Impact of Nitrogen Fertilizer Application on Soil Organic Carbon and Its Active Fractions in Moso Bamboo Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sample Collection
2.3. SOC and Active Organic Carbon Fractions
2.4. Soil Carbon Pool Management Index
2.5. Data Analysis
3. Results
3.1. Characterization of Changes in Soil Organic Carbon and Active Organic Carbon in Response to N Input
3.2. Characterization of Changes in the Proportion of Active Organic Carbon in Response to N Input
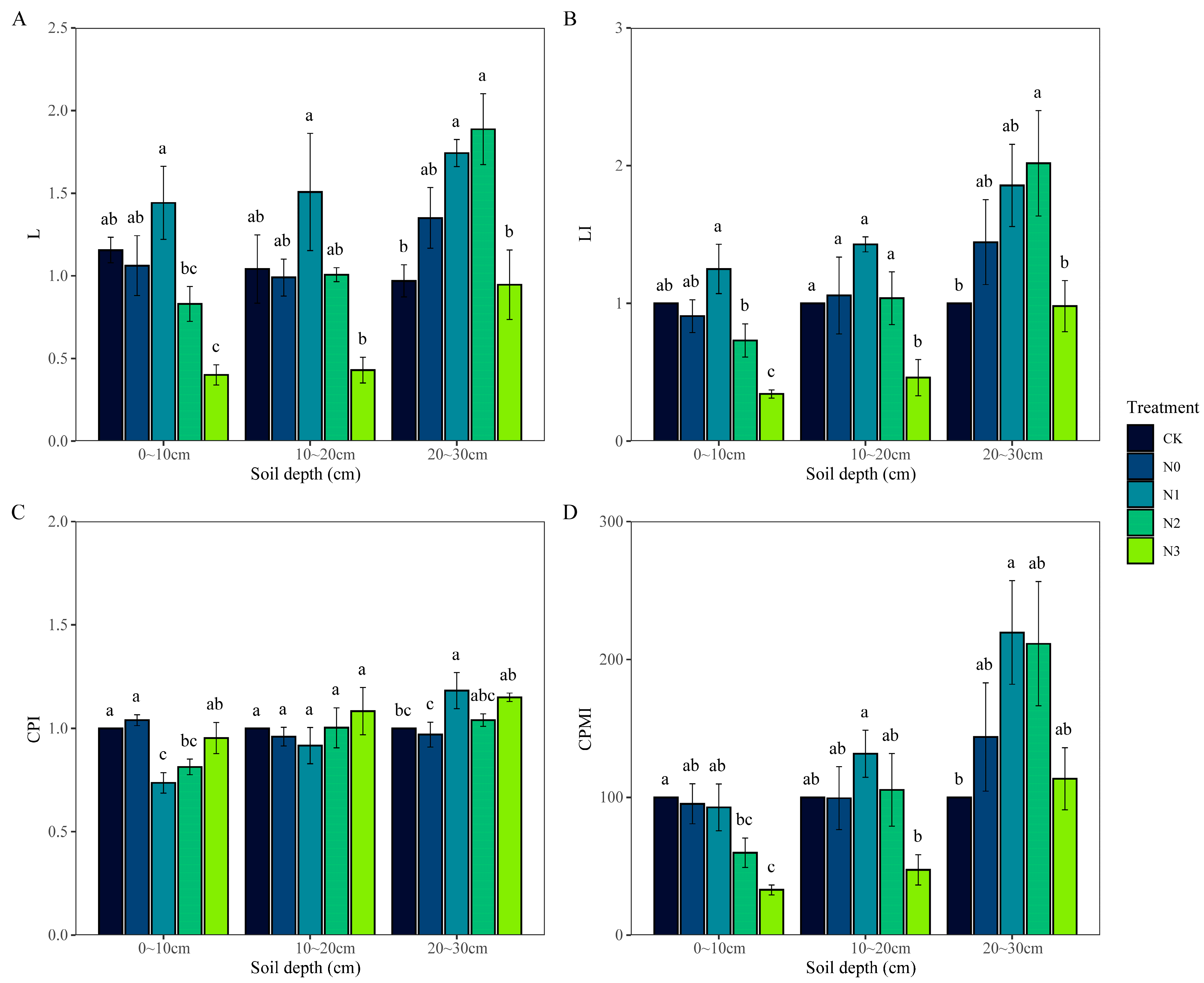
3.3. Characterization of Changes in Soil Carbon Pool Management Indices in Response to N Input
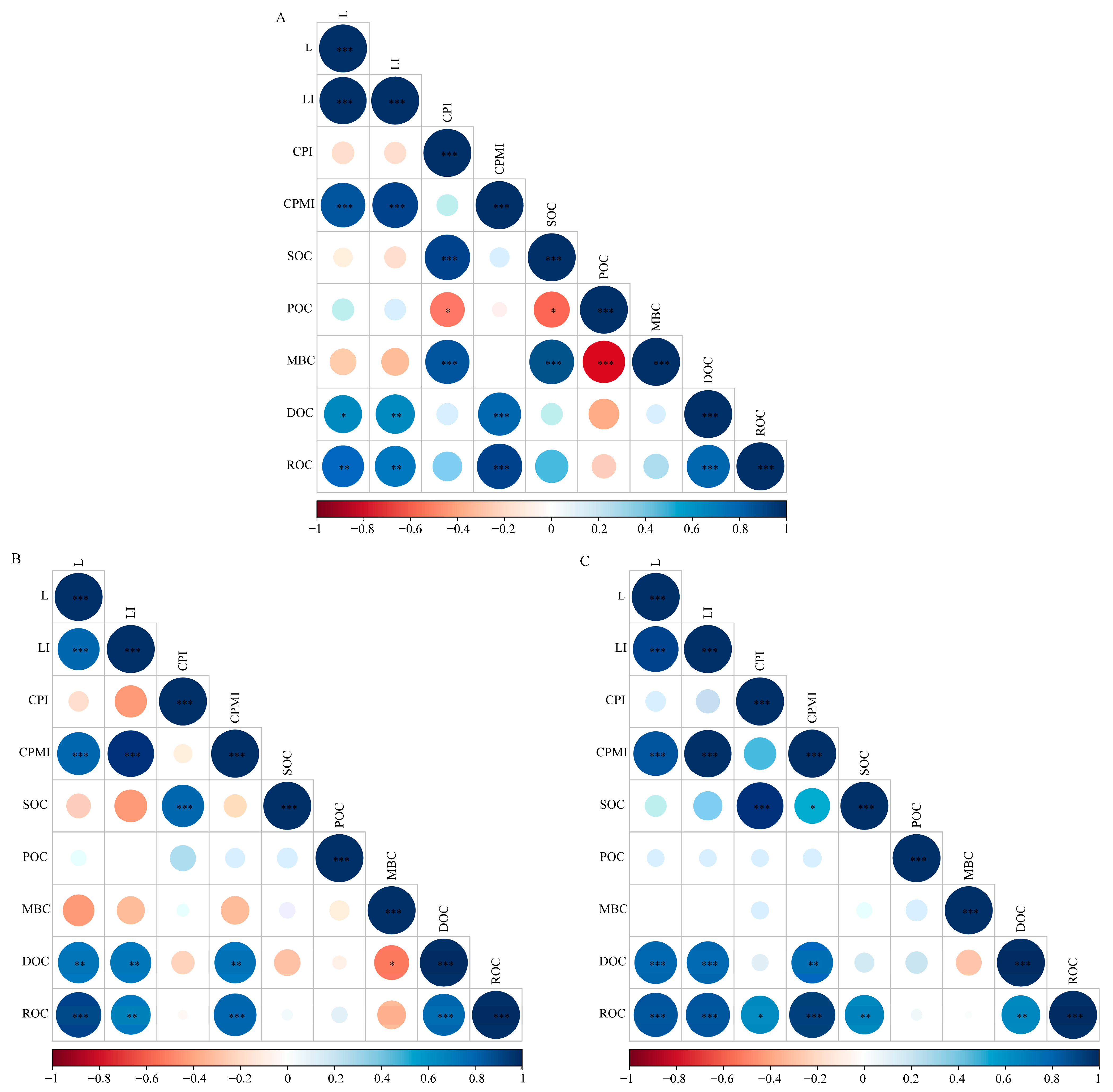
3.4. Correlation between Soil Organic Carbon, Active Organic Carbon, and Carbon Pool Management Indices
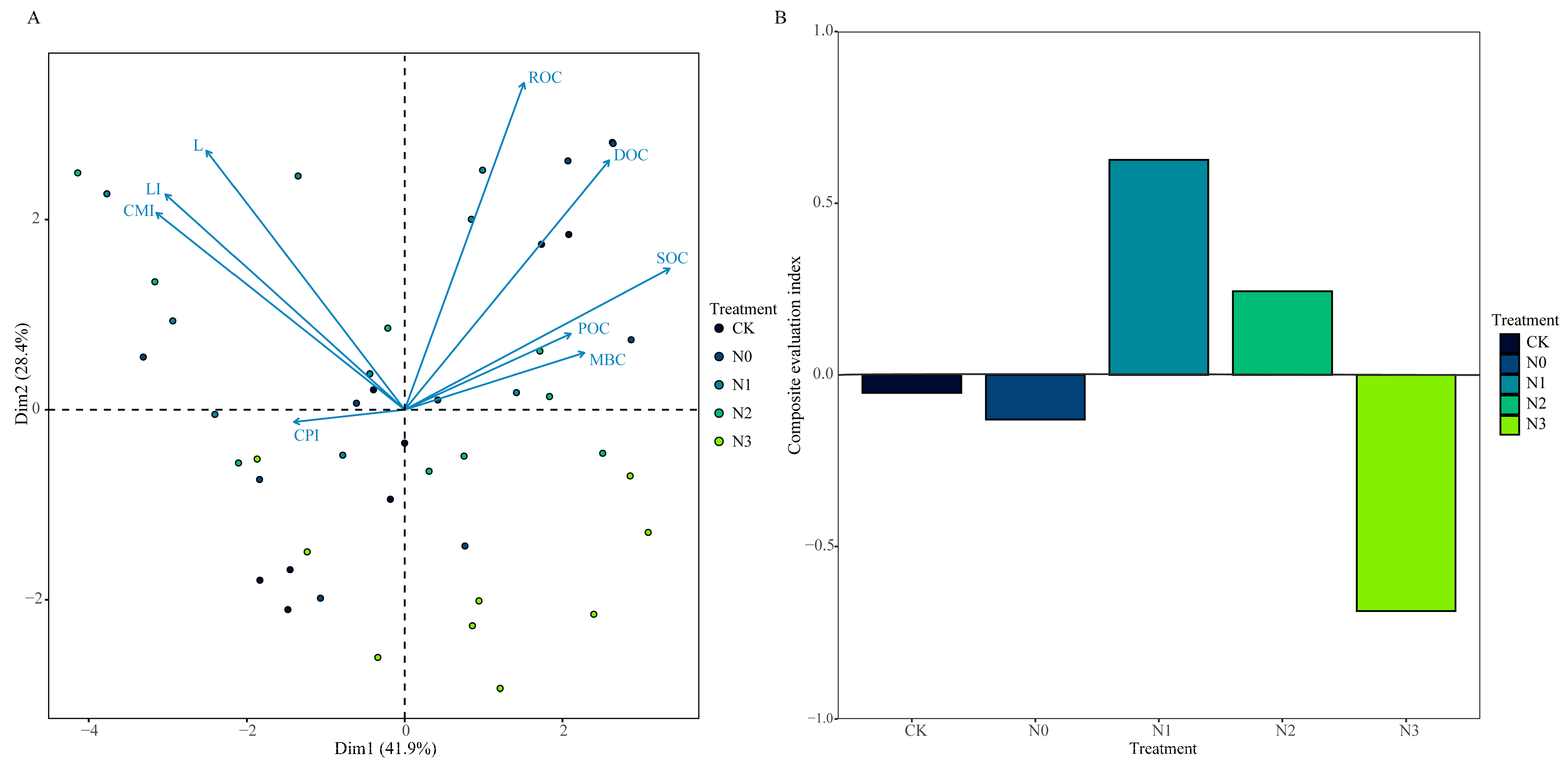
3.5. Comprehensive Evaluation of Soil Organic Carbon and Its Active Fractions with Carbon Pool Management Indices
4. Discussion
4.1. Effects of Nitrogen Application on Soil Organic Carbon in Moso Bamboo Forests
4.2. Effects of Nitrogen Application on Soil Active Organic Carbon and Its Ratio in Moso Bamboo Forests
4.3. Effect of Nitrogen Application on Soil Carbon Pool Management Index in Moso Bamboo Forests
4.4. Innovations and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z. Bamboo and Rattan in the World; China Forest Publishing House: Beijng, China, 2002. [Google Scholar]
- Alamerew, A.N.; Kozak, R.; Shrestha, A.K.; Zhu, Z.; Wang, G. A Way for Livelihood Improvement: Systematic Review on Bamboo Forest Research. Trees For. People. 2024, 16, 100571. [Google Scholar] [CrossRef]
- Feng, P.; Li, Y. China’s Bamboo Resources in 2021. World Bamboo Ratt. 2023, 21, 100–103. [Google Scholar]
- Zheng, Y.; Guan, F.; Fan, S.; Yan, X.; Huang, L. Dynamics of Leaf-Litter Biomass, Nutrient Resorption Efficiency and Decomposition in a Moso Bamboo Forest After Strip Clearcutting. Front. Plant Sci. 2022, 12, 799424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guan, F.; Zhang, X.; Li, C.; Zhou, Y. Response of Moso Bamboo Growth and Soil Nutrient Content to Strip Cutting. Forests 2022, 13, 1293. [Google Scholar] [CrossRef]
- Fan, S.; Liu, G.; Su, W.; Cai, C.; Guan, F. Advances in Research of Bamboo Forest Cultivation. For. Res. 2018, 31, 137–144. [Google Scholar]
- Su, W.; Fan, S.; Zhao, J.; Cai, C. Effects of Various Fertilization Placements on the Fate of Urea-15N in Moso Bamboo Forests. For. Ecol. Manag. 2019, 453, 117632. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, C. Physiological Integration Improves Nitrogen Use Efficiency of Moso Bamboo: An Isotopic (15N) Assessment. For. Ecol. Manag. 2023, 542, 121073. [Google Scholar] [CrossRef]
