Abstract
Nitrogen addition alters soil organic carbon (SOC) and total nitrogen (TN) accumulation in forest ecosystems, but the responses of SOC and TN sequestration rates and dynamics to nitrogen addition in forest ecosystems worldwide remain unclear. This study conducted a global analysis to evaluate the effects of the nitrogen application rate, nitrogen addition duration (time), and humidity on the SOC and TN accumulation rates from 257 data points (63 articles). Nitrogen addition increased SOC and TN by 4.48% and 10.18%, respectively. The SOC and TN accumulation rates were 0.65 and 0.11 g kg−1 yr−1, respectively. Moreover, the percentage changes of SOC and TN overall increased with the nitrogen application rate and duration of nitrogen addition; however, the accumulation rates of SOC and TN overall decreased with the nitrogen application rate and the duration of nitrogen addition. In addition, the percentage changes and change rates of SOC and TN increased overall with the humidity index. In conclusion, nitrogen addition promoted SOC and TN accumulation in forest soil, and the nitrogen application rate and nitrogen addition duration increased the percentage changes in SOC and TN; however, they decreased the accumulation rate, whereas humidity increased the accumulation rates of SOC and TN. These results enhance our understanding of soil carbon and nitrogen cycling in forest soils in the context of global nitrogen deposition.
1. Introduction
Burning fossil fuels and the extensive use of fertilizers (causing nitrogen deposition) [1,2,3] have changed forest community structure [4], forest species composition [5], and forest ecosystem multifunctionality [6]. Nitrogen is a limiting element for plant and microbial growth, and an appropriate amount of exogenous nitrogen is highly important for promoting plant growth, improving plant productivity, and enhancing microbial activity [7,8,9]. However, large or excessive nitrogen inputs may change the physical structure, nutrient balance, and homeostasis of the soil, leading to soil acidification and limiting plant growth and microbial reproduction [10,11]. An increase in nitrogen input is likely to affect the steady state of soil carbon and nitrogen [12,13,14,15].
Nitrogen addition alters SOC and TN accumulation [16,17]. A recent study revealed that nitrogen addition increased the SOC stock and TN content by 5.82% and 6.1%, respectively [18]. However, Ngaba Junior et al. [19] reported that nitrogen addition increased the SOC pools in boreal forests by 17%; however, it decreased the SOC pools in subtropical forests by 0.4%. This difference is due mainly to the different ecosystem types used in the different studies. A unified understanding of the response of SOC and TN to nitrogen addition, particularly the accumulation rates of SOC and TN in forest ecosystems, is lacking.
The changes in SOC and TN accumulation in forest ecosystems involve two main mechanisms: (1) Nitrogen addition changes plant growth and soil nutrient status through nitrogen input, thus affecting forest ecosystem productivity [20,21] and soil microbial activity [22,23]. Changes in microbial activity significantly affect forest litter decomposition and carbon mineralization, thereby affecting SOC and TN accumulation [9,11,24]. (2) The soil microbial community structure affects the release of extracellular enzymes, which in turn affect plant root activities, root exudates, and root turnover [25,26].
The nitrogen application rate and nitrogen addition duration (time) are important factors affecting the SOC and TN accumulation rates [27,28]. Currently, two major perspectives are present. First, plant productivity and the microbial decomposition rate of litter increase with increasing nitrogen application rates and nitrogen addition durations [22], thus increasing SOC and TN. In addition, the heavy nitrogen application rate and long-term nitrogen addition can lead to severe soil acidification [29], resulting in severe nutrient restriction [11] and significantly reduced microbial activity [30], thereby decreasing the accumulation of SOC and TN [11,31]. The nitrogen application rate and duration of nitrogen addition also significantly affect the root turnover rate [22]. Moderate nitrogen addition significantly increases the root turnover rate [7]. Conversely, the heavy nitrogen application rate reduces soil enzymatic activity [32], thereby reducing the root decomposition rate and limiting SOC and TN accumulation [33]. Environmental conditions (humidity indices) also significantly affect SOC and TN accumulation [34,35]. Low humidity leads to soil drought and decreases plant productivity and microbial activity [36,37]. High humidity reduces soil porosity and permeability and negatively affects plant and microbial growth [38,39]. Only moderate soil water availability can improve soil environmental conditions, promote plant growth [40], and increase litter decomposition [41]. However, how the nitrogen application rate, nitrogen addition duration, and environmental conditions affect the SOC and TN in forest ecosystems is insufficiently studied.
