The Effect of Acid Rain and Understory Vegetation Removal on the Biological Activity of the Soils of the Cinnamomum camphora (Linn) Presl Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design and Soil Sampling
2.3. Soil Physicochemical Property, CO2 Release, and Enzyme Analysis
2.4. Soil Microbial Community Analysis
2.5. Statistical Analysis
3. Results
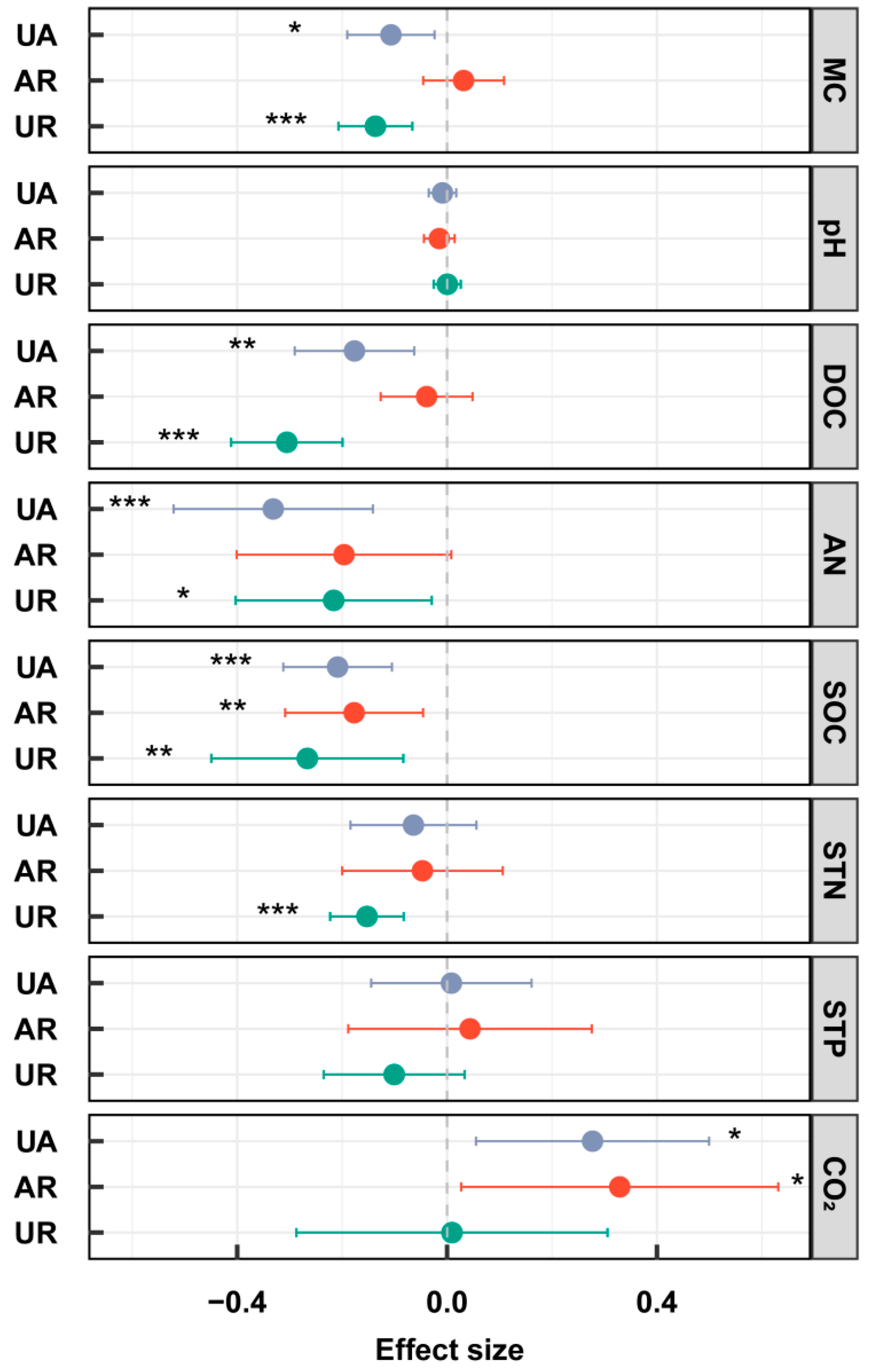
3.1. Soil Physicochemical Properties and CO2 Release
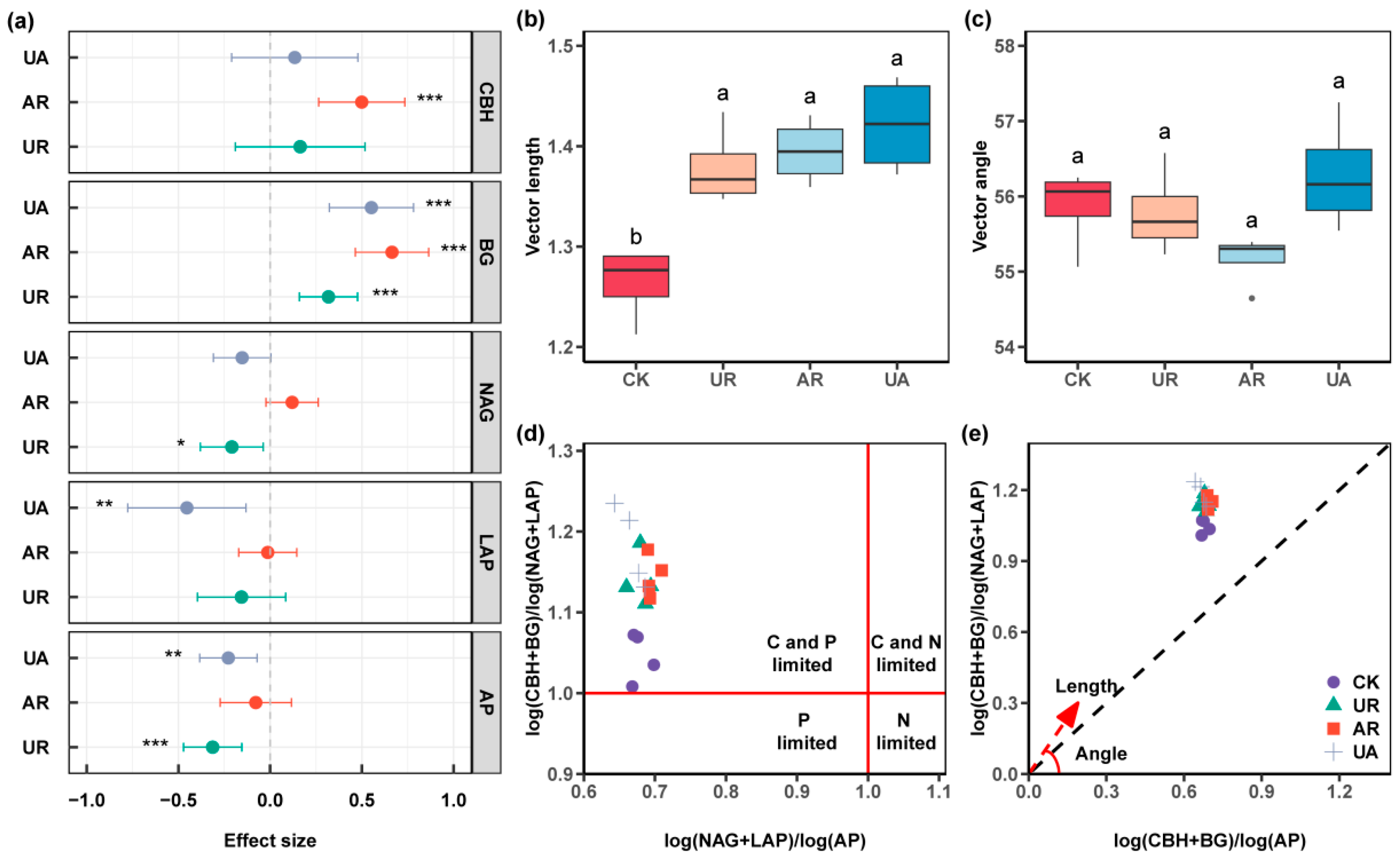
3.2. Soil Enzyme Activities
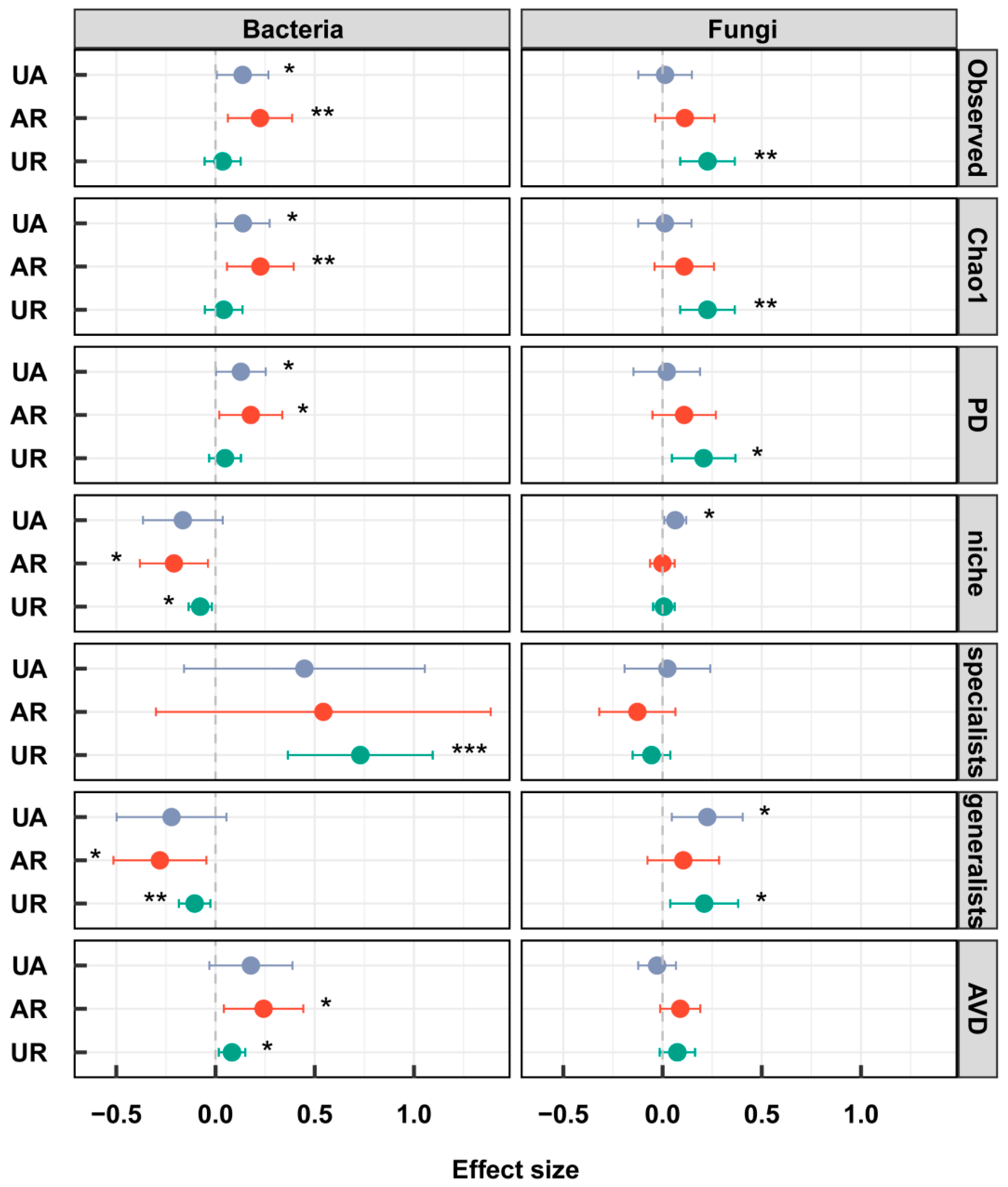
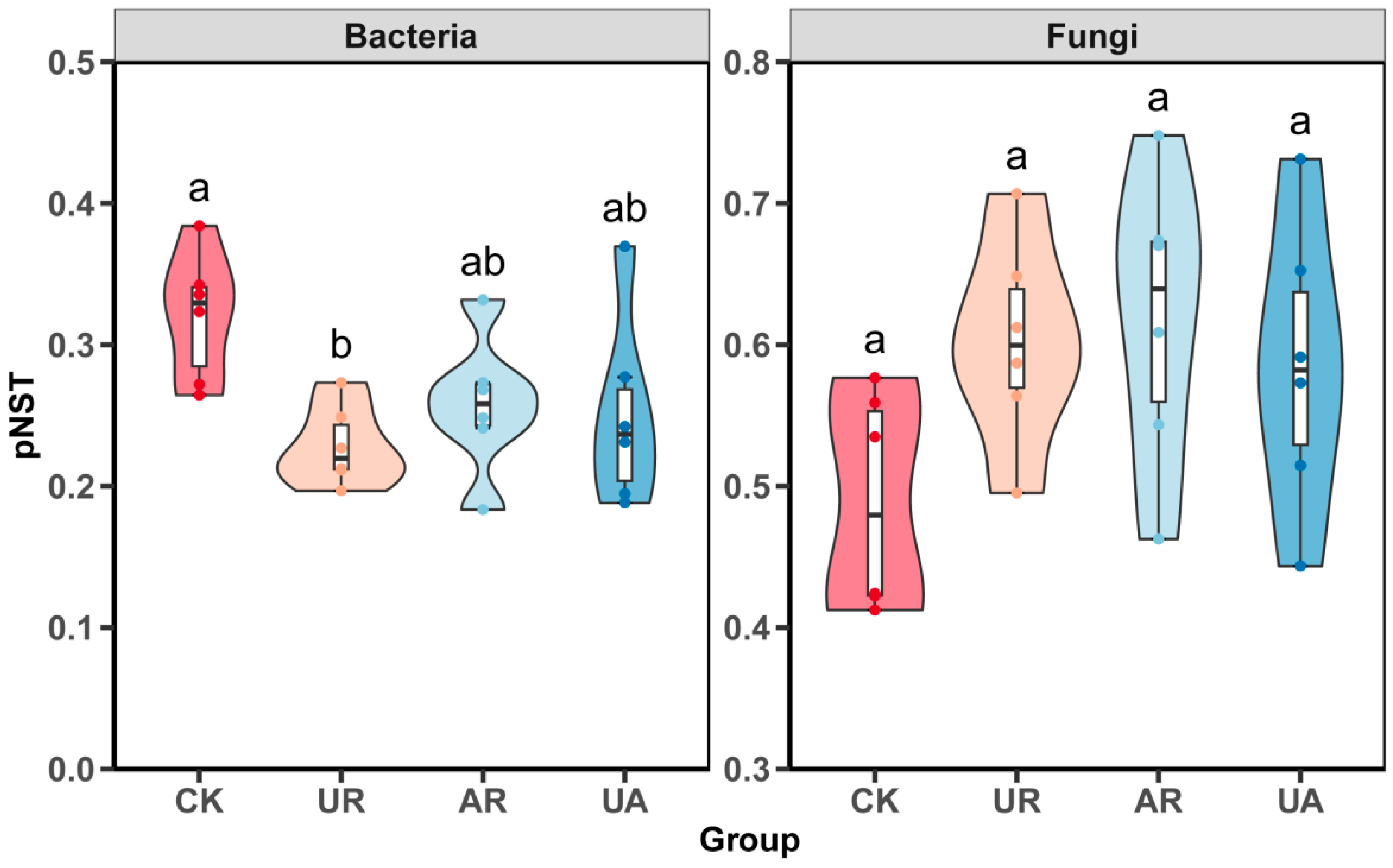
3.3. Soil Microbial Community
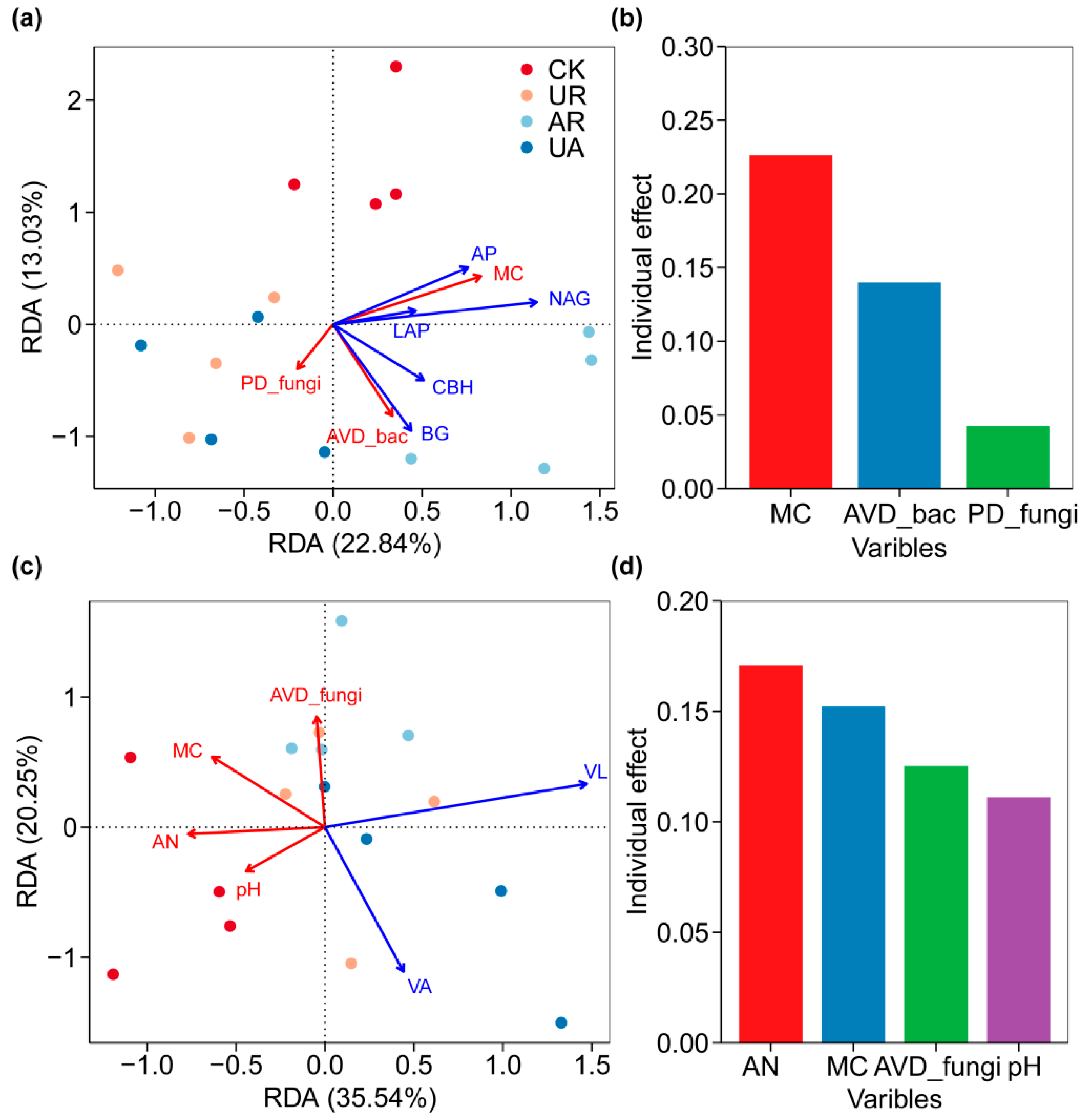
3.4. The Relationship of Soil Physicochemical Properties, Enzyme Activities, and Microbial Community
4. Discussion
4.1. Responses of Soil Physicochemical Properties, CO2 Release, and Enzyme Activities
4.2. Responses of Microbial Community
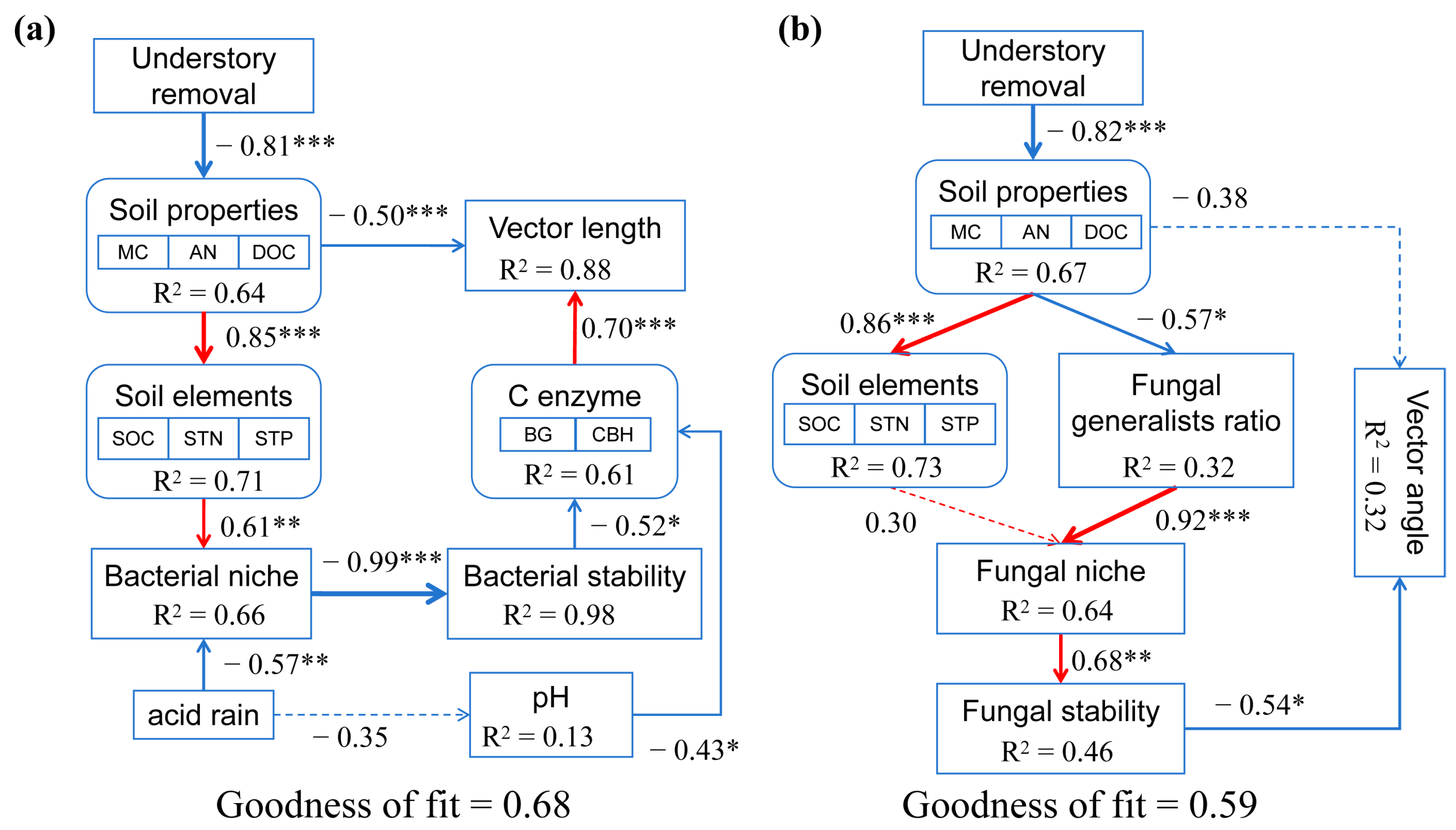
4.3. Relationship Among Measured Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, C.; Tian, D.; Zeng, H.; Li, Z.; Yi, C.; Niu, S. Global soil acidification impacts on belowground processes. Environ. Res. Lett. 2019, 14, 074003. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zheng, Y.; Li, Y. Effects of simulated acid rain on soil enzyme activity and related chemical indexes in woodlands. Forests 2022, 13, 860. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Su, Z.; Liu, Z.; Li, Y.; Wang, J.; Wu, L.; Wei, H.; Zhang, J. Acid rain reduces plant-photosynthesized carbon sequestration and soil microbial network complexity. Sci. Total Environ. 2023, 873, 162030. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, C.; Meng, M.; Zhai, L.; Zhang, B.; Jia, Z.; Gu, Z.; Liu, Q.; Zhang, Y.; Zhang, J. Comparative effects of the recovery from sulfuric and nitric acid rain on the soil enzyme activities and metabolic functions of soil microbial communities. Sci. Total Environ. 2020, 714, 136788. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, L.; Li, C.; Chen, M.; Zhou, Y.; Meng, M.; Li, Z.; Zhang, J.; Liu, X. Effects of short-term simulated acid rain and nitrogen deposition on soil nutrients and enzyme activities in Cunninghamia lanceolata plantation. Front. Ecol. Evol. 2024, 12, 1365954. [Google Scholar] [CrossRef]
- Wang, C.; Guo, P.; Han, G.; Feng, X.; Zhang, P.; Tian, X. Effect of simulated acid rain on the litter decomposition of Quercus acutissima and Pinus massoniana in forest soil microcosms and the relationship with soil enzyme activities. Sci. Total Environ. 2010, 408, 2706–2713. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, C.; Jia, Y.; Wang, W.; Ma, X.; Du, J.; Pu, G.; Tian, X. Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl. Soil Ecol. 2014, 79, 1–9. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Zhang, H.; Wei, H.; Lu, T.; Chen, X.; Li, H.; Yang, J.; Liu, Z. Response and driving factors of soil enzyme activity related to acid rain: A meta-analysis. Environ. Sci. Pollut. Res. 2023, 30, 105072–105083. [Google Scholar] [CrossRef]
- Tang, L.; Lin, Y.; He, X.; Han, G. Acid rain decelerates the decomposition of Cunninghamia lanceolata needle and Cinnamomum camphora leaf litters in a karst region in China. Ecol. Res. 2019, 34, 193–200. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Z.; Wei, H.; Zhang, J. Acid rain reduces soil CO2 emission and promotes soil organic carbon accumulation in association with decreasing the biomass and biological activity of ecosystems: A meta-analysis. Catena 2022, 208, 105714. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Meng, M.; Fu, Z.; Xu, L.; Zha, Y.; Yue, J.; Zhang, S.; Zhang, J. Comparative effects of simulated acid rain of different ratios of SO42− to NO3− on fine root in subtropical plantation of China. Sci. Total Environ. 