- Zhao, J.; Su, W.; Fan, S.; Cai, C.; Su, H.; Zeng, X. Ammonia Volatilization and Nitrogen Runoff Losses from Moso Bamboo Forests after Different Fertilization Practices. Can. J. For. Res. 2019, 49, 213–220. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Gu, H.; Qi, L. Management Practices Amplify the Effects of N Deposition on Leaf Litter Decomposition of the Moso Bamboo Forest. Plant Soil. 2015, 395, 391–400. [Google Scholar]
- Kim, C.; Baek, G.; Yoo, B.O.; Jung, S.-Y.; Lee, K.S. Regular Fertilization Effects on the Nutrient Distribution of Bamboo Components in a Moso Bamboo (Phyllostachys Pubescens (Mazel) Ohwi) Stand in South Korea. Forests 2018, 9, 671. [Google Scholar] [CrossRef]
- Song, Q.; Ouyang, M.; Yang, Q.; Lu, H.; Yang, G.; Chen, F.; Shi, J.-M. Degradation of Litter Quality and Decline of Soil Nitrogen Mineralization after Moso Bamboo (Phyllostachys Pubscens) Expansion to Neighboring Broadleaved Forest in Subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Li, C.; Zhou, G.; Shi, Y.; Zhou, Y.; Zhang, Y. Effects of different management measures on soil carbon in bamboo forest ecosystems. Sci. Silvae Sin. 2015, 51, 26–35. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.E.; Hobbie, S.E.; Bach, E.M.; Hofmockel, K.S.; Kazanski, C.E. Nitrogen Addition Changes Grassland Soil Organic Matter Decomposition. Biogeochemistry 2015, 125, 203–219. [Google Scholar] [CrossRef]
- Wang, M.; Li, F.; Dong, L.; Wang, X.; Han, L.; Olesen, J.E. Effects of Exogenous Organic/Inorganic Nitrogen Addition on Carbon Pool Distribution and Transformation in Grassland Soil. Sci. Total Environ. 2023, 858, 159919. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, C.; Yang, S.; Bai, W.; Liu, L. Microbial Carbon Use Efficiency and Priming Effect Regulate Soil Carbon Storage under Nitrogen Deposition by Slowing Soil Organic Matter Decomposition. Geoderma 2018, 332, 37–44. [Google Scholar] [CrossRef]
- Luo, L.; Yu, J.; Zhu, L.; Gikas, P.; He, Y.; Xiao, Y.; Deng, S.; Zhang, Y.; Zhang, S.; Zhou, W.; et al. Nitrogen Addition May Promote Soil Organic Carbon Storage and CO2 Emission but Reduce Dissolved Organic Carbon in Zoige Peatland. J. Environ. Manag. 2022, 324, 116376. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.Y.H.; Wilsey, B.J.; Luo, Y.; Fan, W. Long-term, Amplified Responses of Soil Organic Carbon to Nitrogen Addition Worldwide. Glob. Change Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef]
