Effect of Stand Age on Soil CO2 Emissions in Pedunculate Oak (Quercus robur L.) Forests
Abstract
1. Introduction
2. Materials and Methods
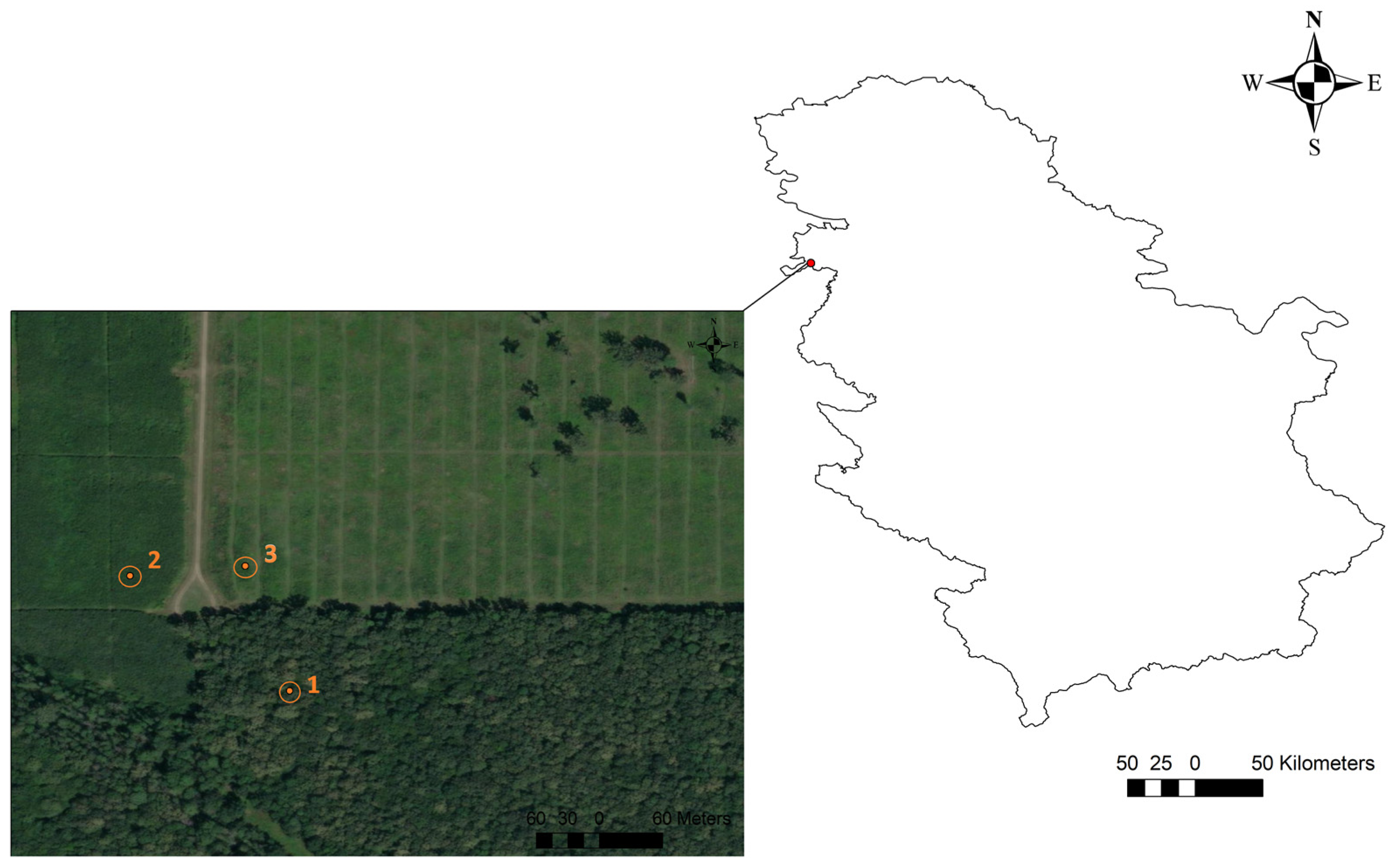
2.1. Site Description
2.2. The Study Design
2.2.1. The Description of Forest Stands within the Study Location
2.2.2. The Description of Forest Soil in the Examined Stands
2.3. Data Collection and Analysis
2.4. Statistical Analyses
3. Results
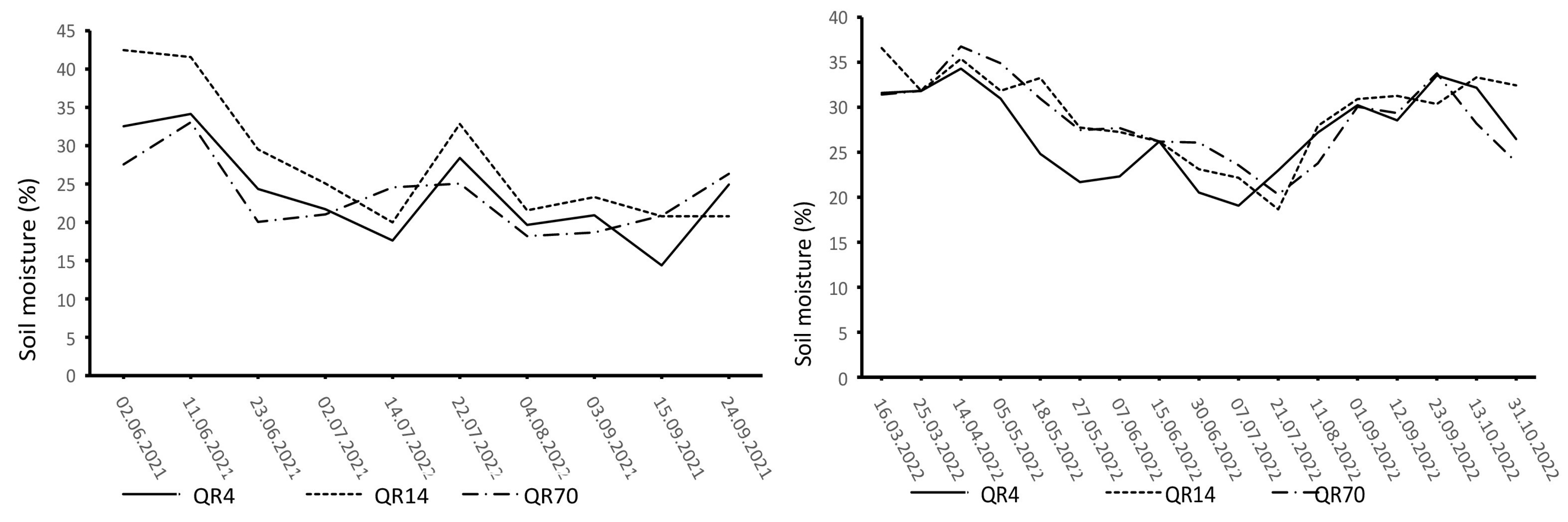
3.1. Dynamics of Soil Temperature and Moisture
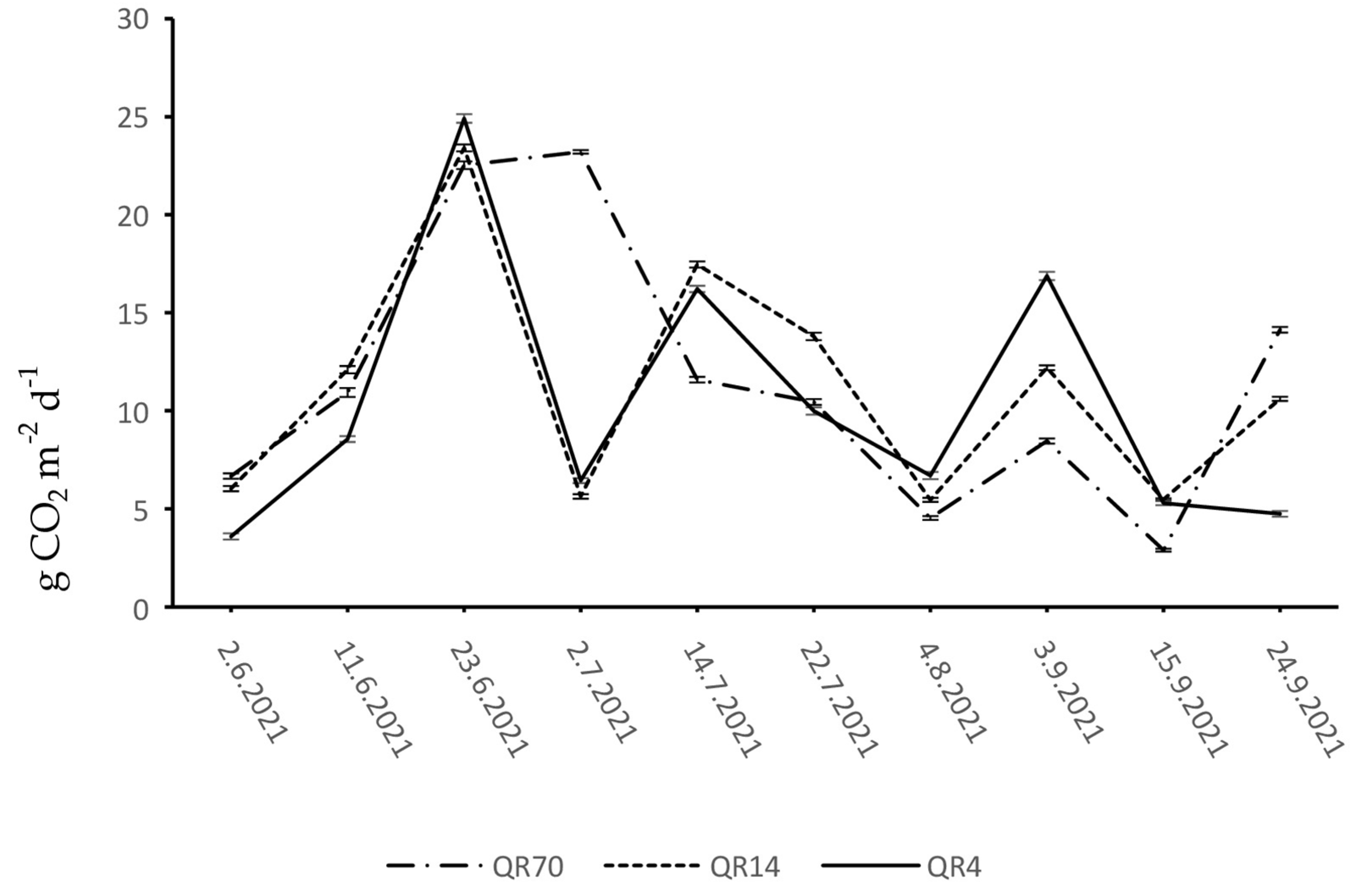
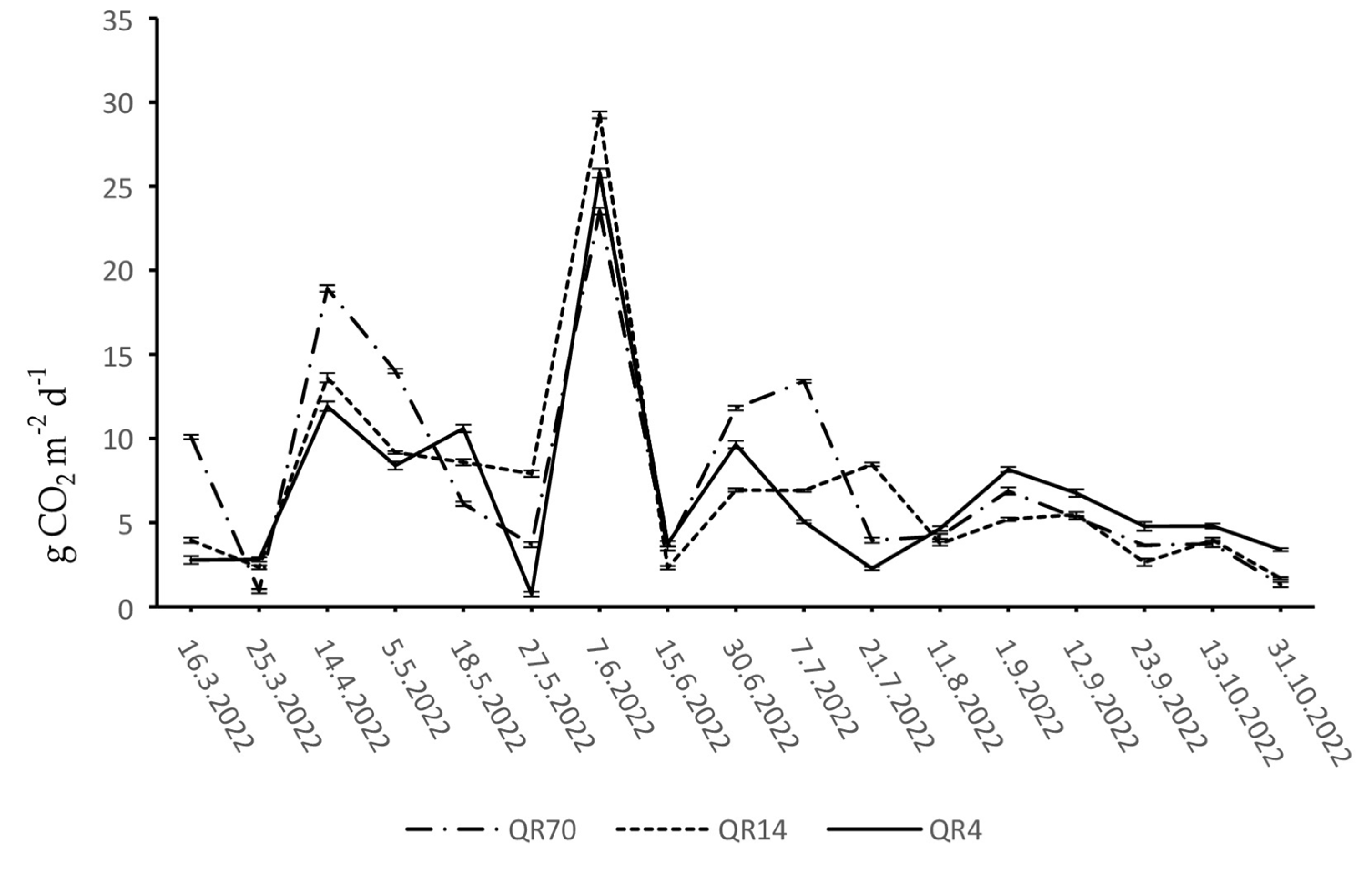
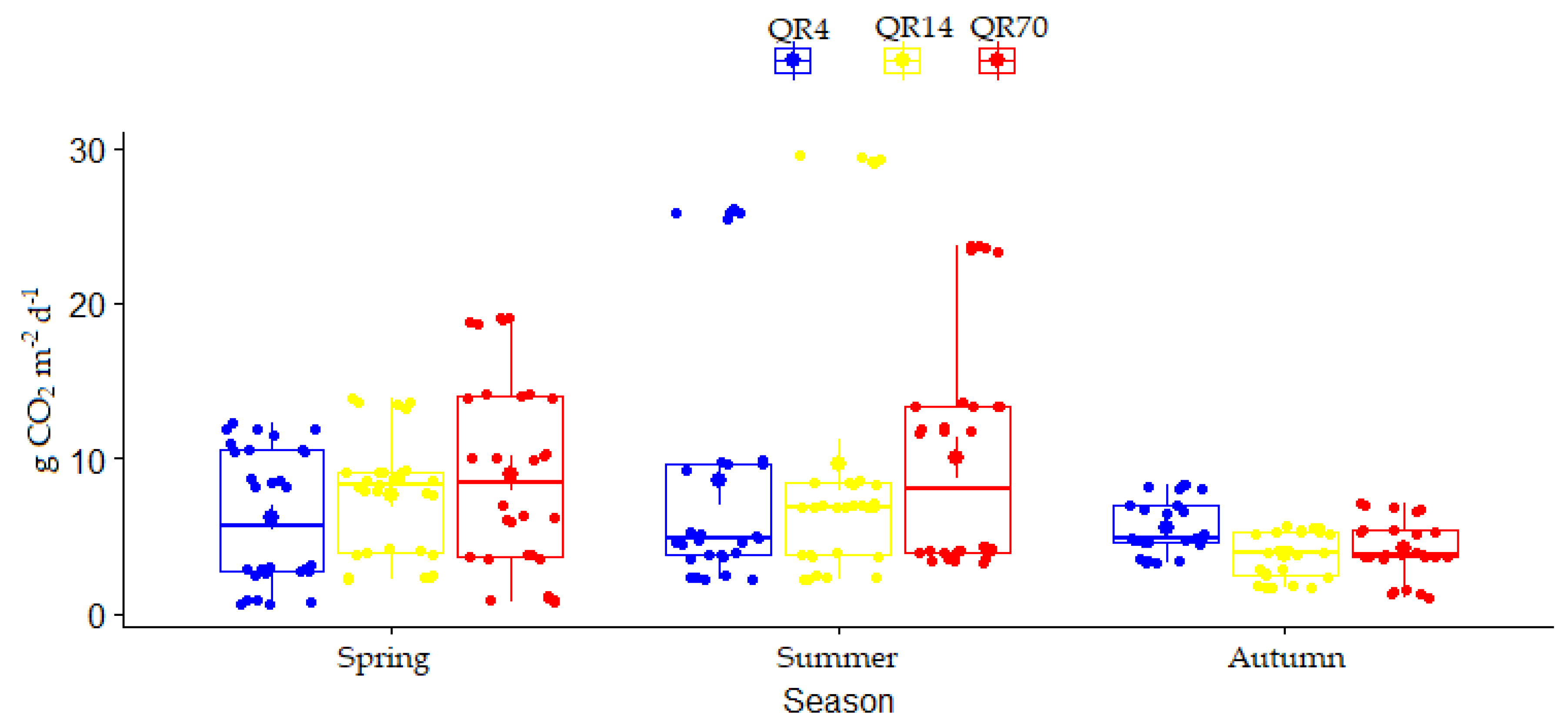
3.2. Dynamics of Soil CO2 Emissions
3.3. The Effects of Stand Age and Season on Soil CO2 Emissions
3.3.1. The Effects of Stand Age and Season on Soil CO2 Emission during 2022
3.3.2. The Effects of Stand Age on Soil CO2 Emission over the Entire Study Period (2021–2022)
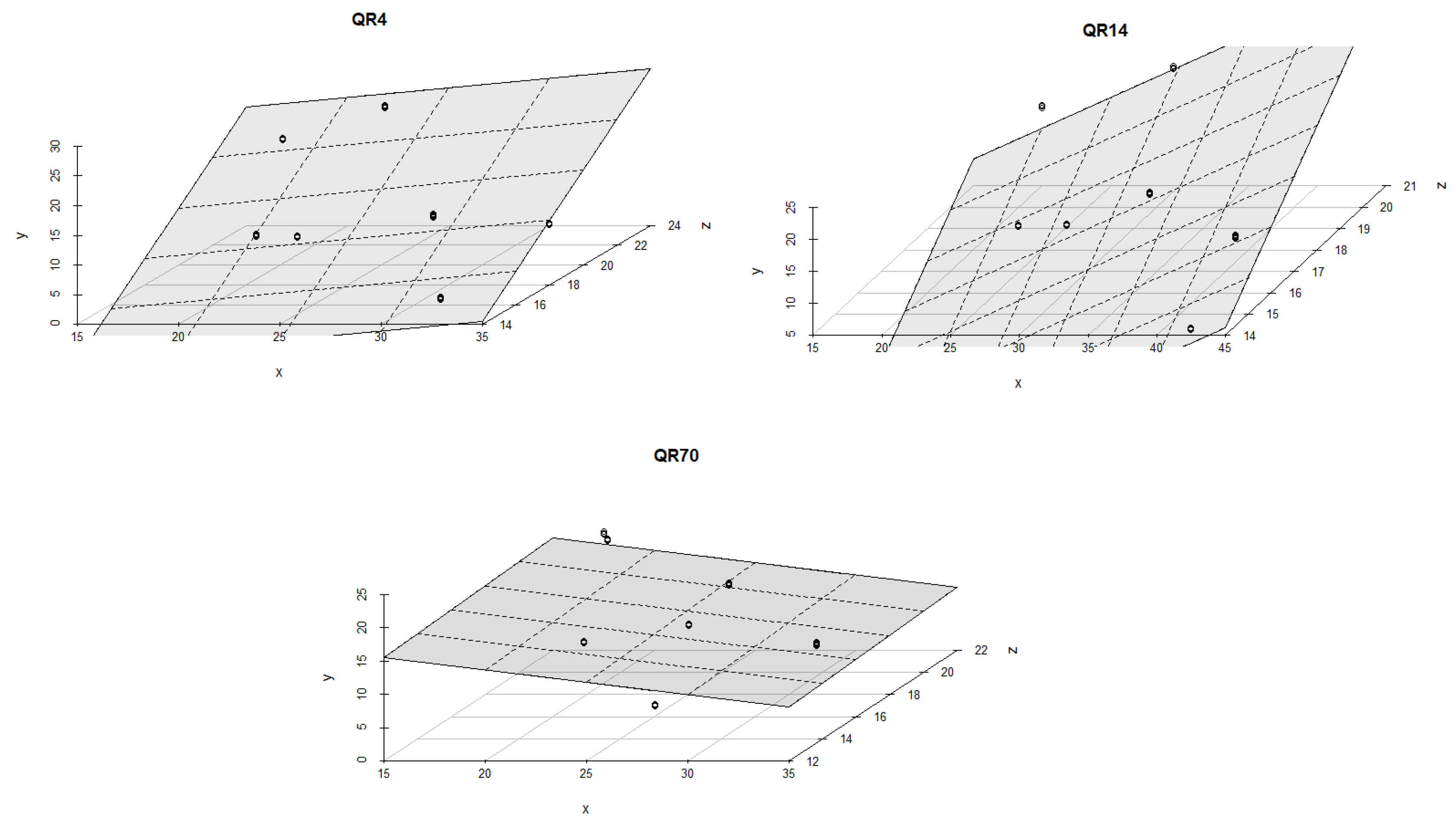
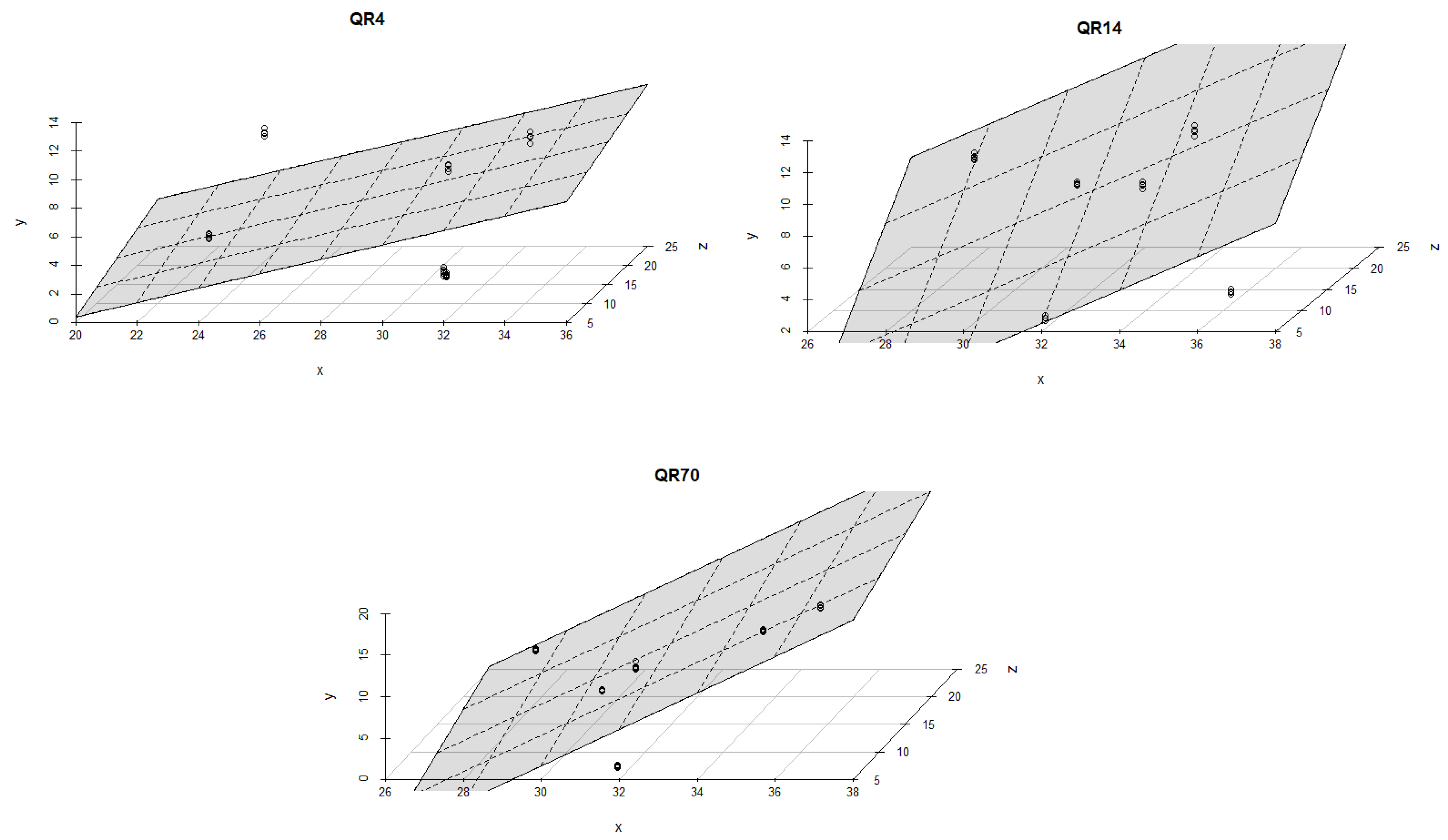
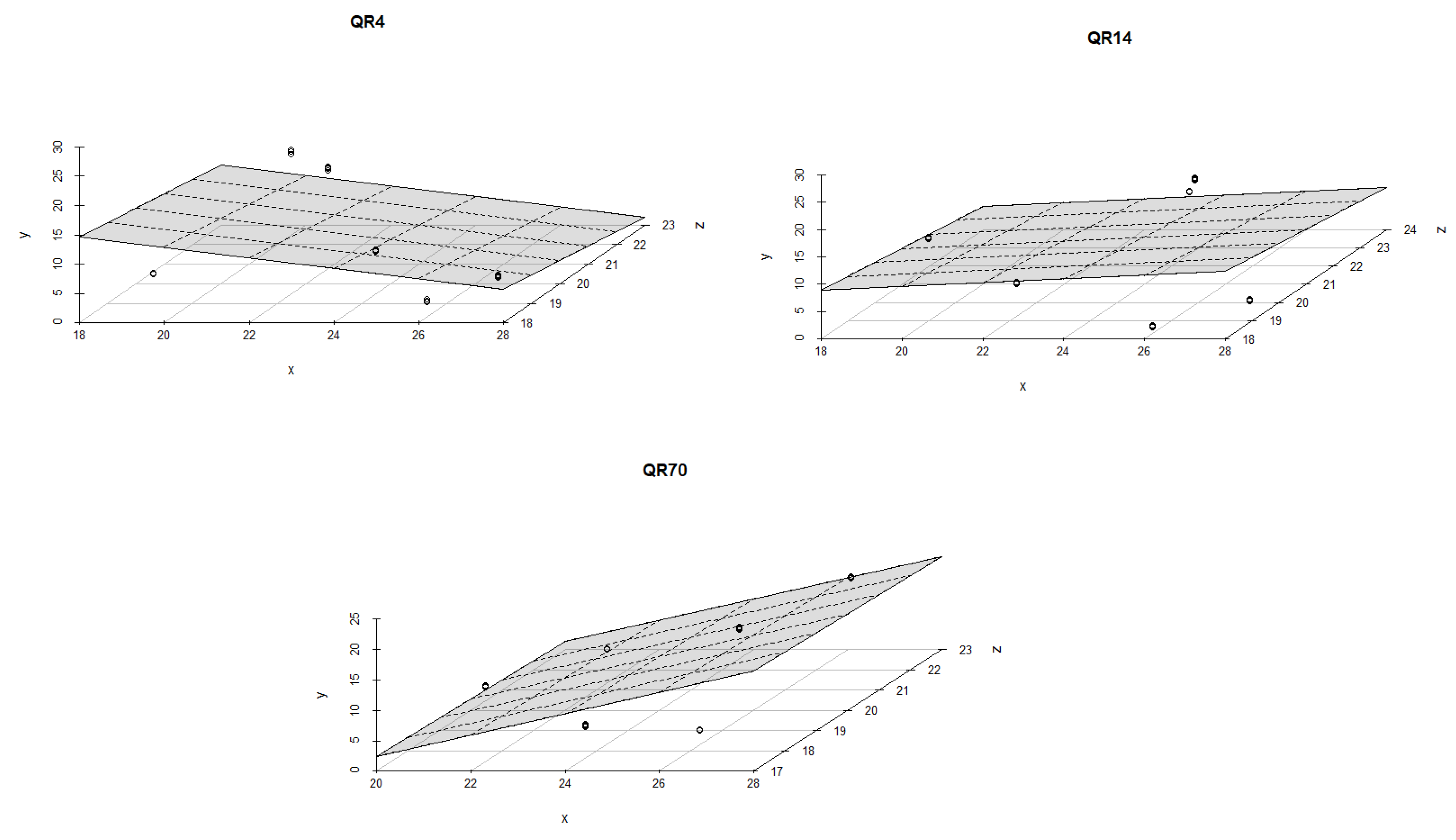
3.4. Influence of Soil Temperature and Soil Moisture on Soil CO2 Emission through Seasons
4. Discussion
4.1. Seasonal Dynamics of Soil CO2 Emission
4.2. The Influence of Soil Temperature and Moisture on Soil CO2 Emission through Seasons
4.3. The Influence of Stand Age on Soil CO2 Emission
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Stand/Season | Mod.eel | Regression Equations | Coefficients | N | R2 | R2adj | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | |||||||
| QR70/Summer of 2021 | 1. | y = a + bT + cW | 19.438 | 0.132 | −0.370 | 35 | 0.08 | 0.03 | 0.25 | |
| 2. | y = a + bT + cW + dTW | 309.848 *** | −15.955 ** | −11.378 *** | 0.619 ** | 35 | 0.35 | 0.29 | <0.05 | |
| QR14/Summer of 2021 | 1. | y = a + bT + cW | −101.547 *** | 4.579 *** | 0.968 *** | 35 | 0.85 | 0.84 | <0.001 | |
