Enhanced Carbon Storage in Mixed Coniferous and Broadleaf Forest Compared to Pure Forest in the North Subtropical–Warm Temperate Transition Zone of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Sample Collection and Processing
2.3. Calculation of Biomass and Carbon Storage
BiBranches = 0.02933(D2H)0.75662
BiLeaves = 0.09220(D2H)0.39445
BiRoots = 0.16723(D2H)0.64106
BiBranches = 0.00550(D2H)1.0439
BiLeaves = 0.00110(D2H)1.12566
BiRoots = 0.00330(D2H)1.0148
2.4. Measurement of Impact Factors
2.5. Data Analysis
3. Results
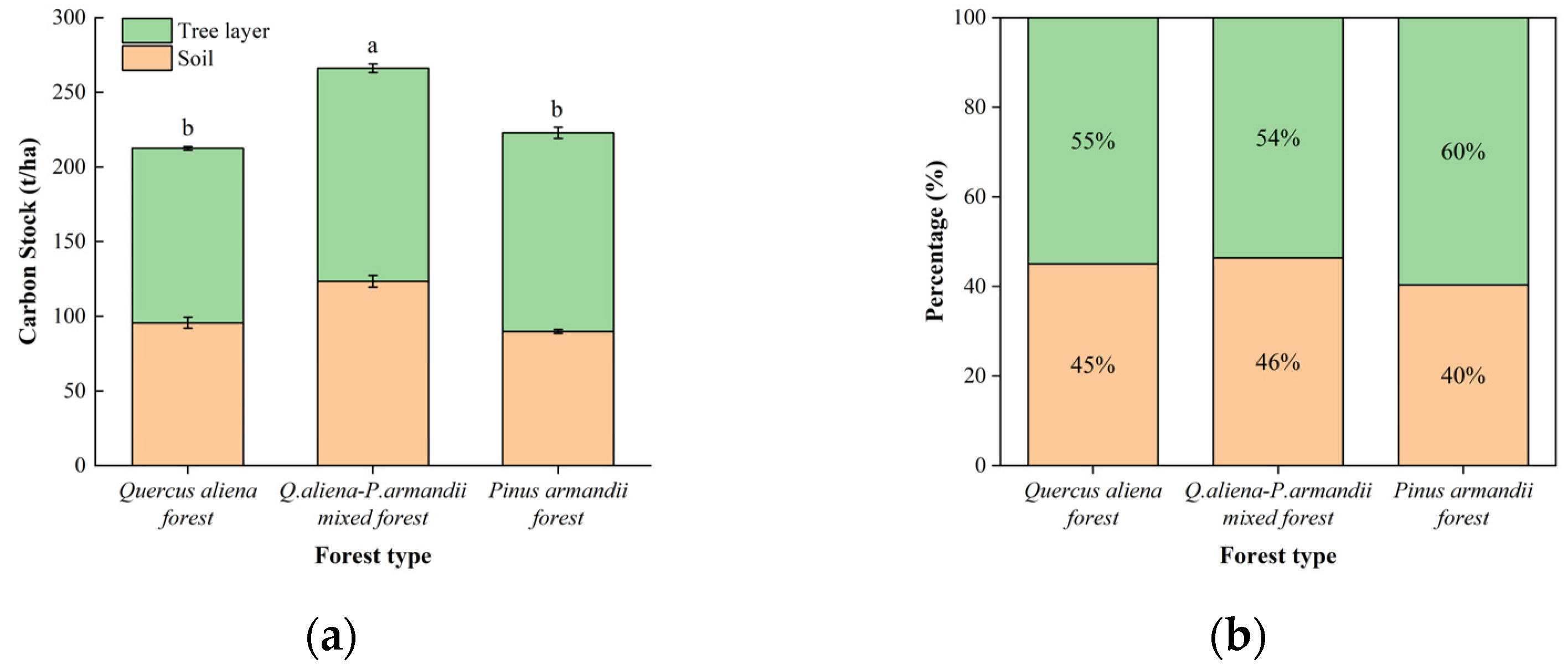
3.1. Carbon Storage and Its Allocation Patterns in the Tree Layer of Different Forest Types
3.2. Soil Carbon Storage and Allocation Patterns in Different Forest Ecosystems
3.3. Total Carbon Storage and Its Allocation Patterns in Different Forest Ecosystems
3.4. Dominant Factors of Carbon Storage in the Tree Layer of Different Forest Types
3.5. Dominant Factors of Soil Carbon Storage in Different Forest Types
4. Discussion
4.1. Carbon Storage and Distribution Patterns in Three Types of Forests
4.2. Dominant Factors of Forest Carbon Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; UNEP. The State of the World’s Forests 2020. In Forests, Biodiversity and People; Sprep Library: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Kumar, R.; Bhardwaj, D.R.; Yadav, R.P.; Negi, V. Budgeting of biomass and carbon stock as ecosystem service from Himalayan dry temperate and alpine forest ecosystem, India. Appl. Ecol. Environ. Res. 2023, 21, 2881–2896. [Google Scholar] [CrossRef]
- Li, Z.R.; Lai, Q.; Bao, Y.H.; Sude, B.; Bao, Z.Y.; Liu, X.Y. Carbon Allocation to Leaves and Its Controlling Factors and Impacts on Gross Primary Productivity in Forest Ecosystems of Northeast China. Forests 2024, 15, 129. [Google Scholar] [CrossRef]
- Bowman, D.; Murphy, B.; Boer, M.; Bradstock, R.; Cary, G.; Cochrane, M.; Fensham, R.; Krawchuk, M.; Price, O.; Williams, R. Forest fire management, climate change, and the risk of catastrophic carbon losses. Front. Ecol. Environ. 2013, 11, 66–68. [Google Scholar] [CrossRef]
- Ruiz-Peinado, R.; Bravo-Oviedo, A.; López-Senespleda, E.; Montero, G.; Río, M. Do thinnings influence biomass and soil carbon stocks in Mediterranean maritime pinewoods? Eur. J. Forest Res. 2013, 132, 253–262. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; Wang, K.L.; Su, Y.R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Wang, J.S.; Sun, J.; Xia, J.Y.; He, N.P.; Li, M.L.; Niu, S.L. Soil and vegetation carbon turnover times from tropical to boreal forests. Funct. Ecol. 2018, 32, 71–82. [Google Scholar] [CrossRef]