Therefore, this study explored the response of SOC and TN accumulation to nitrogen addition in forest ecosystems by collecting 257 data points from 63 articles. We aimed to assess the changes in SOC and TN accumulation rates systematically and to determine how SOC and TN are regulated by the nitrogen application rate, the duration of nitrogen addition, and humidity. We propose three hypotheses: (1) Nitrogen input stimulates plant and microbial growth and enhances forest litter and root decomposition [11,22]. Therefore, we hypothesized that nitrogen addition promotes SOC and TN accumulation in forest ecosystems. (2) Heavy nitrogen application rates and long-term nitrogen addition can promote plant growth; however, they can also lead to soil acidification and microbial activity reduction [42]. Therefore, the SOC and TN accumulation rates decrease with increasing nitrogen application rates and nitrogen addition durations. (3) Soil microorganisms are sensitive to changes in environmental conditions, and relatively high humidity increases soil water availability [40,41]. Therefore, the SOC and TN accumulation rates are significantly positively correlated with humidity.
2. Materials and Methods
2.1. Data Collection
The Web of Science (http://apps.webofknowledge.com/), Google Scholar (https://scholar.google.com), and CNKI (http://www.cnki.net) databases were searched for studies published in the literature. The search terms are shown in Table 1, and the search time was 20 March 2024. The literature screening criteria were as follows:
- The research objects were forests in terrestrial ecosystems.
- The research method was an artificial nitrogen addition experiment, and studies based on mathematical model calculations were excluded.
- Studies that included both a control group and an experimental group and studies without a control group were excluded.
- The nitrogen application rate and duration of nitrogen addition must be clarified.
- The research data included mean values and are presented in the form of tables or figures.
Through screening, 257 data points were obtained from 63 articles. The collected variables included pH, SOC, and TN. The mean annual temperature (MAT) and precipitation (MAP) data of the sample sites were also collected.
2.2. Data Analysis
The percentage change [43] and rate of change [44] in the soil pH, SOC, and TN were calculated as follows:
where Xt and Xc are the pH, SOC, and TN of the nitrogen addition and control groups, respectively. Xi and X0 are the pH, SOC, and TN after i years of nitrogen addition and the initial pH, SOC, and TN, respectively.
The humidity index was used to evaluate the effects of environmental factors (mean annual precipitation [MAP] and temperature [MAT]) [45].
In this study, the humidity index, nitrogen addition duration, and nitrogen application rate were divided into <30, 30–50, and >50; <5, 5–10, and >10 years; and <5, 5–10, and >10 g m−2 yr−1, respectively. One-way ANOVAs were used to test the effects of the humidity index, nitrogen addition duration, and nitrogen application rate on the percentage changes and rates of SOC and TN. Regression analysis was used to analyze the correlations of the nitrogen addition response and change rates of pH, SOC, and TN with the humidity index, nitrogen addition duration, and nitrogen application rate.
3. Results
3.1. Overall Effects
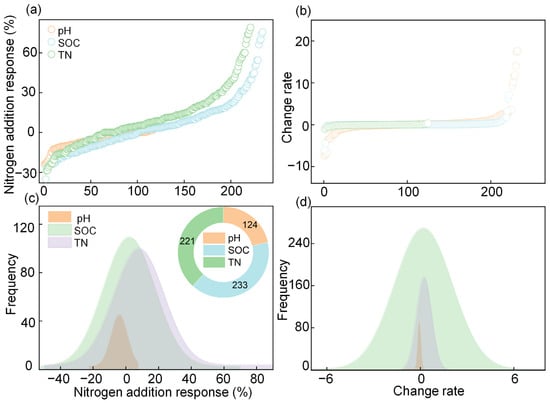
The distributions of the percentage change and change rates of pH, SOC, and TN are shown in Figure 1a,b; all the variables were normally distributed (Figure 1c,d). In total, 124, 221, and 233 data points were obtained for pH, SOC, and TN, respectively (Figure 1c).
Figure 1.
Distribution of the (a) percentage and rate (b) of soil pH, soil organic carbon (SOC), and total nitrogen (TN) and the frequency of the (c) percentage and rate (d) of soil pH, SOC, and TN under nitrogen addition.
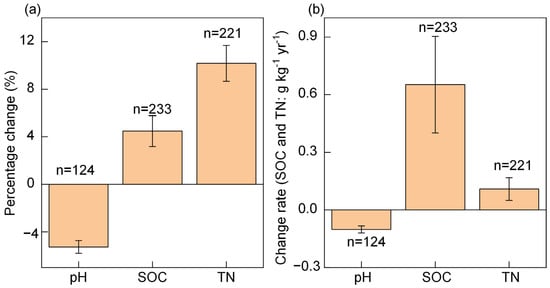
The percentage change (%) and change rate of pH were negative (percentage: −5.27%, change rate: −0.10); however, the percentage change and change rates of SOC (+4.48%, +0.65 g kg−1 yr−1) and TN (+10.18%, +0.11 g kg−1 yr−1) were positive (Figure 2).