| 2. | y = a + bT + cW + dTW | −187.330 *** | 9.079 *** | 3.286 *** | −0.124 *** | 35 | 0.92 | 0.91 | <0.001 | |
| QR4/Summer of 2021 | 1. | y = a + bT + cW | −46.818 *** | 2.581 ’ | 0.319 *** | 35 | 0.57 | 0.54 | <0.001 | |
| 2. | y = a + bT + cW + dTW | −63.991 ’ | 3.447 * | 0.945 | −0.032 | 35 | 0.57 | 0.53 | <0.001 | |
| QR70/Spring of 2022 | 1. | y = a + bT + cW | −66.021 *** | 0.372 *** | 2.193 ** | 30 | 0.75 | 0.73 | <0.001 | |
| 2. | y = a + bT + cW + dTW | −89.608 *** | 2.411 | 2.972 *** | −0.067 | 30 | 0.77 | 0.74 | <0.001 | |
| QR14/Spring of 2022 | 1. | y = a + bT + cW | −33.839 * | 0.570 ** | 1.047 ** | 30 | 0.30 | 0.25 | <0.05 | |
| 2. | y = a + bT + cW + dTW | −10.425 | −1.532 | 0.274 | 0.071 ’ | 30 | 0.39 | 0.32 | <0.05 | |
| QR4/Spring of 2022 | 1. | y = a + bT + cW | −10.330 | 0.145 | 0.502 | 30 | 0.12 | 0.05 | 0.18 | |
| 2. | y = a + bT + cW + dTW | 34.040 * | −3.136 * | −1.146 *** | 0.130 *** | 30 | 0.46 | 0.40 | <0.001 | |
| QR70/Summer of 2022 | 1. | y = a + bT + cW | −29.455 | −0.184 | 1.750 *** | 30 | 0.35 | 0.30 | <0.05 | |
| 2. | y = a + bT + cW + dTW | −630.681 ** | 30.809 ** | 24.721 ** | −1.186 ** | 30 | 0.52 | 0.46 | <0.001 | |
| QR14/Summer of 2022 | 1. | y = a + bT + cW | 16.446 | −0.766 | 0.344 | 30 | 0.07 | - | 0.37 | |
| 2. | y = a + bT + cW + dTW | −689.561 * | 34.755 * | 29.783 * | −1.492 * | 30 | 0.25 | 0.16 | 0.06 | |
| QR4/Summer of 2022 | 1. | y = a + bT + cW | 46.889 | −0.901 | −0.890 | 30 | 0.08 | 0.01 | 0.32 | |
| 2. | y = a + bT + cW + dTW | −6.460 | 1.923 | 1.602 | −0.132 | 30 | 0.08 | - | 0.51 | |
| QR70/Autumn of 2022 | 1. | y = a + bT + cW | −12.653 *** | 0.577 *** | 0.295 *** | 25 | 0.99 | 0.99 | <0.001 | |
| 2. | y = a + bT + cW + dTW | −15.586 ’ | 0.826 | 0.391 | −0.008 | 25 | 0.99 | 0.99 | <0.001 | |
| QR14/Autumn of 2022 | 1. | y = a + bT + cW | −5.253 | 0.553 *** | 0.031 | 25 | 0.89 | 0.88 | <0.001 | |