- Huang, R.; He, J.; Wang, N.; Christakos, G.; Gu, J.; Song, L.; Luo, J.; Agusti, S.; Duarte, C.M.; Wu, J. Carbon Sequestration Potential of Transplanted Mangroves and Exotic Saltmarsh Plants in the Sediments of Subtropical Wetlands. Sci. Total Environ. 2023, 904, 166185. [Google Scholar] [CrossRef]
- Ngaba, M.J.Y.; Uwiragiye, Y.; Bol, R.; de Vries, W.; Zhou, J. Low-Level Nitrogen and Short-Term Addition Increase Soil Carbon Sequestration in Chinese Forest Ecosystems. CATENA 2022, 215, 106333. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.-P.; Baldock, J.A.; Jiang, J.; Mo, J.; Zhou, G.; Yan, J. Divergent Responses of Soil Organic Carbon Accumulation to 14 Years of Nitrogen Addition in Two Typical Subtropical Forests. Sci. Total Environ. 2020, 707, 136104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Wang, S.; He, T.; Liu, L. Fresh Carbon and Nitrogen Inputs Alter Organic Carbon Mineralization and Microbial Community in Forest Deep Soil Layers. Soil. Biol. Biochem. 2014, 72, 145–151. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, L.; Zhong, X.; Yang, Z.; Lin, Y.; Guo, J.; Chen, G.; Yang, Y. N Addition Increased Microbial Residual Carbon by Altering Soil P Availability and Microbial Composition in a Subtropical Castanopsis Forest. Geoderma 2020, 375, 114470. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The Myth of Nitrogen Fertilization for Soil Carbon Sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L.; Gallo, M.; Lauber, C. Nitrogen Deposition Modifies Soil Carbon Storage Through Changes in Enzymatic Activity. Ecol. Appl. 2004, 14, 1172–1177. [Google Scholar] [CrossRef]
- Boot, C.M.; Hall, E.K.; Denef, K.; Baron, J.S. Long-Term Reactive Nitrogen Loading Alters Soil Carbon and Microbial Community Properties in a Subalpine Forest Ecosystem. Soil. Biol. Biochem. 2016, 92, 211–220. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Gurmesa, G.A.; Yu, G.; Li, L.; Zhang, W.; Fang, H.; Mo, J. Effects of Nitrogen Deposition on Carbon Cycle in Terrestrial Ecosystems of China: A Meta-Analysis. Environ. Pollut. 2015, 206, 352–360. [Google Scholar] [CrossRef]
- Balesdent, J.; Basile-Doelsch, I.; Chadoeuf, J.; Cornu, S.; Derrien, D.; Fekiacova, Z.; Hatté, C. Atmosphere–Soil Carbon Transfer as a Function of Soil Depth. Nature 2018, 559, 599–602. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; Luo, Y.; Yang, Y.; Fang, C.; Chen, J.; Li, B. Minor Stimulation of Soil Carbon Storage by Nitrogen Addition: A Meta-Analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Forstner, S.J.; Wechselberger, V.; Müller, S.; Keibinger, K.M.; Díaz-Pinés, E.; Wanek, W.; Scheppi, P.; Hagedorn, F.; Gundersen, P.; Tatzber, M.; et al. Vertical Redistribution of Soil Organic Carbon Pools After Twenty Years of Nitrogen Addition in Two Temperate Coniferous Forests. Ecosystems 2019, 22, 379–400. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Mao, C.; Qin, S.; Wang, J.; Liu, F.; Blagodatsky, S.; Yang, G.; Zhang, Q.; Zhang, D.; et al. Nitrogen Availability Regulates Topsoil Carbon Dynamics after Permafrost Thaw by Altering Microbial Metabolic Efficiency. Nat. Commun. 2018, 9, 3951. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Greaver, T.L. A Global Perspective on Belowground Carbon Dynamics under Nitrogen Enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.D.R.D.; Singh, K.; et al. The Knowns, Known Unknowns and Unknowns of Sequestration of Soil Organic Carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Srinivasa Rao, C.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil Organic Carbon Dynamics: Impact of Land Use Changes and Management Practices: A Review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Wang, H.; Wu, J.; Li, G.; Yan, L.; Wei, X.; Ma, W. Effects of Simulated Nitrogen Deposition on Soil Active Carbon Fractions in a Wet Meadow in the Qinghai-Tibet Plateau. J. Soil. Sci. Plant. Nutr. 2022, 22, 2943–2954. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.; Wang, C.; Pan, T.; Zeng, C. Effects of Rice Straw Incorporation on Active Soil Organic Carbon Pools in a Subtropical Paddy Field. Soil Tillage Res. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Pan, Y.; Cheng, X.; Zhang, K.; Liu, G. Particulate Organic Carbon Is More Sensitive to Nitrogen Addition than Mineral-Associated Organic Carbon: A Meta-Analysis. Soil Tillage Res. 2023, 232, 105770. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, W.; Zheng, C.; Zhu, B. Nitrogen Addition Has Contrasting Effects on Particulate and Mineral-Associated Soil Organic Carbon in a Subtropical Forest. Soil. Biol. Biochem. 2020, 142, 107708. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Feng, W.; Niu, S.; Plante, A.; Luo, Y.; Wang, K. Different Responses of Soil Organic Carbon Fractions to Additions of Nitrogen. Eur. J. Soil. Sci. 2018, 69, 1098–1104. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and Mechanisms of Responses by Soil Microbial Communities to Nitrogen Addition. Soil. Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen Fertilization Weakens the Linkage between Soil Carbon and Microbial Diversity: A Global Meta-analysis. Glob. Chang. Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Tan, S.; Peng, Y.; Wang, D.; Ni, X.; Yue, K.; Wu, F.; Yang, Y. Increased Microbial Sequestration of Soil Organic Carbon under Nitrogen Deposition over China’s Terrestrial Ecosystems. Ecol. Process 2020, 9, 52. [Google Scholar] [CrossRef]