- Ma, Z.L.; Chen, H.Y.H.; Bork, E.W.; Carlyle, C.N.; Chang, S.X. Carbon accumulation in agroforestry systems is affected by tree species diversity, age and regional climate: A global meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1817–1828. [Google Scholar] [CrossRef]
- Raj, A.; Jhariya, M.K. Carbon storage, flux and mitigation potential of tropical Sal mixed deciduous forest ecosystem in Chhattisgarh, India. J. Environ. Manag. 2021, 293, 112829. [Google Scholar] [CrossRef]
- Anudip, G.; Jitendra, A.; Uttam, K.S. Evaluation of ecosystem carbon storage in major forest types of Eastern Himalaya: Implications for carbon sink management. J. Environ. Manag. 2022, 302, 113972. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ai, J.J.; Sun, Q.W.; Li, Z.C.; Hou, L.Y.; Song, L.G.; Tang, G.Y.; Li, L.; Shao, G.D. Soil organic carbon and total nitrogen stocks as affected by vegetation types and altitude across the mountainous regions in the Yunnan Province, south-western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Xiao, R.H.; Man, X.L.; Duan, B.X. Carbon and Nitrogen Stocks in Three Types of Larix gmelinii Forests in Daxing’an Mountains, Northeast China. Forests 2020, 11, 305. [Google Scholar] [CrossRef]
- Yuan, Z.X.; Jin, X.M.; Xiao, W.Y.; Wang, L.; Sun, Y.; Guan, Q.W.; Meshack, A.O. Comparing soil organic carbon stock and fractions under natural secondary forest and Pinus massoniana plantation in subtropical China. Catena 2022, 212, 106092. [Google Scholar] [CrossRef]
- Duan, X.W.; Rong, L.; Hu, J.M.; Zhang, G. Soil organic carbon stocks in the Yunnan Plateau, southwest China: Spatial variations and environmental controls. J. Soil. Sediment. 2014, 14, 1643–1658. [Google Scholar] [CrossRef]
- Wu, X.G.; Wang, W.P.; Li, B.; Liang, Y.L.; Liu, Y.Z. Altitudinal Gradient of Soil Organic Carbon in Forest Soils in the Mid-Subtropical Zone of China. Acta Pedol. Sin. 2020, 57, 1539–1547. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, W.T.; Xie, F.L.; Li, T.; He, N.J.; Zhang, K.R.; Zhang, Q.F.; Dang, H.S. Species-habitat association of a deciduous broadleaved forest in the subtropical and temperate transition zone. Chin. J. Appl. Ecol. 2021, 32, 2755–2762. (In Chinese) [Google Scholar] [CrossRef]
- Yao, Y.T.; Piao, S.L.; Wang, T. Future biomass carbon sequestration capacity of Chinese forests. Sci. Bull. 2018, 63, 1108–1117. [Google Scholar] [CrossRef]
- Favero, A.; Daigneault, A.; Sohngen, B. Forests: Carbon sequestration, biomass energy, or both? Sci. Adv. 2020, 6, eaay6792. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Zhao, X.; Zhao, Z.; Wang, Y.C.; Zhang, X.P.; Wang, D.X. The linkages of plant, litter and soil C: N: P stoichiometry and nutrient stock in different secondary mixed forest types in the Qinling Mountains, China. PeerJ 2020, 8, e9274. [Google Scholar] [CrossRef]