| 2. | y = a + bT + cW + dTW | 153.956 *** | −11.358 *** | −5.091 *** | 0.383 *** | 25 | 0.99 | 0.99 | <0.001 | |
| QR4/Autumn of 2022 | 1. | y = a + bT + cW | −7.177 *** | 0.552 *** | 0.146 ** | 25 | 0.92 | 0.91 | <0.001 | |
| 2. | y = a + bT + cW + dTW | −15.327 | 1.193 | 0.422 | −0.022 | 25 | 0.93 | 0.92 | <0.001 | |
Appendix B






References
- Rahman, M.M. Carbon dioxide emission from soil. Agric. Res. 2013, 2, 132–139. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Orlović, S.; Zlatković, M.; Kostić, S.; Vasić, V.; Miletić, B.; Kesić, L.; Matović, B.; Božanić, D.; Pavlović, L.; et al. Climate change within Serbian forests: Current state and future perspectives. Topola 2021, 208, 39–56. [Google Scholar] [CrossRef]
- Tanovski, V.; Matovic, B.; Kesic, L.; Stojanovic, D.B. A review of the influence of climate change on coniferous forests in the Balkan peninsula. Topola 2022, 210, 41–64. [Google Scholar] [CrossRef]
- Netz, B.; Davidson, O.; Bosch, P.; Dave, R.; Meyer, L. Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M. Forest carbon stocks in woody plants of Chilimo-Gaji Forest, Ethiopia: Implications of managing forests for climate change mitigation. S. Afr. J. Bot. 2019, 127, 213–219. [Google Scholar] [CrossRef]
- Vuletić, D.; Potočić, N.; Krajter, S.; Seletković, I.; Fürst, C.; Makeschin, F.; Galić, Z.; Lorz, C.; Matijašič, D.; Zupanič, M.; et al. How socio-economic conditions influence forest policy development in Central and South-east Europe. Environ. Manag. 2010, 46, 931–940. [Google Scholar] [CrossRef]
- Amundson, R. The carbon budget in soils. Annu. Rev. Earth Planet. Sci. 2001, 29, 535–562. [Google Scholar] [CrossRef]