- Shi, L.; Dech, J.P.; Yao, H.; Zhao, P.; Shu, Y.; Zhou, M. The Effects of Nitrogen Addition on Dissolved Carbon in Boreal Forest Soils of Northeastern China. Sci. Rep. 2019, 9, 8274. [Google Scholar] [CrossRef]
- Wang, J.-J.; Liu, Y.; Bowden, R.D.; Lajtha, K.; Simpson, A.J.; Huang, W.-L.; Simpson, M.J. Long-Term Nitrogen Addition Alters the Composition of Soil-Derived Dissolved Organic Matter. ACS Earth Space Chem. 2020, 4, 189–201. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Shi, Y.; Han, X. Long-term fertilization on indicators of soil active organic carbon. Chin. J. Soil Sci. 2008, 39, 752–755. [Google Scholar]
- Zhao, L.; Han, X.; Wang, S.; Liu, H.; Li, H.; Miao, S.; Wang, F. Change of crop yield and soil fertility under long-term fertilization and nutrients recycling and reutilization on a black soil: IV. soiil organic carbon and its fractions. Chin. J. Appl. Ecol. 2006, 17, 817–821. [Google Scholar]
- Yan, C.; Gong, J.; Zhang, S.; Zhang, W.; Dong, X. Effects of nitrogen addition on soil active organic carbon in a temperate grassland of Nei Mongol. Chin. J. Plant Ecol. 2024, 48, 229. [Google Scholar]
- Blair, G.; Lefroy, R.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 393–406. [Google Scholar] [CrossRef]
- Gai, X.; Zhang, J.; Lv, H.; Huang, Z.; Li, Q. Effects of chicken farming on soil active rrganic carbon and carbon pool management index in the Lei bamboo (Phyllostachys praecox) forest. Sci. Silvae Sin. 2023, 59, 78–86. [Google Scholar]
- Guo, B.; Fan, S.; Du, M.; Liu, G.; Su, W. Effect of land-use type on soil labile carbon pool and carbon management index. Chin. J. Ecol. 2014, 33, 723–728. [Google Scholar]
- Du, M.; Liu, G.; Fan, S.; Feng, H.; Tang, X.; Mao, C. Effects of Fertilization on the Distribution Patterns of Biomass and Carbon Storage in Moso Bamboo Forest, Western Fujian Province, China. Chin. J. Trop. Crops 2015, 36, 872–877. [Google Scholar]
- Lu, Q.; Lin, S.; Liu, S.; Zheng, X.; Bi, Y.; Xiao, Z.; Jiang, J.; Wang, A.; Du, X. Meta-analysis of the effect of fertilization on the yield of Phyllostachys edulis forest. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 88–96. [Google Scholar]
- Chu, H.; Ni, H.; Su, W.; Fan, S.; Long, Y.; Sun, Y. Enhanced Nitrogen Fertilizer Input Alters Soil Carbon Dynamics in Moso Bamboo Forests, Impacting Particulate Organic and Mineral-Associated Carbon Pools. Forests 2023, 14, 2460. [Google Scholar] [CrossRef]
