Abstract
Research Highlights: Environmental abiotic stressors generate secondary stresses in plants, such as osmotic and oxidative stresses, which negatively influence their normal growth, development, and metabolism. Research about other non-enzymatic components with antioxidant capacity has recently focused on polyphenols. However, their role as indicators of drought and shade tolerance in woody species leaves and roots has been poorly explored or was limited to leaves only. Background and Objectives: Under a scenario of increasing drought, understanding the seedling responses in terms of total polyphenols and their antioxidant activity, in particular at the fine root system level, may help to elucidate the native–alien species interaction. Materials and Methods: At the beginning of July, 5-month-old native Quercus robur and alien Quercus rubra seedlings were transferred indoors to the growth chamber and subjected to progressive soil drying for 21 days. Results: The decrease in soil water content was more pronounced for Q. robur (9%) than for Q. rubra (34% of field capacity). Leaf water potential significantly decreased over time in Q. robur but did not differ from the control in Q. rubra. The total polyphenol concentration in Q. robur was markedly lower in the leaves and significantly higher in the fine roots than in Q. rubra. For the leaves, both species showed markedly higher values if well-watered, and the values significantly decreased in response to drought only in Q. rubra. In contrast, the fine root values for both species were markedly higher if droughted and decreased significantly in time only in Q. robur. Differently from the polyphenol concentration, the antioxidant capacity of Q. rubra was always higher in both the leaves and fine roots. Conclusions: The higher antioxidant activity of the alien species Q. rubra revealed by this work, combined with its isohydric behaviour, could further shed some light on our understanding of its competitive performance at the seedling stage against the native Q. robur.
1. Introduction
Environmental stresses, such as drought, low or high temperature, and excessive salinity, are the main factors that influence plant distribution. These abiotic factors induce secondary stresses, such as osmotic and oxidative stresses, which negatively affect the plant, causing changes in its normal growth, development and metabolism [1,2].
Stresses cause disturbances in cellular redox homoeostasis, which in turn, may lead to secondary oxidative stress and the production of reactive oxygen species (ROS) [3] that cause lipid peroxidation, protein denaturation and DNA damage [4,5]. ROS, when present in low concentration, fulfil the roles of signalling molecules to trigger the synthesis of enzymes (catalase, peroxidase, superoxide dismutase), as well as the non-enzymatic molecules (glutathione, α-tocopherol, carotenoids) that take part in antioxidant reactions. In recent years, research about other non-enzymatic components with antioxidant capacity has focused on polyphenols, synonymous with phenolic compounds [6,7]. As by-products of metabolic alteration, polyphenols constitute a large group of molecules with great potential for reducing ROS and avoiding cell damage, complementing the roles played by the enzymatic antioxidant system [8]. Also known as polyphenols, they may be categorised into three different groups: (1) nonflavonoids, molecules with at least one phenolic ring with different hydroxyl and carbonyl reactive groups, including simple phenolic acids, phenyl alcohols, stilbenes, coumarines, chalcones and lignans; (2) flavonoids, molecules with 15 atoms of carbon (C) structured in two aromatic rings that bind with a carbon chain (C6-C3-C6), sub-classified into anthocyanidins, flavonols, flavones, isoflavones, flavanones, flavan-3-ols, etc., depending on the amount, position, and type of reactive group; and (3) tannins, subdivided into (a) condensed tannins: flavonoids polymers type A (C7 → C2 and an ether bond) and type B (C4 → C8 or C4 → C6), and (b) hydrolysable tannins: phenolic acids polymers that bind to a 5- or 6-carbon ring [9]. The mechanism of antioxidant actions involves either hydrogen atom transfer, transfer of a single electron, sequential proton loss electron transfer, and chelation of transition metals [10]. It is worth noting that a large body of literature unequivocally suggests that polyphenols not only play a major role in the defence mechanisms of plants but also exert a healthy influence on humans and animals that feed on products enriched with phenolic compounds [11].
Fast-growing plants typically invest less in defence and protection, leading to lower phenolic compound concentrations. In contrast, slow-growing species have higher phenolic compound concentrations, which could be attributed to the long-term probability of facing biotic and abiotic (primarily water shortage) stress factors ([12] and references therein). Moreover, evidence suggests that the interspecific variations in the concentration of polyphenols reflect the answer to the dilemma “carbon gain vs. water saving”. For instance, as water loss from leaves is an unavoidable consequence of obtaining CO2 for photosynthesis, water-saving plants have evolved to produce protective compounds (such as antioxidants, including phenolic compounds) instead of directing the products of photosynthesis for growth maximisation only [13].
Quercus rubra L. (red oak) is an economically important tree species native to eastern North America and is considered an invasive species in Italy [14]. It causes changes in forest ecosystems associated with decreasing light availability and deposition of large amounts of recalcitrant leaf litter (low N, high C/N ratio), which creates a physical barrier to native species seed germination and seedling growth [15]. As a moderately shade-tolerant species [16,17] with marked isohydricity—water-savings [18], red oak efficiently regenerates under the drier conditions of the exposed soil surface generated by the regular thinning of managed European forests. Several studies and regional reports suggest that in the introduced range, Q. rubra copes with drought more effectively than the native Quercus robur (pedunculate oak) and Quercus petraea (sessile oak) ([19] and references therein). Therefore, under a scenario of increasing drought, it is crucial to understand how seedlings of these two species respond to water limitation, as drought could select more drought-resistant alien species in the future.
It has been proposed that, depending on the ecophysiological strategies adopted, temperate tree species have a gradient of mesophytism that allows them to compete for the same habitat. Considering the severe stress these species face during their establishment at the seedling stage, we hypothesise that polyphenols may be an important component of their antioxidant system. However, the role of polyphenols as indicators of drought and shade tolerance, specifically in woody species leaves and fine roots, has been poorly explored [2,20,21,22,23,24,25,26] or limited to leaves only [27,28,29,30,31].
This study aims to investigate the variation of total polyphenol content and its antioxidant capacity in leaves and fine roots (diameter < 1 mm) of 5-month-old potted seedlings of the native Q. robur and the alien Q. rubra under the additive action of low light intensity and progressive soil drying. As previously stated, these species have shown contrasting drought resistance mechanisms at the seedling stage, with an increasing degree of isohydricity from Q. robur to Q. rubra [18]. Specifically, our objectives were to determine the following:
- (1)
- The extent to which the additive action of low light intensity and progressive soil drying affects the polyphenol content and the oxidative capacity of leaves and fine roots;
- (2)
- Whether the drought intensity correlates with the polyphenol content and the antioxidant capacity;
- (3)
- The extent to which the two investigated species differ in their response.