3.2. Responses of pH, SOC, and TN to Humidity, Duration of Nitrogen Addition, and Nitrogen Application Rate
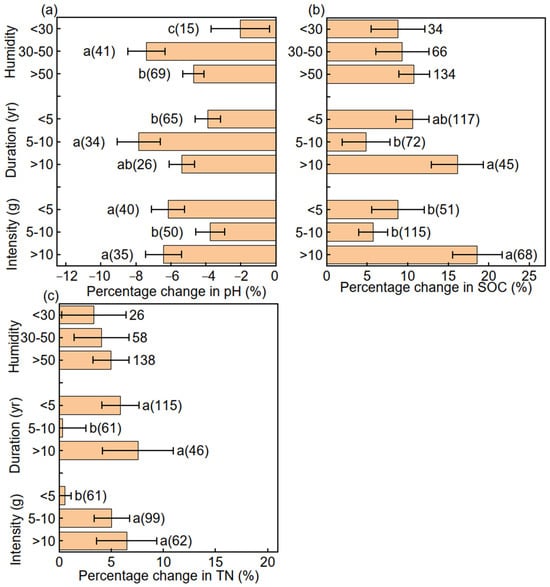
The percentage change in pH was lower at humidities of <30 and >50 than at 30–50, at durations of <5 and >10 years than at 5–10 years, and at application rates of 5–10 g N m−2 yr−1 than at >10 and <5 g N m−2 yr−1 (Figure 3a). The percentage change in SOC was greater at durations >10 years than at 5–10 and <5 years and at application rates >10 g N m−2 yr−1 than at 5–10 and <5 g N m−2 yr−1 (Figure 3b). The percentage change in TN was greater at durations of <5 and >10 years than at 5–10 years and at application rates of 5–10 and >10 g N m−2 yr−1 than at <5 g N m−2 yr−1 (Figure 3c).
Figure 3.
Mean (±SE) percentage changes in (a) soil pH, (b) soil organic carbon (SOC), and (c) total nitrogen (TN) in response to humidity, nitrogen addition duration, and nitrogen application rate. Different letters indicate significant differences in soil pH, SOC, and TN among different groups.
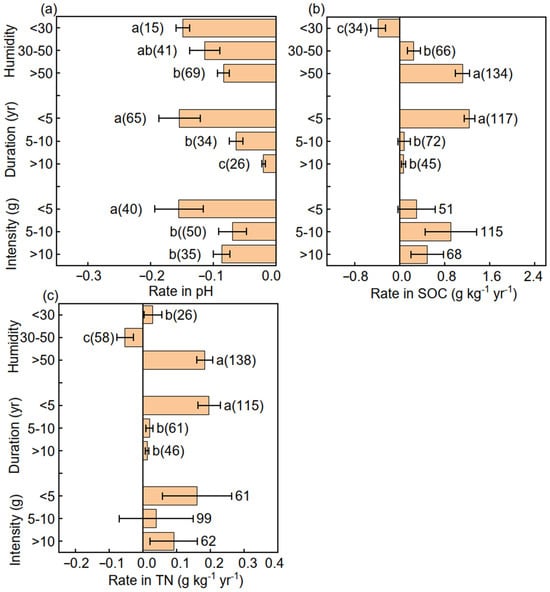
The change rate of pH was greater at humidities of 30–50 and >50 than at <30; at durations of 5–10 and >10 years than at <5 years; and at application rates of 5–10 and >10 g N m−2 yr−1 than at <5 g N m−2 yr−1 (Figure 4a). The change rates of SOC and TN were greater at humidities of >50 than at <30 and 30–50 and at durations of <5 years than at 5–10 and >10 years (Figure 4b,c).
Figure 4.
Mean (±SE) change rates of (a) soil pH, (b) soil organic carbon (SOC), and (c) total nitrogen (TN) in response to humidity, nitrogen addition duration, and nitrogen application rate. Different letters indicate significant differences in soil pH, SOC, and TN among different groups.
The percentage change in pH decreased overall with nitrogen addition duration and nitrogen application rate, whereas the percentage change in SOC and TN increased overall with humidity, nitrogen addition duration, and nitrogen application rate (Figure S1a–i). The change rate of pH tended to increase with humidity, nitrogen addition duration, and nitrogen application rate, whereas the change rates of SOC and TN increased with humidity but decreased with nitrogen addition duration and nitrogen application rate (Figure S2a–i).
