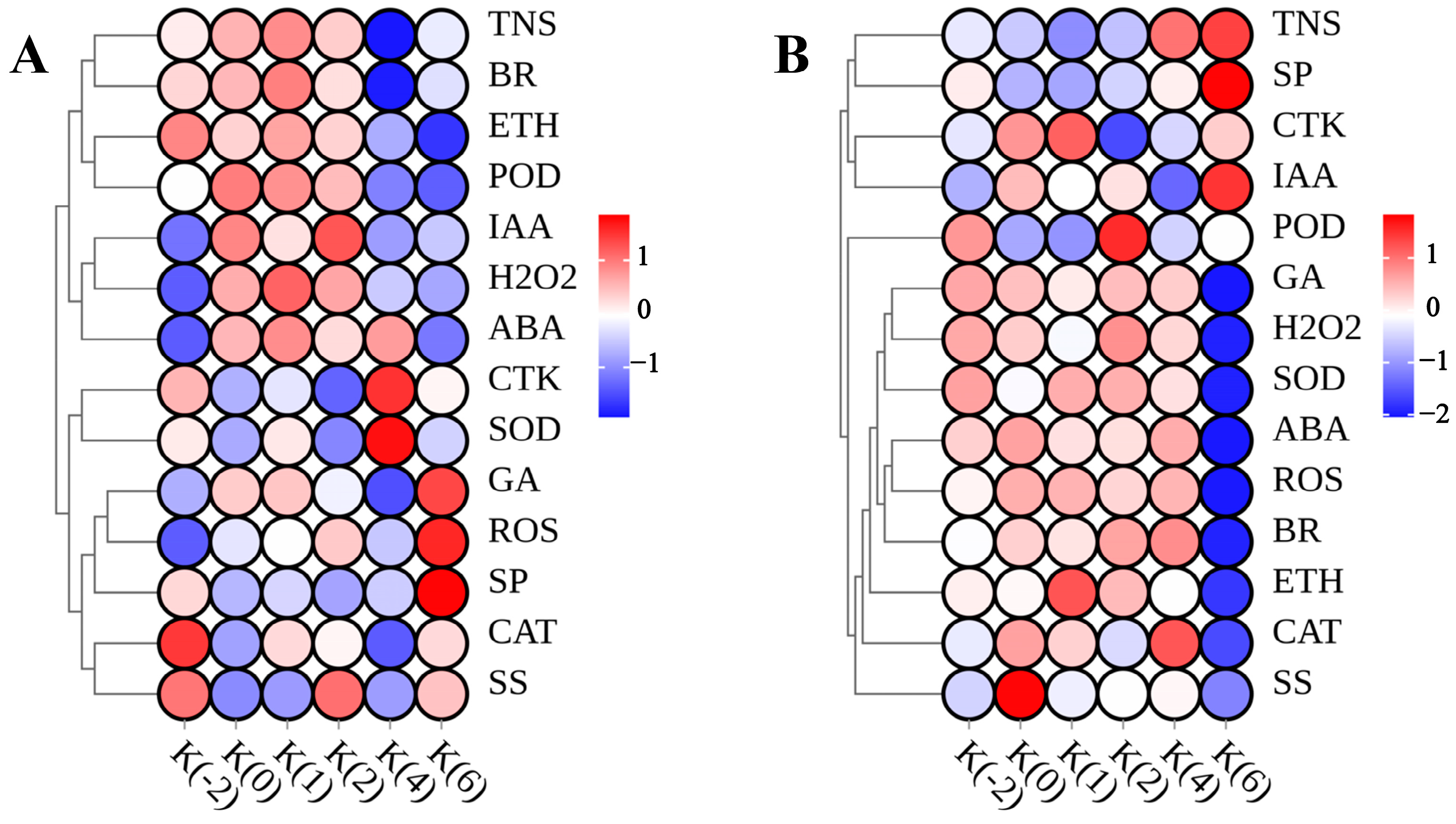
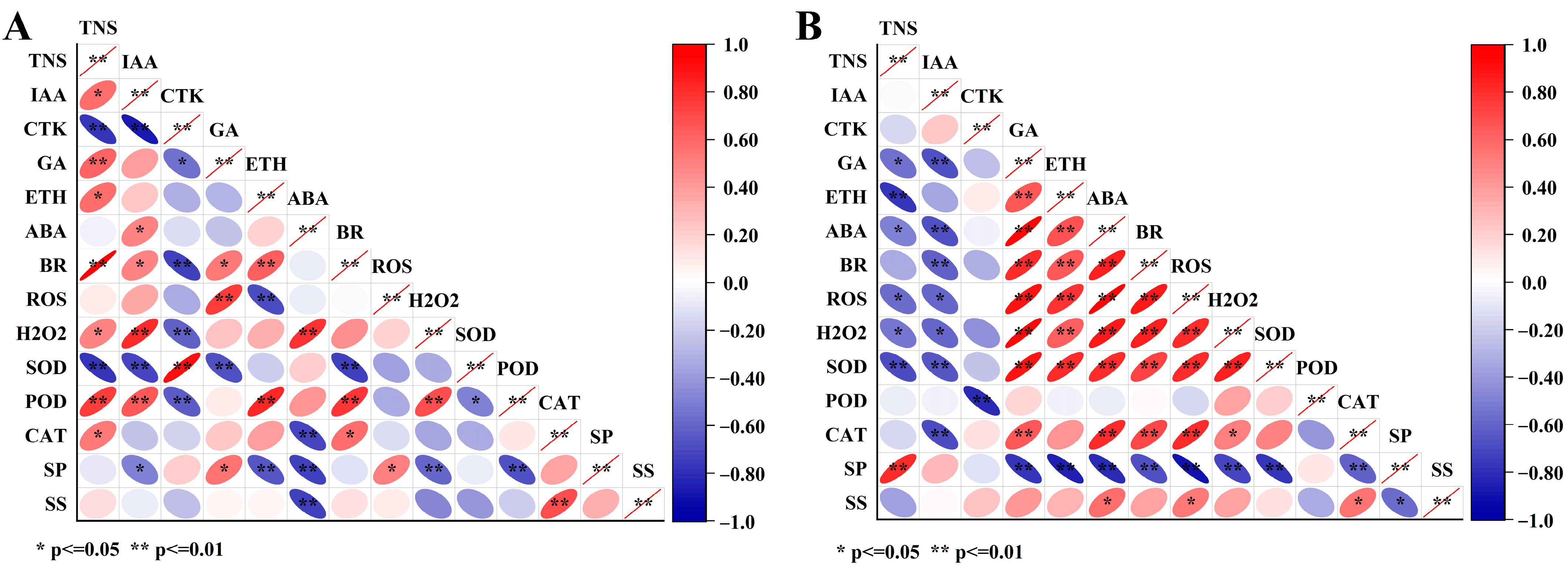
3.6. Correlation Analysis of Endogenous Substances during the Flowering Stage of C. drupifera
4. Discussion
4.1. Dynamic Changes in Endogenous Substances in Floral Organs of C. drupifera during Flowering Stage
4.2. Correlation of Endogenous Substance Contents in Floral Organs of C. drupifera during Flowering
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhen, L.; Xi, R. Sugar Metabolism and Transcriptomic Analysis of Key Enzymes and Transporters during Camellia drupifera Fruit Development. Front. Plant Sci. 2024, 15, 1424284. [Google Scholar]
- Ma, G.; Xia, T.; Sun, X.; Chen, J.; Yao, X.; Wang, C.; Chen, Y.; Feng, Y.; Feng, X.; Xie, S.; et al. Identification and analysis of CdS-RNase in Camellia drupifera: A key determinant of late-acting self-incompatibility. Ind. Crop. Prod. 2023, 203, 116990. [Google Scholar] [CrossRef]
- Li, Y.; Liao, B.; Wang, Y.; Luo, H.; Wang, S.; Li, C.; Song, W.; Zhang, K.; Yang, B.; Lu, S.; et al. Transcriptome and metabolome analyses provide insights into the relevance of pericarp thickness variations in Camellia drupifera and Camellia oleifera. Front. Plant Sci. 2022, 13, 1016475. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.R.; Wu, L.L.; Wang, Y.; Liu, D.; Li, J. Effects of Drought Stress on the Morphological Structure and Flower Organ Physiological Characteristics of Camellia oleifera Flower Buds. Plants 2023, 12, 2585. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Y.; Guo, P.; Li, Z.; Li, J.; Tan, X. Metabonomic and transcriptomic analyses of Camellia oleifera flower buds treated with low-temperature stress during the flowering stage. Ind. Crop. Prod. 2022, 189, 115874. [Google Scholar] [CrossRef]
- Hu, G.; Gao, C.; Fan, X.; Gong, W.; Yuan, D. Pollination compatibility and xenia in Camellia oleifera. Hortscience 2020, 55, 898–905. [Google Scholar] [CrossRef]
- Yuan, B.; Yuan, J.; Huang, C.; Lian, J.; Li, Y.; Fan, X.; Yuan, D. Pseudopollen in Camellia oleifera and its implications for pollination ecology and taxonomy. Front. Plant Sci. 2022, 13, 1032187. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Hu, G.; Zhang, X.; Yuan, J.; Fan, X.; Yuan, D. What are the best pollinator candidates for Camelia oleifera: Do not forget hoverflies and flies. Insects 2022, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Song, Q.; Chen, S.; Wu, Y.; Zheng, G.; Feng, J.; Yang, Z.; Lin, W.; Li, Y.; Chen, H. Transcriptome analysis of self-and cross-pollinated pistils revealing candidate unigenes of self-incompatibility in Camellia oleifera. J. Hortic. Sci. Biotechnol. 