2. Materials and Methods
2.1. Plant Material and Experimental Design
Seedlings of Q. robur and Q. rubra were pot-grown in the common garden of the University of Insubria, Varese, Italy, which has a temperate climate with an annual mean air temperature of 12 °C and annual mean rainfall of 1506 mm. At the beginning of July, 50 homogeneous (25 per species) 5-month-old seedlings of Q. robur (33–40 cm tall) and Q. rubra (30–35 cm tall) were selected and transferred indoors to the growth chamber (Figure S1). The plants were grown under 25/18 °C day/night temperature, 350 μmol m−2 s−1 PPFD, 16 h photoperiod, and 45/70% relative humidity (RH) throughout the experiment. All plants were watered to 70%–80% field capacity (48.66 ± 5.37 vol%) until the start of the experiment. Successively, 15 plants considered as controls were maintained to 70%–80% field capacity and sampled on days 1 (start), 10 and 21, while 10 plants were subjected to progressive soil drying and sampled on days 10 and 21. Soil water content was monitored for every pot with Time Domain Reflectometry (TDR; model ML2x, ΔT Devices, Cambridge, UK).
At each sampling time, 5 seedlings per treatment were harvested. For each plant, the leaf water potential (LWP) was measured on five leaves per species (one per plant) with a Scholander pressure chamber, model SKYE SKPM 1400/(40 Digital Systems). Roots were carefully freed from the soil by shaking, brushing, and rinsing with tap water. All live fine roots (diameter < 1 mm) were excised and placed in water-filled basins; remaining soil particles were carefully removed using soft paint brushes. Upon cleaning (Figure S2), the remaining large and fine roots were weighed separately, and approximately 1 g of fine roots was selected for total polyphenols and antioxidant activity assays. Leaves were cut as well and used for the same assays. The leaves and fine roots < 1 mm were frozen in liquid nitrogen, freeze-dried, weighed and fragmented in a mixer mill. The samples were stored at −20 °C until the time of analysis. The remaining stem and roots were oven-dried at 70 °C for 3–5 days. The resulting dry mass values were pooled with those of the freeze-dried leaves and fine roots for growth measurements.
2.2. Extraction and Quantification of Total Polyphenols
Polyphenols were extracted from 0.25 g of freeze-dried plant material (leaf and fine roots < 1 mm in diameter) in 5 mL of 80% (v/v) methanol for 16 h at 4 °C. After each extraction, the liquid was separated from the solids by centrifugation at 4 °C for 15 min at 15,000× g.
Total polyphenols were determined using the Folin–Ciocalteu method adapted to our samples according to Quettier-Deleu et al. [32]. Briefly, 50 μL of 10-fold diluted extract was pipetted into a 2 mL centrifuge tube, and 50 μL of Folin–Ciocalteu reagent was added, vortexed and left for 6 min; 700 μL of distilled water and 200 μL of Na2CO3 at 20% (w/v) were pipetted, vortexed and left in the dark at room temperature for 90 min. After incubation, the absorbance of the reaction mixture was measured at 765 nm. The total amount of polyphenols was calculated as mg of gallic acid equivalents (Geq) per gram of organ (leaf or fine roots) freeze-dry weight.
2.3. Trolox-Equivalent Antioxidant Capacity (TEAC) Assay
The total antioxidant activity was estimated using the Trolox-equivalent antioxidant capacity (TEAC) assay. In this test, we measured the relative capacity of antioxidants to scavenge the ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical compared to the antioxidant capacity of Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a water-soluble vitamin E analogue used as a standard. The ABTS•+ radical was generated by mixing an ABTS 7 mM solution with 2.45 mM K2S2O8 in the dark for 24 h at room temperature. Before application, the ABTS•+ solution was diluted to obtain an absorbance of 0.700 ± 0.035 at 734 nm with phosphate-buffered saline (PBS) solution (pH 7.4). The spectrophotometer was preliminarily blanked with PBS. Upon adding 1.0 mL of the diluted ABTS•+ solution to 10 μL of the extract or Trolox standard, the absorbance at 734 nm was recorded each minute after initial mixing for 5 min. Trolox 2.5 mM solution was used to obtain the calibration curve. The antioxidant capacity was expressed as mmol of Trolox equivalents (Teq) per gram of organ (leaf or fine roots) freeze-dry weight.
2.4. Statistical Analyses
The experiment was a completely randomised design with factorial treatments (3 sampling points × 2 species × 2 watering regimes). The main and interaction effects for the investigated traits were tested with a three-way ANOVA using a general linear model (SPSS Inc., Chicago, IL, USA). All interactions were initially included in the model, the non-significant ones being stepwise excluded. Before the analysis, all above- and below-ground biomass, physiological and chemical data were tested with the Kolmogorov–Smirnov test for normality and the Levene test for the homoscedasticity assumption. In particular, as the leaf water potential did not meet these requirements, even after transformation, the non-parametric two-groups Mann–Whitney test was carried out on the medians of the main factors. For each variable, one-way ANOVA and the least significant difference (LSD) pairwise multiple comparison tests were performed among treatments within the 10th and 21st sampling days and among sampling days, including day 0, within each treatment. This latter also represents the comparison between the full-sunlight outdoor condition on day 0 and the low-light indoor condition on days 10 and 21. All differences were considered significant at p < 0.05. Principal component analysis (PCA) was performed on all standardised data to detect the pattern of association among the root (fine roots alone and whole root system) and shoot (leaves alone and whole shoot system) dry masses, chemical (polyphenols and antioxidant capacity) variables and the water-status variables leaf water potential and soil water content (software SYN-TAX 2000, Podani, Budapest, Hungary).