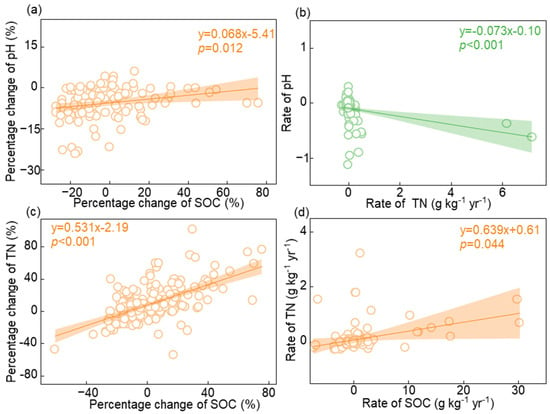
3.3. Relationships between pH, SOC, and TN
4. Discussion
4.1. Effects of Nitrogen Addition on SOC and TN Accumulation
Nitrogen addition increased SOC and TN by 4.48% and 10.18%, respectively, in forest ecosystems, confirming Hypothesis 1. Changes in SOC and TN are regulated mainly by the inputs and outputs of carbon and nitrogen [46,47]. The input process comprises two steps. First, nitrogen addition enhances the input of carbon and nitrogen by promoting tree growth [22,48]. An increase in forest ecosystem productivity increases soil microbial activity [11,49], further accelerating the decomposition of litter on the soil surface [50,51]. Second, nitrogen addition enhances the turnover rate of fine roots and alters root exudates, thus increasing soil fertility [25,26]. The output processes include plant root and soil respiration, with soil respiration being the most important, accounting for more than 70% of the total respiration [23,52,53]. Moreover, a recent meta-analysis confirmed that nitrogen addition reduced soil respiration [22]. The increase in carbon input and decrease in carbon output during nitrogen addition promoted SOC accumulation [54]. Previous studies have indicated that SOC and TN are coupled in terrestrial ecosystems [55]. This study also confirmed that SOC and TN were highly coupled with nitrogen addition.
4.2. Different Effects of Nitrogen Addition Duration and Nitrogen Application Rate on SOC and TN Accumulation
This study revealed that the percentage changes in SOC and TN increased overall with increasing nitrogen addition duration and nitrogen application rate; however, the accumulation rate tended to decrease with increasing nitrogen addition duration and nitrogen application rate in forest ecosystems at the global scale, confirming Hypothesis 2.
This can be explained by three mechanisms: (1) Heavy nitrogen application rates and long-term nitrogen addition significantly increase the aboveground biomass of plants in forest ecosystems [22], and litter regression and subsurface carbon allocation provide more substrates for the soil, promoting microbial decomposition of litter into SOC and TN [12] and thereby increasing the SOC and TN contents. However, excessive nitrogen addition reduces the soil C:N ratio and pH [56]. Soil acidification reduces microbial activity [29], thus limiting litter decomposition by microorganisms and enzymes [30] and reducing SOC and TN accumulation. (2) Heavy nitrogen application rates and long-term nitrogen addition promote SOC and TN accumulation by increasing plant root yield [11]. However, the turnover rate of fine roots is closely related to the nitrogen application rate and nitrogen addition duration, and moderate nitrogen addition significantly improves the root turnover rate, which is related mainly to root quality [7]. In contrast, the heavy nitrogen application rate reduces the activity of soil enzymes involved in the decomposition of root litter [32], thus reducing the accumulation rates of SOC and TN [33]. (3) SOC accumulation is affected by microbial carbon use efficiency, which is regulated by microbial nutrient restriction [57]. Small and short-term nitrogen additions cause microbes to experience weak phosphorus limitations, whereas heavy nitrogen application rates and long-term nitrogen additions aggravate microbial carbon and phosphorus limitations [11,28], thereby reducing microbial activity and the accumulation of SOC and TN. This study revealed that the heavy nitrogen application rate and long-term duration increased the SOC and TN contents but decreased the accumulation rate. The results of this study enhance our understanding of soil carbon and nitrogen turnover against the background of future global nitrogen deposition.
4.3. Effects of Humidity on SOC and TN Accumulation
Under nitrogen addition, climate significantly affected SOC and TN accumulation. This study revealed that the percentage changes in and accumulation rates of SOC and TN tended to increase with the humidity index in forest ecosystems under nitrogen addition. First, low humidity reduces the soil water content and even leads to soil drought, thus limiting plant growth and root productivity in forest ecosystems [25]. A decreased litter content weakens the decomposition capacity of microorganisms and the accumulation of SOC and TN [58,59]. Higher humidity increases the soil water content, and the increased availability of soil water stimulates plant growth and the amount of dead leaves imported into the soil [60,61], improving the decomposition of litter and thus increasing SOC and TN accumulation [62]. Moreover, soil microbial activity is strongly affected by hydrothermal conditions [63], and low humidity is not conducive to soil microbial resource acquisition [64], which reduces microbial activity. The increase in litter content and soil nutrients caused by an increase in soil water provides sufficient carbon and energy sources for microorganisms [34,41], thereby increasing microbial activity, increasing extracellular enzyme secretion [36,65], and accelerating SOC and TN accumulation [66]. In addition, increased humidity improves soil water availability and promotes the formation of soil aggregates [67,68], thus facilitating the survival of microorganisms and the accumulation of SOC and TN in global forest ecosystems.