- Change, I. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies: Hayama, Japan, 2006. [Google Scholar]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.D.; Rebmann, C.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O.; Pilegaard, K.A.; et al. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–865. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Lubbers, I.M.; Van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; Van Groenigen, J.W. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Change 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Phillips, C.L.; Nickerson, N. Soil Respiration. In Reference Module in Earth Systems and Environmental Sciences; Elias, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Teramoto, M.; Liang, N.; Takahashi, Y.; Zeng, J.; Saigusa, N.; Ide, R.; Zhao, X. Enhanced understory carbon flux components and robustness of net CO2 exchange after thinning in a larch forest in central Japan. Agric. For. Meteorol. 2019, 274, 106–117. [Google Scholar] [CrossRef]
- Barba, J.; Cueva, A.; Bahn, M.; Barron-Gafford, G.A.; Bond-Lamberty, B.; Hanson, P.J.; Jaimes, A.; Kulmala, L.; Pumpanen, J.; Scott, R.L.; et al. Comparing ecosystem and soil respiration: Review and key challenges of tower-based and soil measurements. Agric. For. Meteorol. 2018, 249, 434–443. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Teramoto, M.; Liang, N.; Zeng, J.; Saigusa, N.; Takahashi, Y. Long-term chamber measurements reveal strong impacts of soil temperature on seasonal and inter-annual variation in understory CO2 fluxes in a Japanese larch (Larix kaempferi Sarg.) forest. Agric. For. Meteorol. 2017, 247, 194–206. [Google Scholar] [CrossRef]
- Prasad, S.; Baishya, R. Interactive effects of soil moisture and temperature on soil respiration under native and non-native tree species in semi-arid forest of Delhi, India. Trop. Ecol. 2020, 60, 252–260. [Google Scholar] [CrossRef]
- Yu, J.C.; Chiang, P.N.; Lai, Y.J.; Tsai, M.J.; Wang, Y.N. High rainfall inhibited soil respiration in an Asian monsoon forest in Taiwan. Forests 2021, 12, 239. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Kusá, H.; Růžek, P.; Vavera, R. CO2 emissions in a soil under different tillage practices. Plant Soil Environ. 2022, 68, 253–261. [Google Scholar] [CrossRef]