3. Results
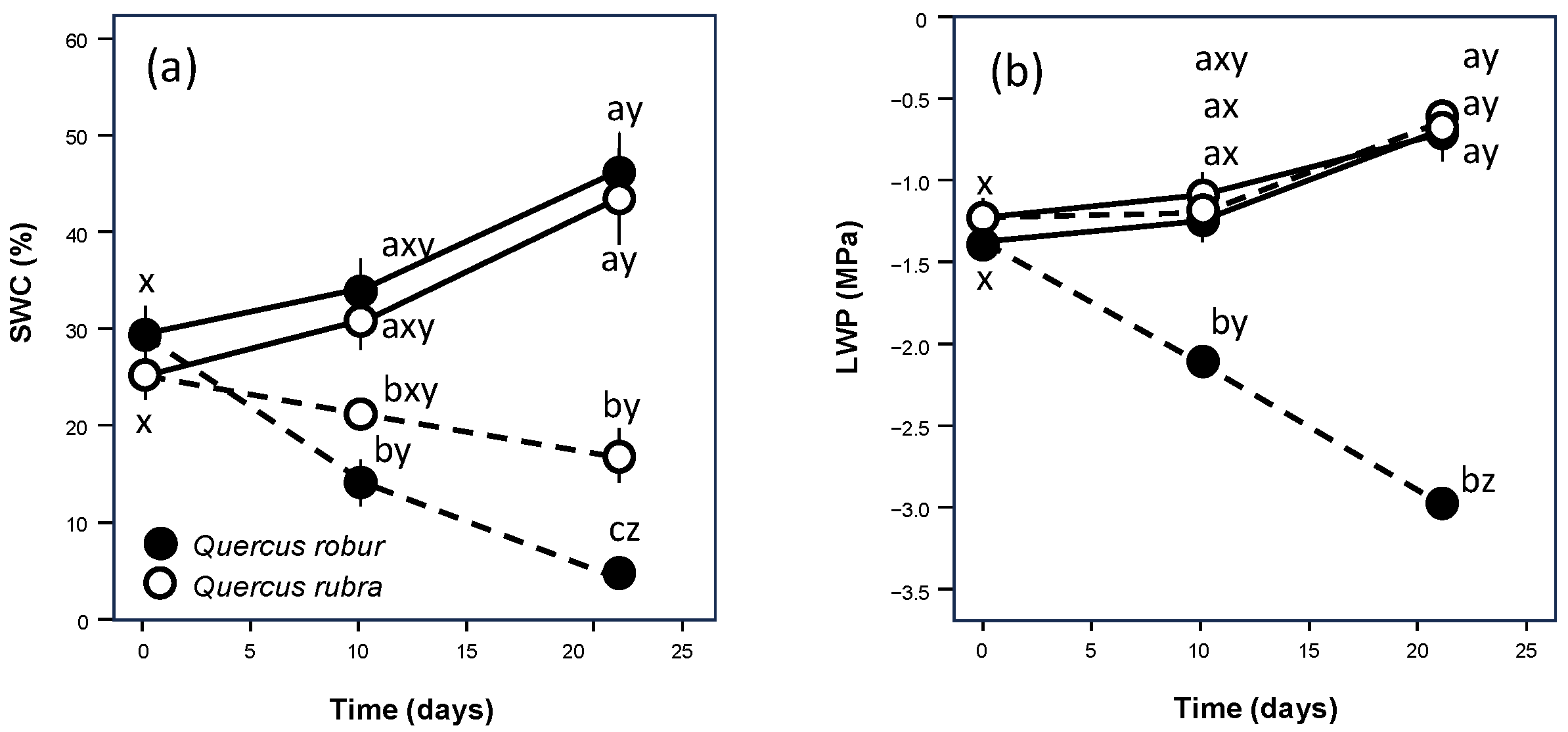
From the beginning of the experiment, SWC decreased significantly in Q. robur droughted pots after 10 days and after the following 11 days, decreasing to 9% of field capacity. For Q. rubra, this decrease became significant only after 21 days, resulting in a higher value equal to 34% of field capacity (Figure 1). The behaviour of leaf water potential was different (Figure 1), with a significant decrease over time for Q. robur and values similar to those of the control for Q. rubra droughted leaves.
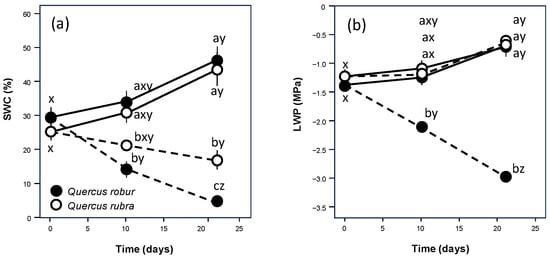
Figure 1.
Time course of soil volumetric water content (SWC) (a) and leaf water potential (LWP) (b) over the experimental period for 5-month-old Quercus robur (filled circle) and Quercus rubra (empty circle) seedlings under two drought intensity levels. Continuous (–) and dashed (– – –) lines indicate watered and drought conditions, respectively. Values are the means of five replicates ±1SE. If shown, a, b, and c indicate significant differences between the different treatments within each sampling point; x, y, and z indicate significant differences between different sampling points within each treatment (LSD, p < 0.05).