4.4. Limitations
Our study explored the response of SOC and TN accumulation to the nitrogen application rate and nitrogen addition duration; however, three limitations are as follows: (1) The selection of research objects in this study was not comprehensive. The terrestrial ecosystems used in this study include forests. There is considerable uncertainty regarding how SOC and TN respond to nitrogen addition in croplands, grasslands, deserts, and wetland ecosystems. (2) The accumulation of SOC and TN is regulated by plant litter, soil physicochemical properties, and soil microorganisms [10,69]; however, systematic assessments of how these factors respond to nitrogen addition and how nitrogen addition indirectly regulates the accumulation rate of SOC and TN by affecting plant growth and microbial activity are lacking. Therefore, future studies should comprehensively consider the effects of nitrogen addition on carbon and nitrogen fixation, emission processes, and driving mechanisms in different ecosystems to enhance the understanding of soil carbon and nitrogen turnover mechanisms.
5. Conclusions
Nitrogen addition promoted SOC and TN accumulation in forest ecosystems. Moreover, the heavy nitrogen application rate and long-term duration increased the SOC and TN contents but decreased the accumulation rate, whereas humidity increased the SOC and TN contents and promoted the accumulation rate in global forest ecosystems. However, this study did not consider the effects of nitrogen addition on carbon and nitrogen emissions or the mechanisms of carbon and nitrogen accumulation, which limits our understanding of the mechanisms of carbon and nitrogen turnover.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15091585/s1, Figure S1: Relationships of humidity and nitrogen addition duration and intensity with percentage changes of soil pH (a–c), soil organic carbon (SOC; d–f), and total nitrogen (TN; g–i) under nitrogen addition; Figure S2: Relationships of humidity and nitrogen addition duration and intensity with change rates of soil pH (a–c), soil organic carbon (SOC; d–f), and total nitrogen (TN; g–i) under nitrogen addition.
Author Contributions
Conceptualization, Y.Y., J.Y. and H.X.; methodology, J.Y., Q.D., D.L. and B.T.; software, Y.Y.; validation, Y.Y. and Z.X.; data curation, H.X.; writing—original draft preparation, Y.Y. and H.X.; writing—review and editing, Y.Y., Q.W. and H.X.; visualization, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Sichuan Province (2024NSFSC1237, 2024NSFSC1189, and 2022NSFSC1175), the Innovation Team Project of Mianyang Normal University (CXTD2023LX01), and the Scientific Research Initiation Project of Mianyang Normal University (QD2020A18 and QD2023A01).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Many thanks to Sha Xue and Chengming You for their statistics assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mou, R.; Jian, Y.; Zhou, D.; Li, J.; Yan, Y.; Tan, B.; Xu, Z.; Cui, X.; Li, H.; Zhang, L.; et al. Divergent responses of woody plant leaf and root non-structural carbohydrates to nitrogen addition in China: Seasonal variations and ecological implications. Sci. Total Environ. 2024, 950, 175425. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.R.; Tsigaridis, K.; Mihalopoulos, N. Past, present, and future atmospheric nitrogen deposition. J. Atmos. Sci. 2016, 73, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.A.; Adams, M.B.; Gilliam, F.S.; Peterjohn, W.T. Non-random species loss in a forest herbaceous layer following nitrogen addition. Ecology 2017, 98, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Siddique, I.; Vieira, I.C.G.; Schmidt, S.; Lamb, D.; Carvalho, C.J.R.; Figueiredo, R.D.; Blomberg, S.; Davidson, E.A. Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories. Ecology 2010, 91, 2121–2131. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of microplastics and nitrogen deposition on soil multifunctionality, particularly C and N cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, G.; Zeng, D. Long-term nitrogen addition modifies fine root growth and vertical distribution by affecting soil nutrient availability in a Mongolian pine plantation. Sci. Total Environ. 2024, 921, 171168. [Google Scholar] [CrossRef]
- Camenzind, T.; Hempel, S.; Homeier, J.; Horn, S.; Velescu, A.; Wilcke, W.; Rillig, M.C. Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob. Chang. Biol. 2014, 20, 3646–3659. [Google Scholar] [CrossRef]