- Wu, G.; Chen, X.M.; Ling, J.; Li, F.; Li, F.Y.; Peixoto, L.; Wen, Y.; Zhou, S.-L. Effects of soil warming and increased precipitation on greenhouse gas fluxes in spring maize seasons in the North China Plain. Sci. Total Environ. 2020, 734, 139269. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Seedre, M.; Chen, H.Y. Carbon dynamics of aboveground live vegetation of boreal mixedwoods after wildfire and clear-cutting. Can. J. For. Res. 2010, 40, 1862–1869. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xie, J.B.; Wang, Y.G.; Li, Y. Biotic and abiotic contribution to diurnal soil CO2 fluxes from saline/alkaline soils. Sci. Rep. 2020, 10, 5396. [Google Scholar] [CrossRef]
- Reichstein, M.; Beer, C. Soil respiration across scales: The importance of a model—Data integration framework for data interpretation. J. Plant Nutr. Soil Sci. 2008, 171, 344–354. [Google Scholar] [CrossRef]
- Tang, J.; Qi, Y.; Xu, M.; Misson, L.; Goldstein, A.H. Forest thinning and soil respiration in a ponderosa pine plantation in the Sierra Nevada. Tree Physiol. 2005, 25, 57–66. [Google Scholar] [CrossRef]
- Pilipović, A.; Orlović, S.; Zhiyanski, M.; Andonovski, V.; Galić, Z. Soil respiration measurement in beech forest during two vegetation periods at Stara planina. Topola 2015, 195–196, 97–105. [Google Scholar]
- Pilipović, A.; Orlović, S.; Galić, Z.; Stojnić, S.; Borišev, M.; Župunski, M. Soil respiration in two different conifer stands during vegetation period 2011–2013. Topola 2014, 193-194, 73–84. [Google Scholar]
- Pilipović, A.; Orlović, S.; Galić, Z.; Stojnić, S.; Borišev, M.; Nikolić, N. The results of monitoring of soil respiration in two different beech associations during vegetation period. Topola 2011, 187–188, 65–75. [Google Scholar]