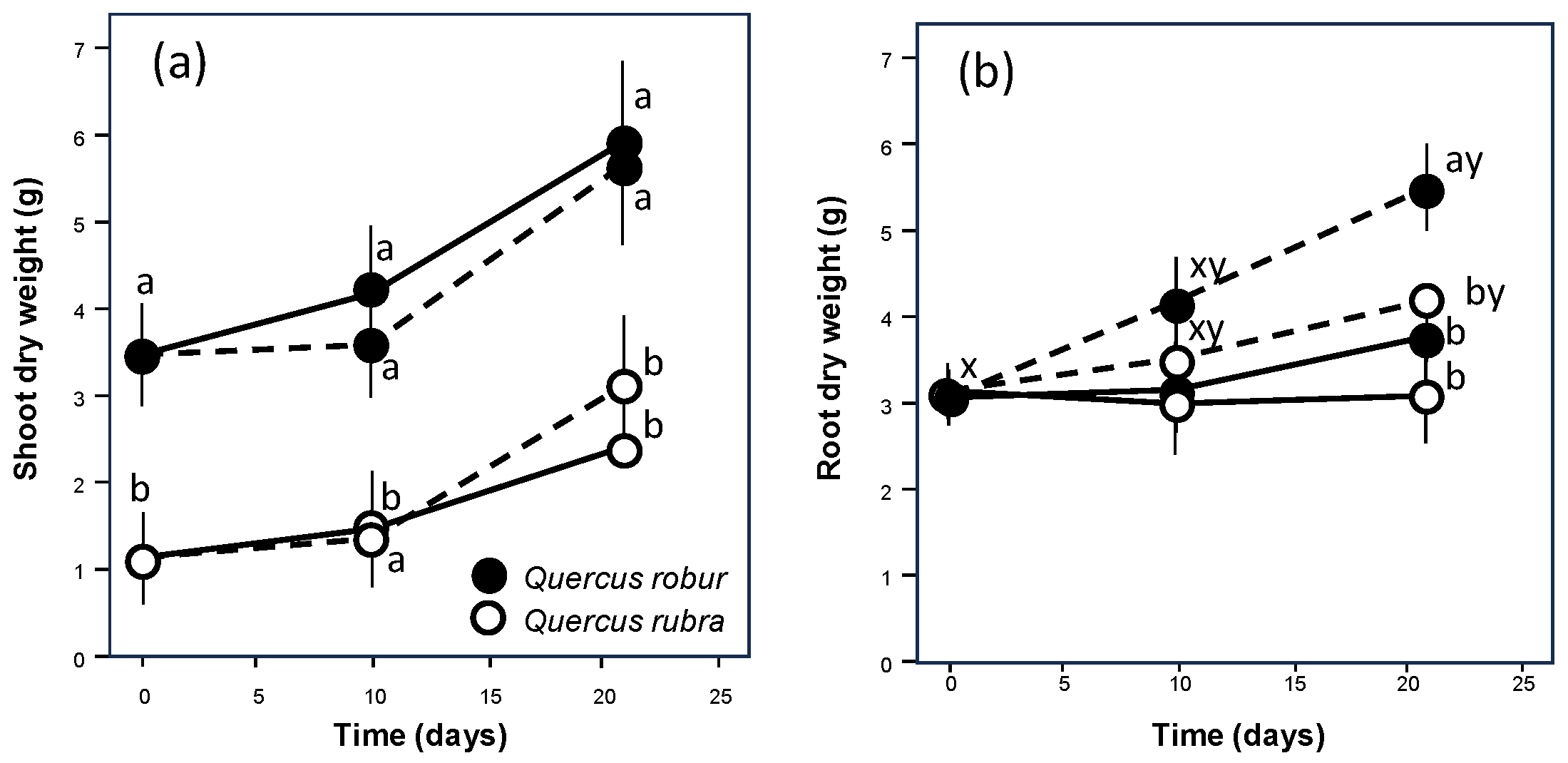
During the experiment, both oaks increased the shoot biomass (Table 1, p = 0.008; Figure 2) independently of the watering regime (Table 1, p = 0.828). However, Q. robur always showed values significantly higher than Q. rubra (Table 1, p < 0.001; Figure 2). The behaviour at the below-ground level was completely different, with no differences in growth between the species (p = 0.082) but a similar significant increase under drought conditions (Table 1, p = 0.006; Figure 2), particularly in Q. rubra.

Table 1.
p-values (in italics) of three-way ANOVA (general linear model) for the effects of time, species and drought intensity on biomass and chemical traits of the leaves and fine roots, and physiological traits of the leaves only of Quercus robur and Quercus rubra. Non-significant interaction effects were excluded from the model (-). Boldface p values are significant at a probability level of p < 0.05.
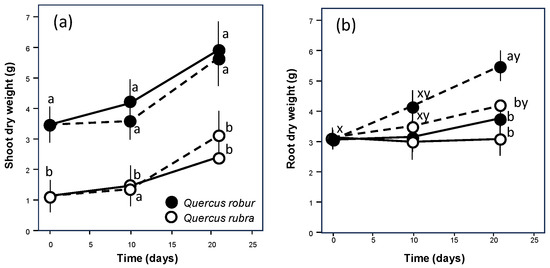
Figure 2.
Time course of shoot (a) and root (b) growth over the experimental period for 5-month-old Quercus robur (filled circle) and Quercus rubra (empty circle) seedlings under two drought intensity levels. Continuous (–) and dashed (– – –) lines indicate watered and drought conditions, respectively. Values are the means of five replicates ±1SE. If shown, a, b and c indicate significant differences between the different treatments within each sampling point; x, y and z indicate significant differences between different sampling points within each treatment (LSD, p < 0.05).
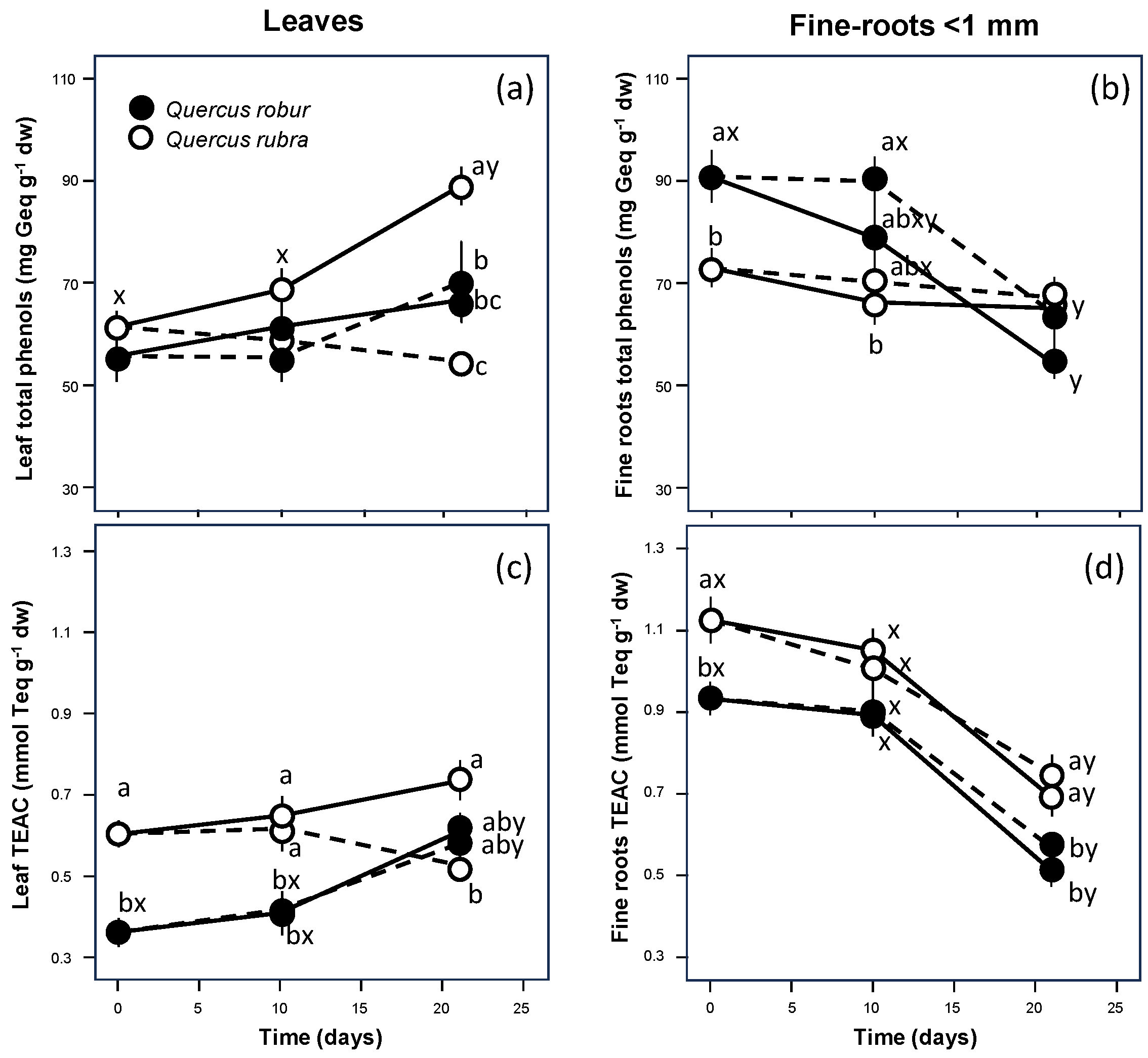
At the fine scale of leaves and fine roots, a different behaviour was also observed for the total polyphenol concentration, as confirmed by the significant inverse correlation between the two organs (Table 2, p = 0.044). Q. robur showed values markedly lower in the leaves (Table 1, p = 0.083; Figure 3a) and significantly higher in the fine roots (Table 1, p = 0.005; Figure 3b) than Q. rubra. For the leaves, both species showed markedly higher values of total polyphenols if well-watered (Table 1, p = 0.007; Figure 3a) but differed