- Abhiram, G.; Eeswaran, R. Legumes for efficient utilization of summer fallow. In Advances in Legumes for Sustainable Intensification; Elsevier: Amsterdam, The Netherlands, 2022; pp. 51–70. [Google Scholar]
- Chen, Y.; Sha, G.; Wei, T.; Ren, K.; Guo, X.; Yu, H.; Jiang, S. Factor contribution to soil carbon and nitrogen accumulation after vegetation restoration on the Loess Plateau, China. Ecol. Eng. 2023, 194, 107016. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C-N-P stoichiometry and microbial nutrient limitation. Soil. Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Yu, X.; Dijkstra, F.A. Carbon and nitrogen dynamics affected by litter and nitrogen addition in a grassland soil: Role of fungi. Eur. J. Soil. Biol. 2020, 100, 103211. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global meta analysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Gaudel, G.; Xing, L.; Shrestha, S.; Poudel, M.; Sherpa, P.; Raseduzzaman, M.; Zhang, X. Microbial mechanisms regulate soil organic carbon mineralization under carbon with varying levels of nitrogen addition in the above-treeline ecosystem. Sci. Total Environ. 2024, 917, 170497. [Google Scholar] [CrossRef] [PubMed]
- Souriol, B.F.A.; Henry, H.A.L. Short-versus long-term effects of nitrogen addition and warming on soil nitrogen mineralization and leaching in a grass-dominated old field. Oecologia 2024, 205, 59–68. [Google Scholar] [CrossRef]
- Kichamu-Wachira, E.; Xu, Z.; Reardon-Smith, K.; Winowiecki, L.A.; Ayele, G.; Biggs, D.; Magaju, C.; Taresh, S.; Hosseini-Bai, S.; Omidvar, N. Effects of planting basins and farmyard manure addition on soil carbon and nitrogen pools under on-farm conditions in Makueni county of Kenya. Soil. Use Manag. 2024, 40, e13008. [Google Scholar] [CrossRef]
- Mann, T.A.; Yanai, R.D.; Fahey, T.J.; Reinmann, A.B. Nitrogen and phosphorus addition affect soil respiration in Northern Hardwood Forests. Ecosystems 2024. [Google Scholar] [CrossRef]
- Yue, K.; Peng, Y.; Peng, C.; Yang, W.; Peng, X.; Wu, F. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: A meta-analysis. Sci. Rep. 2016, 6, 19895. [Google Scholar] [CrossRef]
- Ngaba Junior, M.Y.; Uwiragiye, Y.; Bol, R.; de Vries, W.; Zhou, J. Low-level nitrogen and short-term addition increase soil carbon sequestration in Chinese forest ecosystems. Catena 2022, 215, 106333. [Google Scholar] [CrossRef]
- Avolio, M.L.; Koerner, S.E.; La Pierre, K.J.; Wilcox, K.R.; Wilson, G.W.T.; Smith, M.D.; Collins, S.L. Changes in plant community composition, not diversity, during a decade of nitrogen and phosphorus additions drive above-ground productivity in a tallgrass prairie. J. Ecol. 2014, 102, 1649–1660. [Google Scholar] [CrossRef]
- Ford, C.R.; Mitchell, R.J.; Teskey, R.O. Water table depth affects productivity, water use, and the response to nitrogen addition in a savanna system. Can. J. For. Res. 2008, 38, 2118–2127. [Google Scholar] [CrossRef]
- Liu, H.Y.; Huang, N.; Zhao, C.M.; Li, J.H. Responses of carbon cycling and soil organic carbon content to nitrogen addition in grasslands globally. Soil. Biol. Biochem. 2023, 186, 109164. [Google Scholar] [CrossRef]
- Carrell, A.A.; Hicks, B.B.; Sidelinger, E.; Johnston, E.R.; Jawdy, S.S.; Clark, M.M.; Klingeman, D.M.; Cregger, M.A. Nitrogen addition alters soil fungal communities, but root fungal communities are resistant to change. Front. Microbiol. 2023, 13, 1033631. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, Y.; Li, X.; Zhang, C.; Zeng, Q.; Yuan, X.; Chen, Y.; Yu, Y.; Fu, S. Eleven-year canopy nitrogen addition enhances the uptake of phosphorus by plants and accelerates its depletion in soil. Forests 2024, 15, 416. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Sun, R.; Li, M.; Liu, H.; Shi, Y.; Zhu, D.; Li, J.; Ma, L.; Fu, S. Influence of nitrogen addition on the functional diversity and biomass of fine roots in warm-temperate and subtropical forests. For. Ecol. Manag. 2023, 545, 121309. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhang, B.; Wu, D.; Shi, Y.; Zhang, W.; Ye, Q.; Yan, J.; Fu, J.; Fang, C.; et al. Canopy and understory nitrogen addition have different effects on fine root dynamics in a temperate forest: Implications for soil carbon storage. New Phytol. 2021, 231, 1377–1386. [Google Scholar] [CrossRef]
- Lu, X.; Gilliam, F.S.; Yu, G.; Li, L.; Mao, Q.; Chen, H.; Mo, J. Long-term nitrogen addition decreases carbon leaching in a nitrogen-rich forest ecosystem. Biogeosciences 2013, 10, 3931–3941. [Google Scholar] [CrossRef]