2018, 618, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Fang, S.; Fang, X.; Jin, Y.; Kuang, Y.; Lin, F.; Liu, J.; Ma, J.; Nie, Y.; Ouyang, S.; et al. Forest understory vegetation study: Current status and future trends. For. Res. 2023, 3, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Tan, J.; Li, W.; Singh, B.P.; Yang, X.; Bolan, N.; Chen, X.; Xu, S.; Bao, Y.; et al. An overview of the direct and indirect effects of acid rain on plants: Relationships among acid rain, soil, microorganisms, and plants. Sci. Total Environ. 2023, 873, 162388. [Google Scholar] [CrossRef]
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; De Frenne, P.; Ma, S.; Proesmans, W.; Blondeel, H.; et al. The functional role of temperate forest understorey vegetation in a changing world. Glob. Change Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef]
- Wagner, R.G.; Little, K.M.; Richardson, B.; Mcnabb, K. The role of vegetation management for enhancing productivity of the world’s forests. Forestry 2006, 79, 57–79. [Google Scholar] [CrossRef]
- Giuggiola, A.; Zweifel, R.; Feichtinger, L.M.; Vollenweider, P.; Bugmann, H.; Haeni, M.; Rigling, A. Competition for water in a xeric forest ecosystem—Effects of understory removal on soil micro-climate, growth and physiology of dominant Scots pine trees. For. Ecol. Manag. 2018, 409, 241–249. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhang, C.; Wang, H.; Fu, X.; Chen, F.; Wan, S.; Sun, X.; Wen, X.; Wang, J. Understory vegetation plays the key role in sustaining soil microbial biomass and extracellular enzyme activities. Biogeosciences 2018, 15, 4481–4494. [Google Scholar] [CrossRef]
- Wang, F.; Zou, B.; Li, H.; Li, Z. The effect of understory removal on microclimate and soil properties in two subtropical lumber plantations. J. For. Res. 2014, 19, 238–243. [Google Scholar] [CrossRef]