- Su, W. Fertilization Theory and Practice for Phyllostachys Edulis Stand Based on Growth and Nutrient Accumulation Rules. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2012. [Google Scholar]
- Hyvönen, R.; Persson, T.; Andersson, S.; Olsson, B.; Ågren, G.I.; Linder, S. Impact of Long-Term Nitrogen Addition on Carbon Stocks in Trees and Soils in Northern Europe. Biogeochemistry 2008, 89, 121–137. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, F.C.; Fang, X.M.; He, P.; Zhang, Y.F.; Chen, F.S.; Wang, H.M. Responses of Soil Organic Carbon and Its Labile Fractions to Nitrogen and Phosphorus Additions in Cunninghamia Lanceolata Plantations in Subtropical China. Chin. J. Appl. Ecol. 2017, 28, 449–455. [Google Scholar]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Turner, B.L.; Zhou, G.; Mo, J. Nitrogen Deposition Accelerates Soil Carbon Sequestration in Tropical Forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef]
- Chen, Z.J.; Geng, S.C.; Zhang, J.H.; Setälä, H.; Gu, Y.; Wang, F.; Zhang, X.; Wang, X.X.; Han, S.J. Addition of Nitrogen Enhances Stability of Soil Organic Matter in a Temperate Forest. Eur. J. Soil. Sci. 2017, 68, 189–199. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Fu, S.; Yan, E.; Wu, J.; Lin, L. Long-Term Intensive Management Effects on Soil Organic Carbon Pools and Chemical Composition in Moso Bamboo (Phyllostachys Pubescens) Forests in Subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Qi, P.; Chen, J.; Wang, X.; Zhang, R.; Cai, L.; Jiao, Y.; Li, Z.; Han, G. Changes in Soil Particulate and Mineral-Associated Organic Carbon Concentrations under Nitrogen Addition in China—A Meta-Analysis. Plant Soil 2023, 489, 439–452. [Google Scholar] [CrossRef]
- Gao, G.; Li, Z.; Ge, X.; Huang, R.; LI, A. Effects of nitrogen addition on biomass and root morphology of phyllostachys edulis assdlings under drough stress. Chin. J. Ecol. 2022, 41, 858–864. [Google Scholar]
- Ye, C.; Chen, D.; Hall, S.J.; Pan, S.; Yan, X.; Bai, T.; Guo, H.; Zhang, Y.; Bai, Y.; Hu, S. Reconciling Multiple Impacts of Nitrogen Enrichment on Soil Carbon: Plant, Microbial and Geochemical Controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef]