2020, 95, 19–31. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, J.; Yang, Z.; Xu, C.; Liao, P.; Pu, S.; El-Kassaby, Y.A.; Feng, J. Role of soil nutrient elements transport on Camellia oleifera yield under different soil types. BMC Plant Biol. 2023, 23, 378. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Tang, W.; Peng, S.; Li, M.; Deng, N.; Chen, Y. Predicting potential distribution and evaluating suitable soil condition of oil tea Camellia in China. Forests 2018, 9, 487. [Google Scholar] [CrossRef]
- Wei, W.; Wu, H.; Li, X.; Wei, X.; Lu, W.; Zheng, X. Diversity, daily activity patterns, and pollination effectiveness of the insects visiting Camellia osmantha, C. vietnamensis, and C. oleifera in South China. Insects 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sanz, J.V.; Zuriaga, E.; Cruz-García, F.; McClure, B.; Romero, C. Self-(in) compatibility systems: Target traits for crop-production, plant breeding, and biotechnology. Front. Plant Sci. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Gong, W.; Xu, J.; Gong, H.; Song, Q.; Xiao, S.; Yuan, D. Integration of semi-in vivo assays and multi-omics data reveals the effect of galloylated catechins on self-pollen tube inhibition in Camellia oleifera. Hortic. Res. 2023, 10, uhac248. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hu, S.; Xu, J.; Gong, H.; Guo, X.; Song, Q.; Gong, W.; Yuan, D. Identification of reference genes provides insights into the determinants of self-incompatibility in Camellia oleifera. Sci. Hortic. 2023, 321, 112301. [Google Scholar] [CrossRef]
- Du, M.; Yu, X.; Wu, F. Dynamic changes of endogenous hormones in self-pollinated and cross-pollinated pistils of two Camellia species in Hainan. J. Trop. Biol. 2023, 14, 173–177. [Google Scholar]
- Wang, Y.; Liu, P.; Cai, Y.; Li, Y.; Tang, C.; Zhu, N.; Wang, P.; Zhang, S.; Wu, J. PbrBZR1 interacts with PbrARI2. 3 to mediate brassinosteroid-regulated pollen tube growth during self-incompatibility signaling in pear. Plant Physiol. 2023, 192, 2356–2373. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Guo, X.; Yang, G.; Deng, S.; Chen, Q.; Gong, H.; Song, Q.; Gong, W.; Yuan, D. Tannase increases fruit set by interfering with self-incompatibility of Camellia oleifera. Ind. Crop. Prod. 2024, 210, 118189. [Google Scholar] [CrossRef]