- Bowden, R.D.; Wurzbacher, S.J.; Washko, S.E.; Wind, L.; Rice, A.M.; Coble, A.E.; Baldauf, N.; Johnson, B.; Wang, J.; Simpson, M.; et al. Long-term nitrogen addition decreases organic matter decomposition and increases frest soil carbon. Soil. Sci. Soc. Am. J. 2019, 83, S82–S95. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Weand, M.P.; Arthur, M.A.; Lovett, G.M.; McCulley, R.L.; Weathers, K.C. Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil. Biol. Biochem. 2010, 42, 2161–2173. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Cha, X.; Yang, W.; Zheng, M.; Liu, S.; Wang, Y.; Cai, A.; Han, X.; Yang, G.; et al. Nitrogen addition-driven soil organic carbon stability depends on the fractions of particulate and mineral-associated organic carbon. Nutr. Cycl. Agroecosyst. 2024, 128, 269–281. [Google Scholar] [CrossRef]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil. Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Yang, J.; Wu, F.; Ni, X.; Yue, K.; Wei, X.; Zhang, X.; Zhang, X. Responses of soil nitrogen pools to litter input under nitrogen addition: A meta-analysis. J. Geophys. Res-Biogeo. 2024, 129, e2023JG007699. [Google Scholar] [CrossRef]
- Su, J.; Zhao, Y.; Bai, Y. Asymmetric responses of leaf litter decomposition to precipitation changes in global terrestrial ecosystem. J. Clean. Prod. 2023, 387, 135898. [Google Scholar] [CrossRef]
- Hodges, C.; Araujo, P.I.; Hess, L.J.T.; Vivanco, L.; Kaye, J.; Austin, A.T. Metal cation concentrations improve understanding of controls on soil organic carbon across a precipitation by vegetation gradient in the Patagonian Andes. Geoderma 2023, 440, 116718. [Google Scholar] [CrossRef]
- Li, G.; Si, M.; Zhang, C.; Shen, Z.; Wang, S.; Shao, J. Responses of plant biomass and biomass allocation to experimental drought: A global phylogenetic meta-analysis. Agric. For. Meteorol. 2024, 347, 109917. [Google Scholar] [CrossRef]
- Chang, W.; Song, Q.; Zheng, X.; Li, C.; Wang, L.; Li, H.; Zhang, L.; You, C.; Xu, H.; Xu, L.; et al. Leaf trait variations and correlations across four forests with similar mean annual precipitation in northern China. Ecol. Indic. 2024, 165, 112199. [Google Scholar] [CrossRef]
- Qu, Q.; Xu, H.; Ai, Z.; Wang, M.; Wang, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impacts of extreme weather events on terrestrial carbon and nitrogen cycling: A global meta-analysis. Environ. Pollut. 2023, 319, 120996. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; You, C.; Tan, B.; Xu, L.; Li, H.; Zhang, L.; Wang, L.; Liu, S.; Hou, G.; et al. Warming effects on C:N:P stoichiometry and nutrient limitation in terrestrial ecosystems. Soil. Tillage Res. 2024, 235, 105896. [Google Scholar] [CrossRef]
- Wan, Z.; Ganjurjav, H.; Gu, R.; Hu, G.; Gornish, E.S.; Chun, X.; Zhou, H.; Gao, Q. Changes in plant species dominance maintain community biomass production under warming and precipitation addition in temperate steppe in Inner Mongolia, China. Agric. For. Meteorol. 2023, 341, 109671. [Google Scholar] [CrossRef]
- Martin, P.A.; Fisher, L.; Perez-Izquierdo, L.; Biryol, C.; Guenet, B.; Luyssaert, S.; Manzoni, S.; Menival, C.; Santonja, M.; Spake, R.; et al. Meta-analysis reveals that the effects of precipitation change on soil and litter fauna in forests depend on body size. Glob. Chang. Biol. 2024, 30, e17305. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Soong, J.L.; Castanha, C.; Pries, C.E.H.; Ofiti, N.; Porras, R.C.; Riley, W.J.; Schmidt, M.W.I.; Torn, M.S. Five years of whole-soil warming led to loss of subsoil carbon stocks and increased CO2 efflux. Sci. Adv. 2021, 7, eabd1343. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mohammat, A.; Feng, J.; Zhou, R.; Fang, J. Storage, patterns and environmental controls of soil organic carbon in China. Biogeochemistry 2007, 84, 131–141. [Google Scholar] [CrossRef]
- Ury, E.A.; Wright, J.P.; Ardón, M.; Bernhardt, E.S. Saltwater intrusion in context: Soil factors regulate impacts of salinity on soil carbon cycling. Biogeochemistry 2022, 157, 215–226. [Google Scholar] [CrossRef]
- Morgan, B.S.T.; Tian, G.; Oladeji, O.O.; Cox, A.E.; Granato, T.C.; Zhang, H.; Podczerwinski, E.W. Analysis of effects and factors linked to soil microbial populations and nitrogen cycling under long-term biosolids application. Sci. Total Environ. 2024, 934, 173216. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Arthur, M.A.; Weathers, K.C.; Fitzhugh, R.D.; Templer, P.H. Nitrogen addition increases carbon storage in soils, but not in trees, in an eastern US deciduous forest. Ecosystems 2013, 16, 980–1001. [Google Scholar] [CrossRef]