significantly in response to drought, with decreasing values only for Q. rubra (Table 1, S × D interaction p = 0.015). For the fine roots, despite the higher values observed for Q. robur, both species similarly showed markedly higher values of total polyphenols if droughted (Table 1, p = 0.136; Figure 3b), decreasing significantly in time only for Q. robur (Table 1, S × T interaction p = 0.002).

Table 2.
Pearson’s correlation coefficients between physiological, biomass and chemical traits. Values in normal font refer to correlation coefficients, italics to p-values; boldface p-values are significant at a probability level of p < 0.05 (*) and p < 0.01 (**).
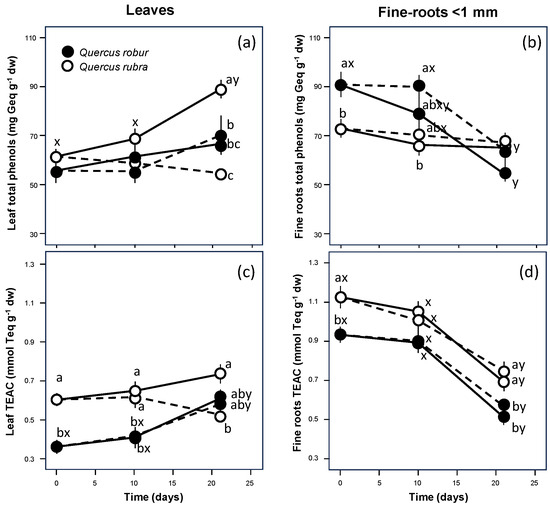
Figure 3.
Time course of total polyphenol concentration (a,b) and ABTS+• free cation radical scavenging activity (c,d) of the leaf (left column) and fine-roots (right column) extracts from 5-month-old Quercus robur (filled circle) and Quercus rubra (empty circle) seedlings under two drought intensity levels. Continuous (–) and dashed (– – –) lines indicate watered and drought conditions, respectively. Values are the means of five replicates ±1SE. If shown, a, b and c indicate significant differences between the different treatments within each sampling point; x, y and z indicate significant differences between different sampling points within each treatment (LSD, p < 0.05).
The antioxidant capacity showed a pattern similar to that observed for the polyphenol content, as demonstrated by the significant positive correlation for both leaves (Table 2, p < 0.001) and fine roots (p = 0.01). For the leaves, the difference became significant, with Q. robur showing values significantly lower than Q. rubra (Table 1, p < 0.001; Figure 3c). In the fine roots, unlike the polyphenol content, the antioxidant capacity was significantly lower in Q. robur than in Q. rubra (Table 1, p < 0.001; Figure 3d). Moreover, the values did not change significantly in either species after 10 days since the beginning of the treatment. Still, at the end of the experiment, they decreased significantly (Table 1, p < 0.001) to values similar to those of leaves.