- Santiago, J.P.; Sharkey, T.D. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant Cell Environ. 2019, 42, 2759–2775. [Google Scholar] [CrossRef]
- Wen, Y.; Su, S.C.; Ma, L.Y.; Wang, X.N. Effects of gibberellic acid on photosynthesis and endogenous hormones of Camellia oleifera Abel. In 1st and 6th leaves. J. Forest Res. 2018, 23, 309–317. [Google Scholar] [CrossRef]
- Pan, X.; Liu, X.; Wang, X.; Ge, L.Y.; Du, Q.H.; Zeng, Y.L. Effects of LED light quality on the physiology and morphological structure of Camellia oleifera leaves. Taiwan J. For. Sci. 2023, 38, 13–26. [Google Scholar]
- Lord, E.M.; Russell, S.D. The mechanisms of pollination and fertilization in plants. Annu. Rev. Cell Dev. Biol. 2002, 18, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Lord, E.M. Adhesion and guidance in compatible pollination. J. Exp. Bot. 2003, 54, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, L.; Lan, Z.; Dresselhaus, T. Peptide signalling during the pollen tube journey and double fertilization. J. Exp. Bot. 2015, 66, 5139–5150. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Pollen biology and hormesis: Pollen germination and pollen tube elongation. Sci. Total Environ. 2021, 762, 143072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Wang, Y.; Liu, S.; Wang, Z.-P.; Yu, X.-J. Integrated approaches to reveal genes crucial for tannin degradation in Aureobasidium melanogenum T9. Biomolecules 2019, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, X.; Wang, R.; Duan, Q.; He, J.; Gao, J.; Wang, J. Disruption of the Contents of Endogenous Hormones Cause Pollen Development Obstruction and Abortion in Male-Sterile Hybrid Lily Populations. Plants 2023, 12, 3804. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Caser, M.; Ghione, G.G.; Mansuino, A.; Giovannini, A.; Scariot, V. Dynamics of abscisic acid and indole-3-acetic acid during the early-middle stage of seed development in Rosa hybrida. Plant Growth Regul. 2015, 75, 265–270. [Google Scholar] [CrossRef]
- Yang, M.; Yarra, R.; Zhang, R.; Zhou, L.; Jin, L.; Martin, J.J.J.; Cao, H. Transcriptome analysis of oil palm pistil during pollination and fertilization to unravel the role of phytohormone biosynthesis and signaling genes. Funct. Integr. Genom. 2022, 22, 261–278. [Google Scholar] [CrossRef]
- Guo, L.; Luo, X.; Li, M.; Joldersma, D.; Plunkert, M.; Liu, Z. Mechanism of fertilization-induced auxin synthesis in the endosperm for seed and fruit development. Nat. Commun. 2022, 13, 3985. [Google Scholar] [CrossRef]