- Bartsch, Z.J.; DeSutter, T.M.; Gasch, C.K.; Casey, F.X.M. Plant growth, soil properties, and microbial community four years after thermal desorption. Agron. J. 2022, 114, 1011–1026. [Google Scholar] [CrossRef]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Grandy, A.S.; Liang, C.; Phillips, R.P. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium promotes persistent soil organic matter by altering microbial transformation of plant litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef]
- Fenn, K.M.; Malhi, Y.; Morecroft, M.D. Soil CO2 efflux in a temperate deciduous forest: Environmental drivers and component contributions. Soil. Biol. Biochem. 2010, 42, 1685–1693. [Google Scholar] [CrossRef]
- Lugli, L.F.; Fuchslueger, L.; Vallicrosa, H.; Van Langenhove, L.; Ranits, C.; Garberi, P.R.F.; Verryckt, L.; Grau, O.; Bréchet, L.; Peguero, G.; et al. Contrasting responses of fine root biomass and traits to large-scale nitrogen and phosphorus addition in tropical forests in the Guiana shield. Oikos 2024, 2024, e10412. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Peng, C.; Kim, D.G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- De Carvalho, S.J.P.; Damin, V.; Dias, A.C.R.; Yamasaki, G.M.; Christoffoleti, P.J. Efficacy and pH of glyphosate spray solutions after the addition of nitrogen fertilizers and the use of CO2 pressurized sprayer. Pesqui. Agropecu. Bras. 2009, 44, 569–575. [Google Scholar]
- Wang, X.; Zhang, H.; Cao, D.; Wu, C.; Wang, X.; Wei, L.; Guo, B.; Wang, S.; Ding, J.; Chen, H.; et al. Microbial carbon and phosphorus metabolism regulated by C:N:P stoichiometry stimulates organic carbon accumulation in agricultural soils. Soil. Tillage Res. 2024, 242, 106152. [Google Scholar] [CrossRef]
- Haettenschwiler, S.; Jorgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Garcia-Palacios, P.; Milla, R.; Gallardo, A.; Maestre, F.T. Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil. Biol. Biochem. 2015, 81, 134–142. [Google Scholar] [CrossRef]
- Zhai, C.; Han, L.; Xiong, C.; Ge, A.; Yue, X.; Li, Y.; Zhou, Z.; Feng, J.; Ru, J.; Song, J.; et al. Soil microbial diversity and network complexity drive the ecosystem multifunctionality of temperate grasslands under changing precipitation. Sci. Total Environ. 2024, 906, 167271. [Google Scholar] [CrossRef]
- Uddin, M.J.; Sherrell, J.; Emami, A.; Khaleghian, M. Application of Artificial Intelligence and Sensor Fusion for Soil Organic Matter Prediction. Sensors 2024, 24, 2357. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Qu, C.; Huang, W.; Zhang, D.; Li, Y.; Yi, Z.; Liu, J. Translocating subtropical forest soils to a warmer region alters microbial communities and increases the decomposition of mineral-associated organic carbon. Soil. Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Ruan, Y.; Ling, N.; Jiang, S.; Jing, X.; He, J.; Shen, Q.; Nan, Z. Warming and altered precipitation independently and interactively suppress alpine soil microbial growth in a decadal-long experiment. eLife 2024, 12, RP89392. [Google Scholar] [CrossRef] [PubMed]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2012, 196, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Sayer, E.J.; Lam, S.K.; Lai, D.Y.F. Precipitation change affects forest soil carbon inputs and pools: A global meta-analysis. Sci. Total Environ. 2024, 908, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.V.; Pinto, P.; Bazzoni, B.; Berenstecher, P.; Casas, C.; Zieher, X.L.; Mallerman, J.; Méndez, M.S.; Omacini, M.; Piñeiro, G.; et al. From plant litter to soil organic matter: A game to understand carbon dynamics. Front. Ecol. Environ. 2024, 22, e2724. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, S.; Lu, X.; Ren, Z.; Wu, Q.; Xu, M.; Ren, C.; Yang, G.; Han, X. Organic carbon, nitrogen accumulation, and soil aggregate dynamics as affected by vegetation restoration patterns in the Loess Plateau of China. Catena 2021, 196, 104867. [Google Scholar] [CrossRef]
- Georgiou, K.; Jackson, R.B.; Vinduskova, O.; Abramoff, R.Z.; Ahlstrom, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef]
- Xu, K. Responses of soil carbon and nitrogen dynamics and GHG fluxes in forest ecosystems to climate change and human activity. Forests 2024, 15, 1235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).