- Fang, X.M.; Wang, G.G.; Xu, Z.J.; Zong, Y.Y.; Zhang, X.L.; Li, J.J.; Wang, H.; Chen, F.S. Litter addition and understory removal influenced soil organic carbon quality and mineral nitrogen supply in a subtropical plantation forest. Plant Soil 2021, 460, 527–540. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Shao, Y.; Xu, G.; Fu, S. Effects of vegetation removal on soil properties and decomposer organisms. Soil Biol. Biochem. 2011, 43, 954–960. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Li, D.; Li, S.; Chen, Z.; Wu, J. A meta-analysis of understory plant removal impacts on soil properties in forest ecosystems. Geoderma 2022, 426, 116116. [Google Scholar] [CrossRef]
- Deng, C.; Lyu, M.; Xiong, X.; Peñuelas, J.; Sardans, J.; Li, X.; Lin, W.; Yang, Y.; Xie, J. Understory ferns removal downregulates microbial carbon use efficiency and carbon accrual in previously degraded lands. Agr. For. Meteorol. 2023, 340, 109631. [Google Scholar] [CrossRef]
- Choma, M.; Tahovská, K.; Kaštovská, E.; Bárta, J.; Růžek, M.; Oulehle, F. Bacteria but not fungi respond to soil acidification rapidly and consistently in both a spruce and beech forest. FEMS Microbiol. Ecol. 2020, 96, fiaa174. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, W. Impact of simulated acid rain on the composition of soil microbial communities and soil respiration in typical subtropical forests in Southwest China. Ecotox. Environ. Saf. 2021, 215, 112152. [Google Scholar] [CrossRef]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K.; Brussard, L.; Mark Dangerfield, J.; Wall, D.H.; Wardle, D.A.; Coleman, D.C.; Giller, K.E.; Lavelle, P.; et al. Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: Patterns, mechanisms, and feedbacks. Bioscience 2000, 50, 1049. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef]
- Reischke, S.; Rousk, J.; Bååth, E. The effects of glucose loading rates on bacterial and fungal growth in soil. Soil Biol. Biochem. 2014, 70, 88–95. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Ebblewhite, N.; Dobson, G. Soil microbial community structure: Effects of substrate loading rates. Soil Biol. Biochem. 1998, 31, 145–153. [Google Scholar] [CrossRef]