- Hao, Z.; Quan, Z.; Han, Y.; Lv, C.; Zhao, X.; Jing, W.J.; Zhu, L.H.; Ma, J.Y. Soil mineralized carbon drives more carbon stock in coniferous-broadleaf mixed plantations compared to pure plantations. PeerJ 2022, 10, e13542. [Google Scholar] [CrossRef]
- Hadley, J.L.; Kuzeja, P.S.; Daley, M.J.; Phillips, N.G.; Mulcahy, T.; Singh, S. Water use and carbon exchange of red oak-and eastern hemlock-dominated forests in the northeastern USA: Implications for ecosystem-level effects of hemlock woolly adelgid. Tree Physiol. 2008, 28, 615–627. [Google Scholar] [CrossRef]
- He, N.P.; Wen, D.; Zhu, J.X.; Tang, X.L.; Xu, L.; Zhang, L.; Hu, H.F.; Huang, M.; Yu, G.R. Vegetation carbon sequestration in Chinese forests from 2010 to 2050. Global Change Biol. 2017, 23, 1575–1584. [Google Scholar] [CrossRef]
- Baddeley, J.A.; Edwards, A.C.; Watson, C.A. Changes in soil C and N stocks and C:N stoichiometry 21 years after land use change on an arable mineral topsoil. Geoderma 2017, 303, 19–26. [Google Scholar] [CrossRef]
- Lutter, R.; Kõlli, R.; Tullus, A.; Tullus, H. Ecosystem carbon stocks of estonian premature and mature managed forests: Effects of site conditions and overstorey tree species. Eur. J. For. Res. 2018, 138, 125–142. [Google Scholar] [CrossRef]
- Wu, X.D.; Zhao, L.; Hu, G.J.; Liu, G.M.; Li, W.P.; Ding, Y.J. Permafrost and land cover as controlling factors for light fraction organic matter on the southern Qinghai-Tibetan plateau. Sci. Total Environ. 2018, 613, 1165–1174. [Google Scholar] [CrossRef]
- Yue, C.; Ciais, P.; Luyssaert, S.; Li, W.; McGrath, M.J.; Chang, J.F.; Peng, S.S. Representing anthropogenic gross land use change, wood harvest, and forest age dynamics in a global vegetation model ORCHIDEE-MICT v8. 4.2. Geosci. Model Dev. 2018, 11, 409–428. [Google Scholar] [CrossRef]
- Yu, Z.; You, W.B.; Agathokleous, E.; Zhou, G.Y.; Liu, S.R. Forest management required for consistent carbon sink in China’s forest plantations. For. Ecosyst. 2021, 8, 54. [Google Scholar] [CrossRef]
- Walker, A.P.; De Kauwe, M.G.; Bastos, A.; Belmecheri, S.; Georgiou, K.; Keeling, R.F.; McMahon, S.M.; Medlyn, B.E.; Moore, D.J.P.; Norby, R.J.; et al. Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. New Phytol. 2021, 229, 2413–2445. [Google Scholar] [CrossRef] [PubMed]
- Haverd, V.; Smith, B.; Canadell, J.G.; Cuntz, M.; Mikaloff-Fletcher, S.; Farquhar, G.; Woodgate, W.; Briggs, P.R.; Trudinger, C.M. Higher than expected CO2 fertilization inferred from leaf to global observations. Glob. Chang. Biol. 2020, 26, 2390–2402. [Google Scholar] [CrossRef]
- Zhang, X.B.; Sun, Z.G.; Liu, J.; Ouyang, Z.; Wu, L.H. Simulating greenhouse gas emissions and stocks of carbon and nitrogen in soil from a long-term no-till system in the North China Plain. Soil Tillage Res. 2018, 178, 32–40. [Google Scholar] [CrossRef]