- Samardžić, M.; Galić, Z.; Orlović, S.; Kovač, M.; Andreeva, I.; Vasenev, I. Environmental assessment of greenhouse gases’ emission in poplar plantation under extreme climate conditions of winter 2019/2020. Topola 2021, 208, 15–19. [Google Scholar] [CrossRef]
- Karaklić, V.; Galić, Z.; Samardžić, M.; Kesić, L.; Orlović, S.; Zorić, M. Carbon dioxide (CO2) emissions from soils during the regeneration of pedunculate oak (Quercus robur L.) stand in the summer period. Šumarski List 2023, 147, 227–237. [Google Scholar] [CrossRef]
- Banković, S.; Medarević, M.; Pantić, D.; Petrović, N. National Forest Inventory of the Republic of Serbia—The Growing Stock of the Republic of Serbia; Ministry of Agriculture, Forestry and Water Management of the Republic of Serbia—Forest Directorate: Belgrade, Serbia, 2009; pp. 1–238.
- Development Plan of Srem Forest Area. Available online: https://psp.vojvodina.gov.rs/wp-content/uploads/2021/01/Plan-razvoja-Sremskog-sumskog-podrucja-knjiga-1.pdf (accessed on 5 December 2023).
- Nikić, Z.; Letić, L.J.; Nikolić, V.; Filipović, V. Procedure for Underground Water Calculation Regime of Pedunculata oak Habitat in Plain Srem. Bull. Fac. For. 2010, 101, 125–138. [Google Scholar]
- Jokanović, D.; Desimirović, I.; Živanović, K. Taxonomy and phytogeography analysis of medicinal plants within forest complex of pedunculate oak at the area of Gornji Srem. Topola 2022, 210, 5–14. [Google Scholar] [CrossRef]
- Cvetićanin, R.; Brujić, J.; Perović, M.; Stupar, V. Dendrology; University of Belgrade—Faculty of Forestry: Belgrade, Serbia, 2016; pp. 1–557. [Google Scholar]
- Rađević, V.; Pap, P.; Vasić, V. Management of the common oak forests in Ravni Srem: Yesterday, today, tomorrow. Topola 2020, 206, 41–52. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Available online: https://www.hidmet.gov.rs/ (accessed on 10 December 2023).