- Deng, W.; Lu, Y.; Lyu, M.; Deng, C.; Li, X.; Jiang, Y.; Zhu, H.; Yang, Y.; Xie, J. Chemical composition of soil carbon is governed by microbial diversity during understory fern removal in subtropical pine forests. Sci. Total Environ. 2024, 914, 169904. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Xuan, C.; Xiaoran, S.; Zhaoji, S.; Jiaen, Z.; Zhong, Q.; Huimin, X.; Hui, W. Analysis of the spatio-temporal changes in acid rain and their causes in China (1998–2018). J. Resour. Ecol. 2021, 12, 593–599. [Google Scholar] [CrossRef]
- He, X.; Lin, Y.; Han, G.; Ma, T. Litterfall interception by understorey vegetation delayed litter decomposition in Cinnamomum camphora plantation forest. Plant Soil 2013, 372, 207–219. [Google Scholar] [CrossRef]
- He, X.; Han, G.; Lin, Y.; Tian, X.; Xiang, C.; Tian, Q.; Wang, F.; He, Z. Diversity and decomposition potential of endophytes in leaves of a Cinnamomum camphora plantation in China. Ecol. Res. 2011, 27, 273–284. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Du, J.; Zhang, Y.; Guo, W.; Li, N.; Gao, C.; Cui, M.; Lin, Z.; Wei, M.; Zhang, H. Chronic impacts of TiO2 nanoparticles on Populus nigra L. leaf decomposition in freshwater ecosystem. J. Hazard. Mater. 2018, 350, 121–127. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2020, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Salazar, G. EcolUtils: Utilities for Community Ecology Analysis. R Package Version 0.1. 2015. Available online: https://github.com/GuillemSalazar/EcolUtils (accessed on 10 May 2023).
- Wu, W.; Logares, R.; Huang, B.; Hsieh, C. Abundant and rare picoeukaryotic sub-communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environ. Microbiol. 2017, 19, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Ning, D.; Wang, Y.; Fan, Y.; Wang, J.; Van Nostrand, J.D.; Wu, L.; Zhang, P.; Curtis, D.J.; Tian, R.; Lui, L.; et al. Environmental stress mediates groundwater microbial community assembly. Nat. Microbiol. 2024, 9, 490–501. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector Analysis of Ecoenzyme Activities Reveal Constraints on Coupled C, N and P Dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Henseler, J.; Sarstedt, M. Goodness-of-fit indices for partial least squares path modeling. Comput. Stat. 2012, 28, 565–580. [Google Scholar] [CrossRef]
- Wetzels, M.; Odekerken-Schröder, G.; Van Oppen, C. Using PLS path modeling for assessing hierarchical construct models: Guidelines and empirical illustration. Mis Quart. 2009, 33, 177–195. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Zhang, J.; Saleem, M.; He, Y.; Zhong, J.; Ma, R. Higher sensitivity of soil microbial network than community structure under acid rain. Microorganisms 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, H.; Zhang, J.; Saleem, M.; He, Y.; Zhong, J.; Ma, R. Seasonality regulates the effects of acid rain on microbial community in a subtropical agricultural soil of Southern China. Ecotox. Environ. Saf. 2021, 224, 112681. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Chen, D.; Hall, S.J.; Pan, S.; Yan, X.; Bai, T.; Guo, H.; Zhang, Y.; Bai, Y.; Hu, S. Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shan, X.; Wei, H.; Zhang, J.; Saleem, M.; Li, D.; Zhang, Y.; Ma, R.; He, Y.; Zhong, J.; et al. Idiosyncratic responses of microbial communities and carbon utilization to acid rain frequency in the agricultural and forest soils. Glob. Ecol. Conserv. 2021, 26, e01429. [Google Scholar] [CrossRef]
- Lyu, M.; Xie, J.; Giardina, C.P.; Vadeboncoeur, M.A.; Feng, X.; Wang, M.; Ukonmaanaho, L.; Lin, T.; Kuzyakov, Y.; Yang, Y. Understory ferns alter soil carbon chemistry and increase carbon storage during reforestation with native pine on previously degraded sites. Soil Biol. Biochem. 2019, 132, 80–92. [Google Scholar] [CrossRef]