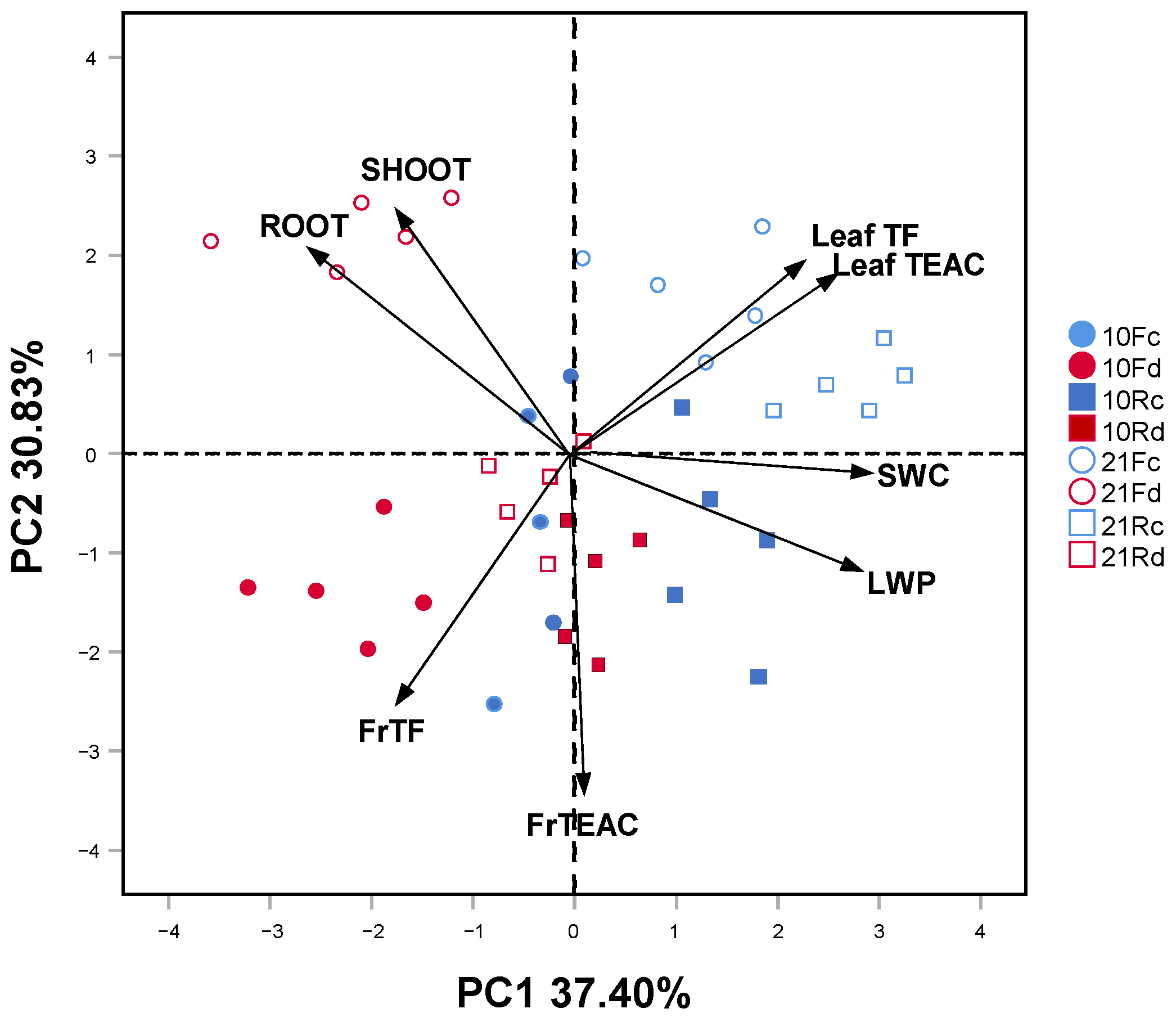
The striking difference among all investigated traits reflected by the first component of PCA (37.40% of total variance) could be attributed to the water availability gradient, from well-watered on the right positive loadings to droughted on the left negative loadings (Figure 4). The overall growth and total polyphenols in fine roots tended to increase more in drought conditions and in Q. robur than in Q. rubra. The second component better explains the time factor (30.83% of total variance). In both oaks, the highest values of growth, total polyphenols, and antioxidant capacity of the leaves were more pronounced on day 21, whereas those for the fine roots were more evident on day 10.
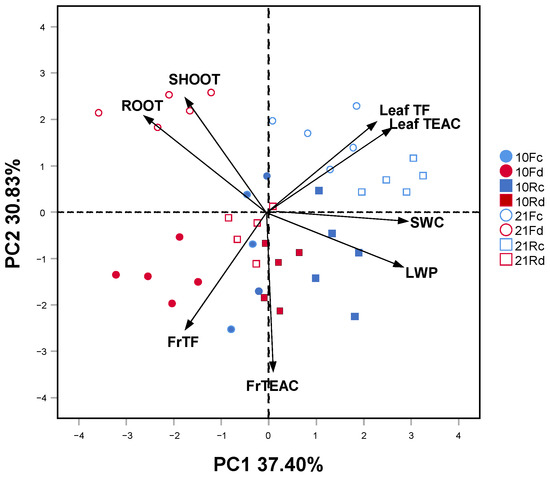
Figure 4.
Principal component analysis (PCA) ordination of the first (37.40% of explained variance) and second (30.83% of explained variance) axes, showing the relationships between physiological traits only for the leaves, and chemical and biomass traits for both leaves and fine roots (arrows) under two drought intensity levels (blue: control (c); red: drought (d); filled: 10 days; empty: 21 days) in 5-month-old Quercus robur (circle, F) and Quercus rubra (square, R) seedlings. SWC, soil water content; LWP, leaf water potential; SHOOT, shoot dry mass; ROOT, root system dry mass; TF, total polyphenols; TEAC, Trolox-equivalent antioxidant capacity; Fr, fine roots.
4. Discussion
According to our previous findings [18] obtained under identical experimental conditions, the only exception being the longer dry period and the adoption of 4-month-old seedlings, i.e., one month younger, Q. rubra showed reduced growth and significantly lower shoot biomass than Q. robur, independently from the drought intensity. Similarly, Q. rubra maintained a significantly higher LWP, even under drier conditions and a slightly higher soil water content at the end of the drying period, which was observed to correlate with a lower stomatal conductance in the previous work [18]. These outcomes confirmed the isohydric behaviour of this species, at least in the seedling stage and in the adopted experimental conditions, whose main effect was the overall reduced growth [18]. Differences between the investigated species emerged even for the biochemical traits investigated; our findings indicate that the polyphenol content in the alien red oak was lower in the fine roots and higher in the leaves compared to the native pedunculate oak. On the other hand, the antioxidant activity was higher for red oak in both leaves and fine roots. Such differences may be expected between phylogenetically distant species. Indeed, chemotaxonomic evidence has highlighted qualitative and quantitative differences in polyphenol chemistry within deciduous trees and among deciduous and evergreen trees [33] and shrubs [22]. These differences appeared rather species-specific since changes in the major phenolic groups during the growing season (hydrolysable and condensed tannins, flavonols, quinic acid) were quantitative rather than qualitative [33], i.e., differing in the concentrations without changes in the proportions between groups. For temperate oak species in particular, despite differences in oxidative capacity with leaf-out time in the spring, by midsummer, mature leaves converged on a phenotype of reduced total polyphenol content and oxidative capacity [34]. In contrast, proanthocyanidin (condensed tannins) content followed the opposite pattern, increasing in concentration by midsummer [34,35,36,37]. This seasonal pattern is important, considering that the oak seedlings used for this experiment were transferred from outdoors to the growth chamber at the beginning of July when leaves were mature.
Numerous reports indicate increases in secondary metabolites under both low- [38] and high-light conditions [27,28,29,30]. The shade-tolerant shrub Labisia pumila showed higher polyphenol content and antioxidant activity under low light intensity but decreased these values as light intensity increased [39]. Similarly, for tropical evergreen tree species, shade-tolerant (late-successional) Cryptocarya concinna showed higher polyphenol content and antioxidant activity than light-demanding (mid-successional) Schima superba under low light intensity but unlike Labisia pumila, even when exposed to full sunlight [29,30].