- Jović, D.; Jović, N.; Jovanović, B.; Tomić, Z.; Banković, S.; Medarević, M.; Knežević, M.; Grbić, P.; Živanov, N.; Ivanišević, P. Forest Types of Ravni Srem—Atlas; University of Belgrade—Faculty of Forestry: Belgrade, Serbia, 1994. [Google Scholar]
- FAO. World Reference Base for Soil Resources 2014, Update 2015; World Soil Resources Reports 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Avilov, K.V.; Barkov, A.V.; Vasenev, I.I.; Vasenev, I.V.; Vizirskaja, M.M.; Paskarev, A.A.; Terekhov, V.A.; Kurbatova, A.J.; Samardzhich, M. Device for Measuring Emission of Greenhouse Gas from Soil and Plants; Russian Federation’s Federal Service for Intellectual Property: Moscow, Russia, 2014; No. RU 2 518 979 C1.
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bergeron, Y.; Chen, H.Y. The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol. Biochem. 2012, 53, 18–27. [Google Scholar] [CrossRef]
- Song, X.; Yuan, H.; Kimberley, M.O.; Jiang, H.; Zhou, G.; Wang, H. Soil CO2 flux dynamics in the two main plantation forest types in subtropical China. Sci. Total Environ. 2013, 444, 363–368. [Google Scholar] [CrossRef]
- Ming, A.; Yang, Y.; Liu, S.; Wang, H.; Li, Y.; Li, H.; Nong, Y.; Cai, D.; Jia, H.; Tao, Y.; et al. Effects of near natural forest management on soil greenhouse gas flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook. plantations. Forests 2018, 9, 229. [Google Scholar] [CrossRef]
- Vanhala, P. Seasonal variation in the soil respiration rate in coniferous forest soils. Soil Biol. Biochem. 2002, 34, 1375–1379. [Google Scholar] [CrossRef]
- Sarzhanov, D.A.; Vasenev, V.I.; Sotnikova, Y.L.; Tembo, A.; Vasenev, I.I.; Valentini, R. Short-term dynamics and spatial heterogeneity of CO2 emission from the soils of natural and urban ecosystems in the Central Chernozemic Region. Eurasian Soil Sci. 2015, 48, 416–424. [Google Scholar] [CrossRef]
- Sarzhanov, A.D.; Vasenev, I.V.; Vasenev, I.I.; Sotnikova, L.Y.; Ryzhkov, V.O.; Morin, T. Carbon stocks and CO2 emissions of urban and natural soils in Central Chernozemic region of Russia. Catena 2017, 158, 131–140. [Google Scholar] [CrossRef]
- Jia, B.; Zhou, G.; Wang, Y.; Wang, F.; Wang, X. Effects of temperature and soil water-content on soil respiration of grazed and ungrazed Leymus chinensis steppes, Inner Mongolia. J. Arid. Environ. 2006, 67, 60–76. [Google Scholar] [CrossRef]
- Cui, Y.B.; Feng, J.G.; Liao, L.G.; Yu, R.; Zhang, X.; Liu, Y.H.; Yang, L.-Y.; Zhao, J.-F.; Tan, Z.-H. Controls of temporal variations on soil respiration in a tropical lowland rainforest in Hainan Island, China. Trop. Conserv. Sci. 2020, 13, 1940082920914902. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Ligges, U.; Mächler, M. Scatterplot3d—An R package for visualizing multivariate data. J. Stat. Softw. 2003, 8, 1–20. [Google Scholar] [CrossRef]
- Adachi, M.; Bekku, Y.S.; Rashidah, W.; Okuda, T.; Koizumi, H. Differences in soil respiration between different tropical ecosystems. Appl. Soil Ecol. 2006, 34, 258–265. [Google Scholar] [CrossRef]
- Li, H.J.; Yan, J.X.; Yue, X.F.; Wang, M.B. Significance of soil temperature and moisture for soil respiration in a Chinese mountain area. Agric. For. Meteorol. 2008, 148, 490–503. [Google Scholar] [CrossRef]
- Ma, S.; Concilio, A.; Oakley, B.; North, M.; Chen, J. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. For. Ecol. Manag. 2010, 259, 904–915. [Google Scholar] [CrossRef]
- Yuste, J.C.; Nagy, M.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Soil respiration in a mixed temperate forest and its contribution to total ecosystem respiration. Tree Physiol. 2005, 25, 609–619. [Google Scholar] [CrossRef]
- Tang, X.L.; Zhou, G.Y.; Liu, S.G.; Zhang, D.Q.; Liu, S.Z.; Li, J.; Zhou, C.Y. Dependence of soil respiration on soil temperature and soil moisture in successional forests in southern China. J. Integr. Plant Biol. 2006, 48, 654–663. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A. Tree species influence soil respiration in a temperate forest of Uttrakhand Himalaya, India. J. Sustain. For. 2021, 40, 820–830. [Google Scholar] [CrossRef]
- Wan, S.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
- Lee, M.S.; Nakane, K.; Nakatsubo, T.; Koizumi, H. Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant Soil 2003, 255, 311–318. [Google Scholar] [CrossRef]
- Nevedrov, N.P.; Sarzhanov, D.A.; Protsenko, E.P.; Vasenev, I.I. Seasonal dynamics of CO2 emission from soils of Kursk. Eurasian Soil Sci. 2021, 54, 80–88. [Google Scholar] [CrossRef]
- Rey, A.; Pegoraro, E.; Oyonarte, C.; Were, A.; Escribano, P.; Raimundo, J. Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain. Soil Biol. Biochem. 2011, 43, 393–403. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Goncharova, O.; Matyshak, G.; Udovenko, M.; Semenyuk, O.; Epstein, H.; Bobrik, A. Temporal dynamics, drivers, and components of soil respiration in urban forest ecosystems. Catena 2020, 185, 1–8. [Google Scholar] [CrossRef]
- Díaz-Pinés, E.; Schindlbacher, A.; Pfeffer, M.; Jandl, R.; Zechmeister-Boltenstern, S.; Rubio, A. Root trenching: A useful tool to estimate autotrophic soil respiration? A case study in an Austrian mountain forest. Eur. J. For. Res. 2010, 129, 101–109. [Google Scholar] [CrossRef]
- Tang, J.; Baldocchi, D.D. Spatial-temporal variation in soil respiration in an oak–grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 2005, 73, 183–207. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, X.; McLaughlin, N.B.; Liang, A.; Jia, S.; Chen, X.; Chen, X. Effect of soil temperature and soil moisture on CO2 flux from eroded landscape positions on black soil in Northeast China. Soil Tillage Res. 2014, 144, 119–125. [Google Scholar] [CrossRef]
- Chen, W.; Jia, X.; Zha, T.; Wu, B.; Zhang, Y.; Li, C.; Wang, X.; He, G.; Yu, H.; Chen, G. Soil respiration in a mixed urban forest in China in relation to soil temperature and water content. Eur. J. Soil Biol. 2013, 54, 63–68. [Google Scholar] [CrossRef]