- Jiang, M.; Lin, T.; Shaner, P.L.; Lyu, M.; Xu, C.; Xie, J.; Lin, C.; Yang, Z.; Yang, Y. Understory interception contributed to the convergence of surface runoff between a Chinese fir plantation and a secondary broadleaf forest. J. Hydrol. 2019, 574, 862–871. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Shang, H.; Wang, J.; Zhang, P. Impact of simulated acid rain on soil microbial community function in Masson pine seedlings. Electron. J. Biotechnol. 2014, 17, 199–203. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Zhang, J.; Saleem, M.; Zhang, Y.; Ma, R.; He, Y.; Yang, J.; Xiang, H.; Wei, H. Effect of simulated acid rain on soil CO2, CH4 and N2O emissions and microbial communities in an agricultural soil. Geoderma 2020, 366, 114222. [Google Scholar] [CrossRef]
- Wu, Y.T.; Gutknecht, J.; Nadrowski, K.; Geißler, C.; Kühn, P.; Scholten, T.; Both, S.; Erfmeier, A.; Böhnke, M.; Bruelheide, H.; et al. Relationships between soil microorganisms, plant communities, and soil characteristics in Chinese subtropical forests. Ecosystems 2012, 15, 624–636. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Datta, R.; Chen, J.; Du, Y.; Du, D. Key factors shaping prokaryotic communities in subtropical forest soils. Appl. Soil Ecol. 2022, 169, 104162. [Google Scholar] [CrossRef]
- Cline, L.C.; Hobbie, S.E.; Madritch, M.D.; Buyarski, C.R.; Tilman, D.; Cavender-Bares, J.M. Resource availability underlies the plant-fungal diversity relationship in a grassland ecosystem. Ecology 2017, 99, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Xue, Y.; Cui, Y.; Moorhead, D.L.; Lambers, H.; Wang, D. Nutrient limitation mediates soil microbial community structure and stability in forest restoration. Sci. Total Environ. 2024, 935, 173266. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhang, X.; Chen, W.; Liang, Z.; Wang, K.; Zhang, Y.; Song, Y. Differential responses of bacterial and fungal community structure in soil to nitrogen deposition in two planted forests in southwest China in relation to pH. Forests 2024, 15, 1112. [Google Scholar] [CrossRef]