In this study, leaves of control plants, particularly Q. rubra, increased their polyphenols and antioxidant capacity when transferred from outdoors to the growth chamber under low irradiance intensity. The opposite behaviour regarding trend and species involved occurred in the fine roots, with a significant decrease only in Q. robur (Figure 3). The relatively low concentrations of polyphenols and antioxidant capacity in July at the experiment start, which agrees with the above-mentioned lower values in midsummer compared to those in spring [34], may have triggered such increases, highlighting the shade-tolerant trait of both species and Q. rubra in particular. Moreover, the higher values observed in the leaves of Q. rubra may be explained by its limited growth, which should have increased the availability of the carbon pool allocated to secondary metabolism [38] compared to Q. robur. The opposite behaviour observed in the fine roots of both investigated oaks highlighted differing mechanisms between the two organs, with fine roots not inclined to invest in polyphenols and/or antioxidant activity if water was available and the photosynthates from above were reduced in response to the low light intensity.
In contrast to the increase-response in the well-watered plants, the additive action of low light intensity and prolonged drought stress appeared to inhibit polyphenol synthesis, slowing down the increase in leaves and the decrease in fine roots, particularly in Q. rubra. Indeed, in Q. rubra, drought did not affect the phenolic content of fine roots as it did for leaves, significantly inhibiting the synthesis of polyphenols and antioxidant capacity in the latter despite the high (close to control) leaf water potential. In contrast, in the leaves and fine roots of Q. robur when transferred into the growth chamber, the response to drought mimicked that of the control plants but was delayed in time.
The slow reduction in polyphenols in the droughted fine roots of both species allowed them to maintain a slightly higher concentration than the control, highlighting ongoing oxidative stress. Evidence suggests that a general trend of increasing polyphenol production in fine roots (diameter < 1 mm) similarly occurs along declining soil fertility stress gradients between forest types [40]. In response to drought stress conditions, the literature reports contrasting results for the total polyphenol content of woody species. There was an increase in fine roots of Vitis vinifera [19] and Rehmannia glutinosa [41] after one week at 30% of field capacity and −1.18 ± 0.04 MPa soil water potential or under general abiotic stress [42,43], but a decrease both in leaves and fine roots of Vitis vinifera after a prolonged drought (two weeks) at 30% of field capacity [2] and in leaves of the shrub Ligustrum vulgare after eight weeks at 40% of field capacity [23]. Varela et al. [22] showed contrasting results between the evergreen Larrea divaricata and the deciduous Lycium chilense shrubs during the growing season, with total polyphenol and flavonoid contents higher in the former and in the leaves than in the roots. Such divergences in experimental results can be attributed to differences between the evergreen and deciduous habits, the type of abiotic stresses (drought, low or high temperature), their intensity and duration, the stages of plant development, or the organ investigated, i.e., fine roots (and between diameter sub-classes) or leaves, which are characterised by a great heterogeneity of secondary metabolites [20]. On the other hand, even if the polyphenol concentration is reduced, as in the leaves of Ligustrum vulgare, caution must be exercised because the ratio to net CO2 assimilation may increase, suggesting a counterbalance in favour of the secondary metabolites [27].
Water-saving/isohydric plants evolved to accumulate protective compounds (synthesising antioxidants, including phenolic compounds) rather than diverting their carbon toward maximising growth [13]. In red oak seedlings, all these aspects, i.e., the higher antioxidant activity observed in the leaves and fine roots, the isohydric behaviour and the reduced growth, simultaneously occurred. On the other hand, the higher antioxidant activity would appear more polyphenol-related in the leaves than in the fine roots. Other important endogenous antioxidants such as glutathione, ascorbic acid, α-tocopherol and carotenoids, or enzymatic antioxidants such as catalase, peroxidase and superoxide dismutase [5], which were not quantified in the present study, may have also contributed to the protection process [31], partially explaining the higher antioxidant activity found in the leaves and fine roots of the alien Q. rubra than the native Q. robur, despite the similar concentrations of polyphenols.
5. Conclusions
The higher antioxidant activity of the alien species Q. rubra revealed by this work, combined with its isohydric behaviour, could further shed some light on our understanding of its competitive performance at the seedling stage against the native Q. robur, especially given the drier conditions in the canopy openings during the summer season. Further investigations on the possible interaction between antioxidant activity and the production of secondary metabolites involved in plant–plant interactions at the root level also worth pursuing.
Overall, the relationship between polyphenols and antioxidant activity in plants is complex and multifaceted, involving various mechanisms contributing to plant tissues’ overall antioxidant capacity. Comparing the seasonal pattern of the polyphenol content between seedlings grown outdoors and seedlings transferred indoors under low light intensity at different moments of the growing season, which also implies a drought period, will surely improve our understanding of the interaction between polyphenol content and variation in water availability and light intensity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15091647/s1. Figure S1. Sub-samples of Quercus robur (left) and Quercus rubra (right) 5-month-old seedlings when transferred in the growth chamber. Figure S2. Quercus robur (left) and Quercus rubra (right) 5-month-old control seedlings sampled on day 21 after cleaning procedure.
Author Contributions
Conceptualization, A.D.I. and T.M.; methodology, A.D.I. and T.M.; data curation, S.C., T.M. and A.D.I.; investigation, T.M., S.C. and A.D.I.; formal analysis, A.D.I.; visualization, A.D.I.; writing—original draft preparation, A.D.I., S.C. and T.M.; writing—review and editing, A.D.I.; project administration, A.D.I.; funding acquisition, A.D.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Research Fund of the University of Insubria (FAR no. 2019).