- Li, Y.Y.; Dong, S.K.; Wen, L.; Wang, X.X. Soil carbon and nitrogen pools and their relationship to plant and soil dynamics of degraded and artificially restored grasslands of the Qinghai-Tibetan Plateau. Geoderma 2014, 213, 178–184. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, Z.; Li, X.; Wang, Y.Y.; Bai, Z.K. Characteristics of labile organic carbon fractions in reclaimed mine soils: Evidence from three reclaimed forests in the Pingshuo opencast coal mine, China. Sci. Total Environ. 2018, 613, 1196–1206. [Google Scholar] [CrossRef]
- Simard, M.; Lecomte, N.; Bergeron, Y.; Bernier, P.Y.; Paré, D. Forest productivity decline caused by successional paludification of boreal soils. Ecol. Appl. 2007, 17, 1619–1637. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.N.; Chen, S.F.; Zheng, X.; Ge, X.M.; Li, Y.; Fang, Y.M.; Cui, P.; Ding, H. Neighborhood diversity structure and neighborhood species richness effects differ across life stages in a subtropical natural secondary forest. For. Ecosyst. 2022, 9, 100075. [Google Scholar] [CrossRef]
- Seidl, R.; Turner, M.G. Post-disturbance reorganization of forest ecosystems in a changing world. Proc. Natl. Acad. Sci. USA 2022, 119, e2202190119. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Pena-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Özcan, M.; Gökbulak, F.; Hizal, A. Exclosure effects on recovery of selected soil properties in a mixed broadleaf forest recreation site. Land Degrad. Dev. 2013, 24, 266–276. [Google Scholar] [CrossRef]
- Sun, L.; Yang, L.; Chen, L.; Li, S.; Zhao, F.; Sun, S. Tracing the soil water response to autumn rainfall in different land uses at multi-day timescale in a subtropical zone. Catena 2019, 180, 355–364. [Google Scholar] [CrossRef]
- Chen, C.G.; Gong, L.Q.; Peng, H.; Liu, X.Z. Biomass and Productivity of the Sharptooth Oak Forests in the Qinling Moutains. J. Northwest For. Univ. 1996, 11 (Suppl. S1), 103–114. (In Chinese) [Google Scholar]
- Chen, C.G. Study on the Production of Pinus armandii Forests in the Qinling Mountains Biomass and Production of the Arbor-Layers in Pinus armandii Forests (1). J. Northwest For. Univ. 1984, 1, 1–18. (In Chinese) [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Solymos, P.; Steven, M.H.H.; Wagner, H.; Vegan: Community Ecology Package. R Package Version 1.15–1. 2008. Available online: http://www.R-project.org (accessed on 19 December 2008).
- Leo, B.; Adele, C.; Andy, L.; Matthew, W.; Package “randomForest” title Breiman and cutler’s random forests for classification and regression. R Package Version 4.6–14. 2018. Available online: https://cran.r-project.org/src/contrib/Archive/randomForest (accessed on 25 March 2018).