- Pold, G.; Domeignoz-Horta, L.A.; Morrison, E.W.; Frey, S.D.; Sistla, S.A.; DeAngelis, K.M. Carbon Use Efficiency and Its Temperature Sensitivity Covary in Soil Bacteria. mBio 2020, 11, e02293-19. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z.; Chen, J.; Xu, H.; Ma, S.; Dippold, M.A.; Kuzyakov, Y. Long-Term Nitrogen and Phosphorus Fertilization Reveals That Phosphorus Limitation Shapes the Microbial Community Composition and Functions in Tropical Montane Forest Soil. Sci. Total Environ. 2023, 854, 158709. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-M.; Zhang, X.-L.; Chen, F.-S.; Zong, Y.-Y.; Bu, W.-S.; Wan, S.-Z.; Luo, Y.; Wang, H. Phosphorus Addition Alters the Response of Soil Organic Carbon Decomposition to Nitrogen Deposition in a Subtropical Forest. Soil. Biol. Biochem. 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Li, J.H.; Cheng, B.H.; Zhang, R.; Li, W.J.; Shi, X.M.; Han, Y.W.; Ye, L.F.; Ostle, N.J.; Bardgett, R.D. Nitrogen and Phosphorus Additions Accelerate Decomposition of Slow Carbon Pool and Lower Total Soil Organic Carbon Pool in Alpine Meadows. Land. Degrad. Dev. 2021, 32, 1761–1772. [Google Scholar] [CrossRef]
- Chu, H.; Su, W.; Zhou, Y.; Wang, Z.; Long, Y.; Sun, Y.; Fan, S. Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests. Forests 2024, 15. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Zhang, W.; Shao, Y.; Duan, H.; Chen, B.; Wei, X.; Fan, H. Long-Term Nitrogen Addition Changes Soil Microbial Community and Litter Decomposition Rate in a Subtropical Forest. Appl. Soil. Ecol. 2019, 142, 43–51. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil Extracellular Enzyme Activities, Soil Carbon and Nitrogen Storage under Nitrogen Fertilization: A Meta-Analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Qu, X.; Wang, X.; Wu, J.; He, P. Both Carbon Sequestration and Yield Are Related to Particulate Organic Carbon Stability Affected by Organic Amendment Origins in Mollisol. J. Soils Sediments 2021, 21, 3044–3056. [Google Scholar] [CrossRef]
- Li, J.; Pan, W.; Wang, J.; Xue, L.; Zhang, X.; Li, S. Response of active organic carbon and carbon pool management index of karst soil to rocky desertification control measures. For. Res. 2022, 35, 156–163. [Google Scholar]
- Jin, B.; Derrick Yuk Fo, L.; Gao, D.; Tong, C.; Zeng, C. Changes in Soil Organic Carbon Dynamics in a Native C4 Plant-Dominated Tidal Marsh Following Spartina Alterniflora Invasion. Pedosphere 2017, 27, 856–867. [Google Scholar] [CrossRef]
- Li, Q.; Lv, J.; Peng, C.; Xiang, W.; Xiao, W.; Song, X. Nitrogen-Addition Accelerates Phosphorus Cycling and Changes Phosphorus Use Strategy in a Subtropical Moso Bamboo Forest. Environ. Res. Lett. 2021, 16, 024023. [Google Scholar] [CrossRef]
- Rezaei, S.; Gilkes, R.; Andrews, S.; Arzani, H. Soil Quality Assessment in Semiarid Rangeland in Iran. Soil. Use Manag. 2005, 21, 402–409. [Google Scholar] [CrossRef]