- Li, D.; Wu, J. Canopy nitrogen addition and understory removal destabilize the microbial community in a subtropical Chinese fir plantation. J. Environ. Manag. 2024, 354, 120407. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Shen, C.; Zhou, G.; Delgado-Baquerizo, M.; Ge, Y. Microbial diversity losses constrain the capacity of soils to mitigate climate change. Glob. Change Biol. 2024, 30, e17601. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. T. R. Soc. B. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Xi, D.; Jin, S.; Wu, J. Soil bacterial community is more sensitive than fungal community to canopy nitrogen deposition and understory removal in a Chinese fir plantation. Front. Microbiol. 2022, 13, 1015936. [Google Scholar] [CrossRef]
- Cline, L.C.; Zak, D.R. Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 2015, 96, 3374–3385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Liu, Y.; Lin, Y.; Kong, X.; Lin, H.; He, X. The Effect of Acid Rain and Understory Vegetation Removal on the Biological Activity of the Soils of the Cinnamomum camphora (Linn) Presl Plantation. Forests 2025, 16, 525. https://doi.org/10.3390/f16030525
He Z, Liu Y, Lin Y, Kong X, Lin H, He X. The Effect of Acid Rain and Understory Vegetation Removal on the Biological Activity of the Soils of the Cinnamomum camphora (Linn) Presl Plantation. Forests. 2025; 16(3):525. https://doi.org/10.3390/f16030525
Chicago/Turabian StyleHe, Zaihua, Yini Liu, Yonghui Lin, Xiangshi Kong, Hong Lin, and Xingbing He. 2025. "The Effect of Acid Rain and Understory Vegetation Removal on the Biological Activity of the Soils of the Cinnamomum camphora (Linn) Presl Plantation" Forests 16, no. 3: 525. https://doi.org/10.3390/f16030525
APA StyleHe, Z., Liu, Y., Lin, Y., Kong, X., Lin, H., & He, X. (2025). The Effect of Acid Rain and Understory Vegetation Removal on the Biological Activity of the Soils of the Cinnamomum camphora (Linn) Presl Plantation. Forests, 16(3), 525. https://doi.org/10.3390/f16030525