- Zhou, Y.R.; Yu, Z.L.; Zhao, S.D. Carbon storage and budget of major Chinese forest types. Chin. J. Plant Ecol. 2000, 24, 518–522. (In Chinese) [Google Scholar]
- Wei, X.H.; Blanco, J.A.; Hui, D.F. Significant Increase in Ecosystem C Can Be Achieved with Sustainable Forest Management in Subtropical Plantation Forests. PLoS ONE 2014, 9, e89688. [Google Scholar] [CrossRef]
- Köhl, M.; Neupane, P.R.; Lotfiomran, N. The impact of tree age on biomass growth and carbon accumulation capacity: A ret-rospective analysis using tree ring data of three tropical tree species grown in natural forests of Suriname. PLoS ONE 2017, 12, e0181187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Gu, F.X.; Liu, S.R.; Liu, Y.C.; Li, C. Variations of carbon stock with forest types in subalpine region of southwestern China. Forest Ecol. Manag. 2013, 300, 88–95. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. Forest Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Falster, D.S.; Duursma, R.A.; FitzJohn, R.G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, E6789–E6798. [Google Scholar] [CrossRef]
- Kaushal, S.; Baishya, R. Stand structure and species diversity regulate biomass carbon stock under major Central Himalayan forest types of India. Ecol. Process 2021, 10, 14. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, Plasticity and Allometry in Plants. Perspect. Plant Ecol. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Biomass Allocation in Plants: Ontogeny or Optimality? A Test along Three Resource Gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Niklas, K.J.; Enquist, B.J. Canonical rules for plant organ biomass partitioning and annual allocation. Am. J. Bot. 2002, 89, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I. Ecological and physiological processes in mixed versus monospecific stands. In Mixed-Species Forests; Pretzsch, H., Forrester, D., Bauhus, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, P.; Ding, J.Z.; Zhao, X.; Ma, W.H.; Ji, C.J.; Fang, J.Y. Increased topsoil carbon stock across China’s forests. Global Change Biol. 2014, 20, 2687–2696. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.J.; Saeed, S.; Zhang, B.; Luo, M. The difference of soil properties between pure and mixed Chinese fir (Cunninghamia lanceolata) plantations depends on tree species. Glob. Ecol. Conserv. 2020, 22, e01009. [Google Scholar] [CrossRef]
- Brunn, M.; Hafner, B.D.; Zwetsloot, M.J.; Weikl, F.; Pritsch, K.; Hikino, K.; Ruehr, N.K.; Sayer, E.J.; Bauerle, T.L. Carbon allocation to root exudates is maintained in mature temperate tree species under drought. New Phytol. 2022, 235, 965–977. [Google Scholar] [CrossRef]
- Díaz-Pinés, E.; Rubio, A.; Van Miegroet, H.; Montes, F.; Benito, M. Does tree species composition control soil organic carbon pools in Mediterranean mountain forests? Forest Ecol. Manag. 2011, 262, 1895–1904. [Google Scholar] [CrossRef]
- Cremer, M.; Kern, N.V.; Prietzel, J. Soil organic carbon and nitrogen stocks under pure and mixed stands of European beech, Douglas fir and Norway spruce. Forest Ecol. Manag. 2016, 367, 30–40. [Google Scholar] [CrossRef]
- McElligott, K.M.; Seiler, J.R.; Strahm, B.D. The Impact of Water Content on Sources of Heterotrophic Soil Respiration. Forests 2017, 8, 299. [Google Scholar] [CrossRef]