- Yakovlev, A.; Gendugov, V.; Glazunov, G.; Evdokimova, M.; Shulakova, E. Methodology for the Environmental Assessment of the Soil State and Regulation of the Soil Quality. Eurasian Soil. Sci. 2009, 42, 916–925. [Google Scholar] [CrossRef]

| Soil Depth | Nitrogen Application Treatments | POC/SOC (%) | MBC/SOC (%) | DOC/SOC (%) | ROC/SOC (%) |
|---|---|---|---|---|---|
| 0~10 cm | CK | 6.47 ± 0.35 cd | 1.73 ± 0.06 b | 1.38 ± 0.07 b | 53.5 ± 1.64 ab |
| N0 | 5.41 ± 0.1 d | 2.06 ± 0.08 a | 1.38 ± 0.06 b | 50.66 ± 4.75 ab | |
| N1 | 12.89 ± 0.6 a | 0.85 ± 0.03 c | 1.69 ± 0.11 a | 58.32 ± 3.99 a | |
| N2 | 10.49 ± 0.61 b | 1.06 ± 0.11 c | 1.46 ± 0.11 ab | 44.98 ± 3.25 b | |
| N3 | 7.85 ± 1.03 c | 1.86 ± 0.09 ab | 0.9 ± 0.06 c | 28.34 ± 3.04 c | |
| 10~20 cm | CK | 8.6 ± 0.94 b | 1.07 ± 0.11 c | 1.18 ± 0.06 b | 50.07 ± 4.65 a |
| N0 | 8.64 ± 1.1 b | 2.49 ± 0.19 a | 1.13 ± 0.11 b | 49.42 ± 2.88 a | |
| N1 | 10.82 ± 0.14 ab | 1.05 ± 0.11 c | 1.41 ± 0.07 a | 58.64 ± 5.27 a | |
| N2 | 11.66 ± 0.23 a | 1.4 ± 0.05 bc | 1.23 ± 0.01 ab | 50.14 ± 1.07 a | |
| N3 | 10.01 ± 0.96 ab | 1.69 ± 0.16 b | 0.65 ± 0.04 c | 29.64 ± 3.83 b | |
| 20~30 cm | CK | 10.75 ± 1 a | 0.85 ± 0.12 a | 1.15 ± 0.07 b | 48.97 ± 2.64 b |
| N0 | 11.45 ± 1.74 a | 2.47 ± 0.28 b | 1.09 ± 0.11 b | 56.88 ± 3.64 ab | |
| N1 | 10.81 ± 0.97 a | 1.37 ± 0.09 b | 1.19 ± 0.06 ab | 63.48 ± 1.06 a | |
| N2 | 10.71 ± 1.24 a | 2.07 ± 0.13 a | 1.45 ± 0.09 a | 64.95 ± 2.81 a | |
| N3 | 10.32 ± 1.24 a | 2.45 ± 0.14 a | 0.81 ± 0.07 c | 47.47 ± 5.35 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.; Su, W.; Fan, S.; He, X.; Huang, Z. Impact of Nitrogen Fertilizer Application on Soil Organic Carbon and Its Active Fractions in Moso Bamboo Forests. Forests 2024, 15, 1483. https://doi.org/10.3390/f15091483
Chu H, Su W, Fan S, He X, Huang Z. Impact of Nitrogen Fertilizer Application on Soil Organic Carbon and Its Active Fractions in Moso Bamboo Forests. Forests. 2024; 15(9):1483. https://doi.org/10.3390/f15091483
Chicago/Turabian StyleChu, Haoyu, Wenhui Su, Shaohui Fan, Xianxian He, and Zhoubin Huang. 2024. "Impact of Nitrogen Fertilizer Application on Soil Organic Carbon and Its Active Fractions in Moso Bamboo Forests" Forests 15, no. 9: 1483. https://doi.org/10.3390/f15091483
APA StyleChu, H., Su, W., Fan, S., He, X., & Huang, Z. (2024). Impact of Nitrogen Fertilizer Application on Soil Organic Carbon and Its Active Fractions in Moso Bamboo Forests. Forests, 15(9), 1483. https://doi.org/10.3390/f15091483