- Rounds, C.M.; Winship, L.J.; Hepler, P.K. Pollen tube energetics: Respiration, fermentation and the race to the ovule. AoB Plants 2011, 2011, plr019. [Google Scholar] [CrossRef]
- Zhao, W.; Hou, Q.; Qi, Y.; Wu, S.; Wan, X. Structural and molecular basis of pollen germination. Plant Physiol. Biochem. 2023, 203, 108042. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive oxygen species as mediators of gametophyte development and double fertilization in flowering plants. Front. Plant Sci. 2020, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Lodde, V.; Morandini, P.; Costa, A.; Murgia, I.; Ezquer, I. cROStalk for life: Uncovering ROS signaling in plants and animal systems, from gametogenesis to early embryonic development. Genes 2021, 12, 525. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Dolferus, R. Pollen developmental arrest: Maintaining pollen fertility in a world with a changing climate. Front. Plant Sci. 2019, 10, 679. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, X.S.; Gao, X. ROS in the male–female interactions during pollination: Function and regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant. Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N. Role of plant nutrients in plant growth and physiology. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 51–93. [Google Scholar]
- Ma, L.; Zhao, C.; Chen, S.; Lin, X.; Mao, Y.; Zhang, C.; Li, L.; Zhu, H.; Hu, Y.; Zhang, Z.; et al. High-yield Cultivation Technology of Regenerated Rice in Boluo County. J. Mod. Crop. Sci. 2023, 2, 53–58. [Google Scholar]
- Liu, M.L.; Xie, H.F.; Ma, Y.; Li, H.; Li, C.; Chen, L.; Jiang, B.; Nian, B.; Guo, T.; Zhang, Z.; et al. High Performance Liquid Chromatography and Metabolomics Analysis of Tannase Metabolism of Gallic Acid and Gallates in Tea Leaves. J. Agric. Food Chem. 2020, 68, 4946–4954. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Motyka, V.; Pokorná, E.; Filepová, R.; Dobrev, P.I.; Handa, A.K.; Mattoo, A.K. A comprehensive endogenous phytohormone metabolite landscape identifies new metabolites associated with tomato fruit. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, C.; Liu, Y.; Shahid, M.O.; Wang, C.; Gao, J. Petal senescence: A hormone view. J. Exp. Bot. 2018, 69, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ding, J.; Li, J.; Li, H.; Ruan, C. Co-regulatory effects of hormone and mRNA-miRNA module on flower bud formation of Camellia oleifera. Front. Plant Sci. 2023, 14, 1109603. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, H.; Xu, H.; Yao, X. Effects of phytohormones on pollen germination and pollen tube growth of 4 Camellia plants. Chin. J. Oil Crop. Sci. 2021, 43, 700. [Google Scholar]