- Novick, K.A.; Miniat, C.F.; Vose, J.M. Drought limitations to leaf-level gas exchange: Results from a model linking stomatal optimization and cohesion tension theory. Plant Cell Environ. 2016, 39, 583–596. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Sussmilch, F.C.; Brodribb, T.J. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ. 2016, 39, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mund, M.; Kummetz, E.; Hein, M.; Bauer, G.A.; Schulze, E.D. Growth and carbon stocks of a spruce forest chronosequence in central Europe. Forest Ecol. Manag. 2002, 171, 275–296. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Law, B.E.; Quandt, D.; Williams, M. Effects of heat and drought on carbon and water dynamics in a regenerating semi-arid pine forest: A combined experimental and modeling approach. Biogeosciences 2014, 11, 4139–4156. [Google Scholar] [CrossRef]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, Z.; Wu, C.Y.; Song, L.S.; Ma, M.G.; Yu, P.J.; Lu, B.Q.; Tang, X.G. Divergent responses of ecosystem water-use efficiency to extreme seasonal droughts in Southwest China. Sci. Total Environ. 2021, 760, 143427. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Hipólito, M.; Bota, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Laclau, P.B.; Laclau, J.P. Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Res. 2009, 114, 351–360. [Google Scholar] [CrossRef]
- Luo, Z.K.; Feng, W.T.; Luo, Y.Q.; Baldock, J.; Wang, E.L. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef]
- Wang, T.; Kang, F.; Cheng, X.; Han, H.R.; Ji, W.J. Soil organic carbon and total nitrogen stocks under different land uses in a hilly ecological restoration area of North China. Soil Tillage Res. 2016, 163, 176–184. [Google Scholar] [CrossRef]
- de Groot, C.C.; van den Boogaard, R.; Marcelis, L.F.M.; Harbinson, J.; Lambers, H. Contrasting effects of N and P deprivation on the regulation of photosynthesis in tomato plants in relation to feedback limitation. J. Exp. Bot. 2003, 54, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Wassen, M.J.; Venterink, H.O.; Lapshina, E.D.; Tanneberger, F. Endangered plants persist under phosphorus limitation. Nature 2005, 437, 547–550. [Google Scholar] [CrossRef] [PubMed]
- van Groenigen, K.J.; Six, J.; Hungate, B.A.; de Graaff, M.A.; van Breemen, N.; van Kessel, C. Element interactions limit soil carbon storage. Proc. Natl. Acad. Sci. USA 2006, 103, 6571–6574. [Google Scholar] [CrossRef]
- Tang, X.Y.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Van der Sande, M.T.; Arets, E.J.; Peña-Claros, M.; Hoosbeek, M.R.; Cáceres-Siani, Y.; van der Hout, P.; Poorter, L. Soil fertility and species traits, but not diversity, drive productivity and biomass stocks in a Guyanese tropical rainforest. Funct. Ecol. 2018, 32, 461–474. [Google Scholar] [CrossRef]




| Plot | Main Species | Geographic Location | Altitude (m) | Density (Tree/ha) | Average Height (m) | Average DBH (cm) |
|---|---|---|---|---|---|---|
| Quercus aliena forest | Quercus aliena | 111°55′59″ E 33°29′51″ N | 1320–1323 | 692 | 21.19 ± 0.39 | 28.06 ± 0.68 |
| Q. aliena–P. armandii mixed forest | Quercus aliena, Pinus armandii | 111°55′51″ E 33°30′49″ N | 1296–3120 | 501 592 | 20.03 ± 0.45 16.38 ± 0.48 | 24.38 ± 0.97 21.61 ± 0.86 |
| Pinus armandii forest | Pinus armandii | 111°55′44″ E 33°30′55″ N | 1274–1281 | 738 | 18.62 ± 0.68 | 25.62 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhang, B.; Xu, Q.; Gao, D.; Zuo, H.; Ren, R.; Diao, K.; Chen, Z. Enhanced Carbon Storage in Mixed Coniferous and Broadleaf Forest Compared to Pure Forest in the North Subtropical–Warm Temperate Transition Zone of China. Forests 2024, 15, 1520. https://doi.org/10.3390/f15091520
Xu W, Zhang B, Xu Q, Gao D, Zuo H, Ren R, Diao K, Chen Z. Enhanced Carbon Storage in Mixed Coniferous and Broadleaf Forest Compared to Pure Forest in the North Subtropical–Warm Temperate Transition Zone of China. Forests. 2024; 15(9):1520. https://doi.org/10.3390/f15091520
Chicago/Turabian StyleXu, Wenbin, Beibei Zhang, Qing Xu, Deqiang Gao, Haijun Zuo, Ranran Ren, Ke Diao, and Zhicheng Chen. 2024. "Enhanced Carbon Storage in Mixed Coniferous and Broadleaf Forest Compared to Pure Forest in the North Subtropical–Warm Temperate Transition Zone of China" Forests 15, no. 9: 1520. https://doi.org/10.3390/f15091520