- Koizumi, H.; Kontturi, M.; Mariko, S.; Nakadai, T.; Bekku, Y.; Mela, T. Soil respiration in three soil types in agricultural ecosystems in Finland. Acta Agric. Scand. Sect. B-Plant Soil Sci. 1999, 49, 65–74. [Google Scholar] [CrossRef]
- Wiseman, P.E.; Seiler, J.R. Soil CO2 efflux across four age classes of plantation loblolly pine (Pinus taeda L.) on the Virginia Piedmont. For. Ecol. Manag. 2004, 192, 297–311. [Google Scholar] [CrossRef]
- Piao, S.; Luyssaert, S.; Ciais, P.; Janssens, I.A.; Chen, A.; Cao, C.; Fang, J.; Friedlingstein, P.; Luo, Y.; Wang, S. Forest annual carbon cost: A global-scale analysis of autotrophic respiration. Ecology 2010, 91, 652–661. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Lin, C.; Liu, X.; Chen, G.; Yang, Y. Conversion of a natural evergreen broadleaved forest into coniferous plantations in a subtropical area: Effects on composition of soil microbial communities and soil respiration. Biol. Fertil. Soils 2016, 52, 799–809. [Google Scholar] [CrossRef]
- Yu, K.; Yao, X.; Deng, Y.; Lai, Z.; Lin, L.; Liu, J. Effects of stand age on soil respiration in Pinus massoniana plantations in the hilly red soil region of Southern China. Catena 2019, 178, 313–321. [Google Scholar] [CrossRef]
- Xiao, W.; Ge, X.; Zeng, L.; Huang, Z.; Lei, J.; Zhou, B.; Li, M. Rates of litter decomposition and soil respiration in relation to soil temperature and water in different-aged Pinus massoniana forests in the three gorges reservoir area, China. PLoS ONE 2014, 9, e101890. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Liu, S.; Wang, J.; Zhu, X.; Shi, Z. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 2011, 43, 503–512. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Song, Q.; Lin, Y.; Zhou, R.; Dong, Y.; Zhou, L.; Li, J.; Jin, Y.; Zhou, W.; et al. Stand age-related effects on soil respiration in rubber plantations (Hevea brasiliensis) in southwest China. Eur. J. Soil Sci. 2019, 70, 1221–1233. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-Bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Chang. Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Klopatek, J.M. Belowground carbon pools and processes in different age stands of Douglas-fir. Tree Physiol. 2002, 22, 197–204. [Google Scholar] [CrossRef]
- Gong, J.; Ge, Z.; An, R.; Duan, Q.; You, X.; Huang, Y. Soil respiration in poplar plantations in northern China at different forest ages. Plant Soil 2012, 360, 109–122. [Google Scholar] [CrossRef]
- Law, B.E.; Ryan, M.G.; Anthoni, P.M. Seasonal and annual respiration of a ponderosa pine ecosystem. Glob. Chang. Biol. 1999, 5, 169–182. [Google Scholar] [CrossRef]
- Striegl, R.G.; Wickland, K.P. Effects of a clear-cut harvest on soil respiration in a jack pine-lichen woodland. Can. J. For. Res. 1998, 28, 534–539. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Zhang, W.; Ai, Z.; Shen, H.; Liu, X.; Cao, J.; Manevski, K. Soil respiration at different stand ages (5, 10, and 20/30 years) in coniferous (Pinus tabulaeformis Carrière) and deciduous (Populus davidiana Dode) plantations in a sandstorm source area. Forests 2016, 7, 153. [Google Scholar] [CrossRef]
- Tedeschi, V.; Rey, A.N.A.; Manca, G.; Valentini, R.; Jarvis, P.G.; Borghetti, M. Soil respiration in a Mediterranean oak forest at different developmental stages after coppicing. Glob. Chang. Biol. 2006, 12, 110–121. [Google Scholar] [CrossRef]
- Ma, Y.; Piao, S.; Sun, Z.; Lin, X.; Wang, T.; Yue, C.; Yang, Y. Stand ages regulate the response of soil respiration to temperature in a Larix principis-rupprechtii plantation. Agric. For. Meteorol. 2014, 184, 179–187. [Google Scholar] [CrossRef]



| Examined Stands | Soil Depth | Total Sand >0.02 mm | Silt 0.02–0.002 mm | Clay <0.002 mm | Textural Class | C | N | CaCO3 | pH (H2O) |
|---|---|---|---|---|---|---|---|---|---|
| cm | % | % | % | - | % | % | % | - | |
| QR70 | 0–30 | 26.8 | 35.6 | 37.6 | clay loam | 0.89 | 0.16 | 0.58 | 7.32 |
| 30–80 | 25.2 | 28.6 | 46.2 | clay | 0.16 | 0.07 | 1.21 | 7.65 | |
| >80 | 28.4 | 33.2 | 38.3 | clay loam | 0.04 | - | 0.92 | 7.97 | |
| QR14 | 0–30 | 27.8 | 38.6 | 33.6 | clay loam | 2.54 | 0.16 | 1.33 | 6.47 |
| 30–80 | 27.0 | 27.0 | 46.0 | clay | 0.19 | 0.06 | 1.16 | 7.32 | |
| >80 | 25.7 | 33.6 | 40.7 | clay | 0.19 | - | 0.17 | 7.36 | |
| QR4 | 0–30 | 40.3 | 35.7 | 24.0 | loam | 5.30 | 0.13 | 2.01 | 7.56 |
| 30–80 | 26.7 | 29.1 | 44.2 | clay | 0.22 | 0.04 | 1.34 | 7.86 | |
| >80 | 27.6 | 34.1 | 38.4 | clay loam | 0.01 | - | 129 | 7.90 |
| Source of Variation | df | F | p |
|---|---|---|---|
| Stand age | 2 | 0.82 | 0.44 |
| Season | 2 | 14.70 | <0.001 |
| Stand age × Season | 4 | 2.6 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaklić, V.; Samardžić, M.; Orlović, S.; Zorić, M.; Kesić, L.; Perendija, N.; Galić, Z. Effect of Stand Age on Soil CO2 Emissions in Pedunculate Oak (Quercus robur L.) Forests. Forests 2024, 15, 1574. https://doi.org/10.3390/f15091574
Karaklić V, Samardžić M, Orlović S, Zorić M, Kesić L, Perendija N, Galić Z. Effect of Stand Age on Soil CO2 Emissions in Pedunculate Oak (Quercus robur L.) Forests. Forests. 2024; 15(9):1574. https://doi.org/10.3390/f15091574
Chicago/Turabian StyleKaraklić, Velisav, Miljan Samardžić, Saša Orlović, Martina Zorić, Lazar Kesić, Nikola Perendija, and Zoran Galić. 2024. "Effect of Stand Age on Soil CO2 Emissions in Pedunculate Oak (Quercus robur L.) Forests" Forests 15, no. 9: 1574. https://doi.org/10.3390/f15091574
APA StyleKaraklić, V., Samardžić, M., Orlović, S., Zorić, M., Kesić, L., Perendija, N., & Galić, Z. (2024). Effect of Stand Age on Soil CO2 Emissions in Pedunculate Oak (Quercus robur L.) Forests. Forests, 15(9), 1574. https://doi.org/10.3390/f15091574