- Shen, S.; Yan, W.; Xie, S.; Yu, J.; Yao, G.; Xia, P.; Wu, Y.; Yang, H. Physiological and Transcriptional Analysis Reveals the Response Mechanism of Camellia vietnamensis Huang to Drought Stress. Int. J. Mol. Sci. 2022, 23, 11801. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Faleri, C.; Del Duca, S.; Cai, G. Depletion of sucrose induces changes in the tip growth mechanism of tobacco pollen tubes. Ann. Bot. 2018, 122, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Weng, S.; Ma, Y.; Wu, R.; Kang, X.; Du, L. Study on physiological and biochemical characteristics during in vitro flowering of Rosa ‘Yametsu-Hime’. Plant Cell Tissue Org. 2024, 157, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zhang, X.; Wang, C.; Zhang, Z.; Sun, P. Exogenous application of ethephon regulates flower abscission, shoot growth, and secondary metabolites in Camellia sinensis. Sci. Hortic. 2022, 304, 111333. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.; Chen, Y.; Xu, Y.; Tang, W.; Chen, L.; Li, Z. Physiological and Biochemical Effects of Exogenous Calcium on Camellia oleifera Abel under Drought Stress. Forests 2023, 14, 2082. [Google Scholar] [CrossRef]
- Sheng, J.; Li, X.; Zhang, D. Gibberellins, brassinolide, and ethylene signaling were involved in flower differentiation and development in Nelumbo nucifera. Hortic. Plant J. 2022, 8, 243–250. [Google Scholar] [CrossRef]
- Breygina, M.; Schekaleva, O.; Klimenko, E.; Luneva, O. The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination. Plants 2022, 11, 993. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tao, J.; Ma, C.; Wen, M.; Xi, R.; Deng, X. Dynamic Changes in Endogenous Substances in Flowering Organs of Camellia drupifera during the Flowering Stage. Forests 2024, 15, 1391. https://doi.org/10.3390/f15081391
Liu Z, Tao J, Ma C, Wen M, Xi R, Deng X. Dynamic Changes in Endogenous Substances in Flowering Organs of Camellia drupifera during the Flowering Stage. Forests. 2024; 15(8):1391. https://doi.org/10.3390/f15081391
Chicago/Turabian StyleLiu, Zhen, Jialu Tao, Chunhua Ma, Mengling Wen, Ruchun Xi, and Xiaomei Deng. 2024. "Dynamic Changes in Endogenous Substances in Flowering Organs of Camellia drupifera during the Flowering Stage" Forests 15, no. 8: 1391. https://doi.org/10.3390/f15081391