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author upon reasonable request. The data are not publicly available as some sections must remain confidential to support the publication of other thematic articles.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.Z.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Motilva, M.-J.; Serra, A.; Macià, A. Analysis of food polyphenols by ultra high-performance liquid chromatography coupled to mass spectrometry: An overview. J. Chromatogr. A 2013, 1292, 66–82. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P.; Stavroulaki, V.; Sumbele, S. “Carbon gain vs. water saving, growth vs. defence”: Two dilemmas with soluble phenolics as a joker. Plant Sci. 2014, 227, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Karabourniotis, G.; Liakopoulos, G. Phenolic compounds in plant cuticles: Physiological and ecological aspects. Adv. Plant Physiol. 2005, 8, 33–47. [Google Scholar]
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora alien to Italy. Plant Biosyst. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- Stanek, M.; Zubek, S.; Stefanowicz, A.M. Differences in phenolics produced by invasive Quercus rubra and native plant communities induced changes in soil microbial properties and enzymatic activity. For. Ecol. Manag. 2021, 482, 118901. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Francis Salifu, K.; Davis, A.S. Drought susceptibility and recovery of transplanted Quercus rubra seedlings in relation to root system morphology. Ann. For. Sci. 2009, 66, 504. [Google Scholar] [CrossRef]
- Sander, I. Quercus rubra L. In Silvics of North America: Volume 2. Hardwoods; USDA Agricultural Handbook 654; Burns, R.M., Honkala, B.H., Eds.; USDA: Washington, DC, USA, 1990; Volume 2, pp. 727–738. ISBN 654. [Google Scholar]
- Di Iorio, A.; Caspani, A.C.; Beatrice, P.; Montagnoli, A. Drought-related root morphological traits and non-structural carbohydrates in the seedlings of the alien Quercus rubra and the native Quercus robur: Possible implication for invasiveness. Front. For. Glob. Chang. 2024, 7, 1307340. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Vor, T.; Mason, W.L.; Bastien, J.-C.; Brus, R.; Henin, J.-M.; Kupka, I.; Lavnyy, V.; La Porta, N.; Mohren, F.; et al. Ecology and management of northern red oak (Quercus rubra L. syn. Q. borealis F. Michx.) in Europe: A review. For. Int. J. For. Res. 2020, 93, 481–494. [Google Scholar] [CrossRef]
- Weidner, S.; Karolak, M.; Karamać, M.; Kosińska, A.; Amarowicz, R. Phenolic compounds and properties of antioxidants in grapevine roots (Vitis vinifera L.) under drought stress followed by recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar] [CrossRef]
- Giertych, M.J.; Karolewski, P.; Oleksyn, J. Carbon allocation in seedlings of deciduous tree species depends on their shade tolerance. Acta Physiol. Plant. 2015, 37, 216. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.E.A.; Kobe, R.K.; McCarthy-Neumann, S. Tree seedling shade tolerance arises from interactions with microbes and is mediated by functional traits. Front. Ecol. Evol. 2023, 11, 1224540. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Munakata, R.; Larbat, R.; Duriot, L.; Olry, A.; Gavira, C.; Mignard, B.; Hehn, A.; Bourgaud, F. Polyphenols from Plant Roots. Recent Adv. Polyphen. Res. 2019, 6, 207–236. [Google Scholar] [CrossRef]
- Kecis, H.; Abdelouahab, Y.; Bagues, M.; Gali, L.; Mekircha, F.; Alloun, W.; Nagaz, K. Phenolic profile and bioactivity of the aerial part and roots of Mentha rotundifolia L. grown in two different localities in northeastern Algeria: A comparative study. Biocatal. Agric. Biotechnol. 2023, 47, 102581. [Google Scholar]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Zhang, T.J.; Zheng, J.; Yu, Z.C.; Huang, X.D.; Zhang, Q.L.; Tian, X.S.; Peng, C.L. Functional characteristics of phenolic compounds accumulated in young leaves of two subtropical forest tree species of different successional stages. Tree Physiol. 2018, 38, 1486–1501. [Google Scholar] [CrossRef]
- Yu, Z.C.; Zheng, X.T.; Lin, W.; Cai, M.L.; Zhang, Q.L.; Peng, C.L. Different photoprotection strategies for mid- and late-successional dominant tree species in a high-light environment in summer. Environ. Exp. Bot. 2020, 171, 103927. [Google Scholar] [CrossRef]
- Yu, Z.C.; Lin, W.; Zheng, X.T.; Cai, M.L.; Zhang, T.J.; Luo, Y.N.; Peng, C.L. Interpretation of the difference in shade tolerance of two subtropical forest tree species of different successional stages at the transcriptome and physiological levels. Tree Physiol. 2021, 41, 1669–1684. [Google Scholar] [CrossRef]
- Lassouane, N.; Aïd, F.; Quinet, M.; Lutts, S. Phenolic acids and flavonoids classes in Acacia arabica (Lam) Willd. seedling during water stress and subsequent re-hydration. Plant Soil 2024, 496, 449–471. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Thierry, D.; Claude, B.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vanhakylä, S.; Salminen, J.-P. Mass Spectrometric Fingerprint Mapping Reveals Species-Specific Differences in Plant Polyphenols and Related Bioactivities. Molecules 2023, 28, 6388. [Google Scholar] [CrossRef] [PubMed]
- Barber, N.A.; Fahey, R.T. Consequences of phenology variation and oxidative defenses in Quercus. Chemoecology 2015, 25, 261–270. [Google Scholar] [CrossRef]
- Salminen, J.P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Ossipov, V.; Salminen, J.P.; Ossipova, S.; Haukioja, E.; Pihlaja, K. Gallic acid and hydrolysable tannins are formed in birch leaves from an intermediate compound of the shikimate pathway. Biochem. Syst. Ecol. 2003, 31, 3–16. [Google Scholar] [CrossRef]
- Riipi, M.; Ossipov, V.; Lempa, K.; Haukioja, E.; Koricheva, J.; Ossipova, S.; Pihlaja, K. Seasonal changes in birch leaf chemistry: Are there trade-offs between leaf growth and accumulation of phenolics? Oecologia 2002, 130, 380–390. [Google Scholar] [CrossRef]
- Jaafar, H.Z.E.; Ibrahim, M.H.; Fakri, N.F.M. Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), malondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 2012, 17, 7305–7322. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Reduced photoinhibition under low irradiance enhanced kacip fatimah (Labisia pumila Benth) secondary metabolites, phenyl alanine lyase and antioxidant activity. Int. J. Mol. Sci. 2012, 13, 5290–5306. [Google Scholar] [CrossRef]
- Muller, R.N.; Kalisz, P.J.; Luken, J.O. Fine root production of astringent phenolics. Oecologia 1989, 79, 563–565. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.J.; Lim, J.D.; Yu, C.Y.; Kim, S.H.; Hahn, S.J. Comparison of resveratrol, SOD activity, phenolic compounds and free amino acids in Rehmannia glutinosa under temperature and water stress. Environ. Exp. Bot. 2006, 56, 44–53. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Janas, K.M.; Cvikrová, M.; Palagiewicz, A.; Szafranska, K.; Posmyk, M.M. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002, 163, 369–373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).