Abstract
Nitrogen deposition can significantly impact soil biogeochemical cycling; however, its effects on the decomposition processes and nutrient release from leaf and twig litter in subtropical plantations remain inadequately understood. In this study, we focused on the Pinus yunnanensis Franch. forest in the central Yunnan Plateau, southwestern China, and explored how nitrogen addition influences litter decomposition nutrient release over two years, under four levels: control (CK, 0 g·m−2·a−1), low nitrogen (LN, 10 g·m−2·a−1), medium nitrogen (MN, 20 g·m−2·a−1), and high nitrogen (HN, 25 g·m−2·a−1). The results indicate that after 24 nitrogen application treatments, the rates of remaining mass in both leaf and twig litters followed the pattern: LN < CK = MN < HN. Under all nitrogen application treatments, the rate of remaining mass in leaf litters was significantly lower than that of twig litters (p < 0.05). Under LN, the mass retention in leaf and twig litters decreased by 3.96% and 8.41%, respectively, compared to CK. In contrast, under HN treatments, the rates of remaining mass in leaf and twig litters increased by 8.57% and 5.35%, respectively. This demonstrates that low nitrogen accelerates decomposition, whereas high nitrogen inhibits it. Significant differences in the remaining amounts of lignin and cellulose in both leaf and twig litters were observed when compared to CK (p < 0.05). Additionally, decomposition time and nitrogen deposition had significant effects on the remaining rates of nutrients (C, N, P) and their C/N, C/P, and N/P in litters (p < 0.05). Following nitrogen application, the C/N of the litters significantly reduced, while the N/P increased. The results suggest that nitrogen addition alleviates the nitrogen limitation on the litters while intensifying the phosphorus limitation.
1. Introduction
Nitrogen has significant application value in both nature and human activities, serving as a key component for biosynthesis, an industrial raw material, and a protective gas. However, since the mid-20th century, increasing nitrogen demand in industry, agriculture, and livestock, combined with low nitrogen utilization and frequent disruptions from human activities, has significantly altered the global nitrogen cycle [1]. Over the past century, global nitrogen deposition has tripled due to both dry and wet deposition [2]. In 2019, the global nitrogen deposition flux reached 103 Tg N/a, which is expected to surpass the global nitrogen flux load by 2050 [3]. As the largest developing country, China’s nitrogen deposition has greatly exceeded the global average, making it a hotspot for nitrogen deposition [4]. In the future, under short-term or even long-term conditions, high nitrogen deposition may delay litter decomposition, thereby altering ecosystem balance and affecting nutrient turnover and cycling processes.
Numerous studies have been conducted on nitrogen deposition, focusing on ecosystems such as watersheds, grasslands, farmlands, and forests, in conjunction with factors such as phosphorus addition [5,6], climate [7], light intensity and temperature [8], precipitation [9,10], and ultraviolet radiation [11,12] to explore impacts on plant and animal diversity [13,14], physiological and ecological adaptation strategies [15], nutrient release from the litter [16], substrate stoichiometric characteristics and yield [4], soil microbial and enzyme activities [17], and N2O emission [18]. Among these, forest ecosystems, which are the primary recipients of nitrogen deposition, are particularly affected [19]. Litter decomposition, an important process in forest ecosystems, is influenced by nitrogen deposition. Some studies have suggested that nitrogen deposition indirectly affects litter decomposition by altering forest ground cover composition and chemical composition of the litter [20], although the results remain uncertain. Wang et al. [21] conducted a study in Daxing’anling with four nitrogen addition treatments (0, 25, 50, and 75 kg·N·ha−1·a−1) on coniferous and mixed litters. The study suggests that nutrient limitation in litter is crucial in regulating its decomposition, with nitrogen deposition contributing to decomposition. Trentini et al. [22] observed in subtropical torch pine forests in Argentina that nitrogen addition accelerated litter decomposition. In contrast, Zhou et al. [23] reported that simulated nitrogen deposition inhibited leaf litter decomposition in a natural evergreen broadleaf forest in western China, with the inhibitory effect increasing with higher nitrogen deposition levels. Meanwhile, Gill et al. [24] conducted a global assessment and found that nitrogen application initially accelerated early-stage decomposition but slowed down the process. Overall, studies on nitrogen deposition’s effects on litter decomposition have not reached a consensus. Liu et al. [14] found that nitrogen addition in the understory did not significantly affect litter decomposition in temperate and subtropical forests in China. Lo Cascio [25] studied the long-term impacts of nitrogen addition on litter decomposition, soil properties, and microbial activity in Mediterranean shrublands. The results indicated no consistent pattern in litter decomposition, as nitrogen deposition did not uniformly affect the process.
Litter decomposition is essential in forest ecosystems, as it regulates nutrient cycling, soil health, and primary productivity. The rate and process of litter breakdown directly influence how plants and microorganisms absorb and utilize nutrients. Therefore, understanding the mechanisms of nutrient release during litter decomposition under nitrogen addition is crucial. Luo et al. [26] conducted a meta-analysis of 3434 paired observations and found that phosphorus release slowed under nitrogen enrichment, with a stronger response over time. Nitrogen application also inhibited lignin decomposition, and the inhibitory effect increased with higher nitrogen application and longer test durations. Zhang et al. [27] observed a 15%–40% reduction in decomposition rates in a tropical forest in southern China during a nutrient fertilization trial, varying the magnitude by species. Species with higher C/P and cellulose contents exhibited lower litter decomposition rates. Zhuang et al. [28] found that increasing nitrogen deposition might slow nutrient release from decomposing litter in a subtropical forest in southwestern China, where nitrogen deposition is significant. Previous studies have established that C, N, P, lignin, and cellulose are key variables influencing the quality of the litter. Investigating the decomposition process of lignin and cellulose in forest ecosystems under nitrogen deposition and examining changes in litter substrate quality (C, N, P) are current research priorities. However, few studies have explored the response of decomposition characteristics of leaf and twig litter to nitrogen deposition in forest ecosystems in the subtropical Dianzhong high-elevation region of southern China.
Schwede et al. [29] used a model to calculate global nitrogen deposition and found that the highest total nitrogen deposition occurred in southern and eastern China, Japan, the eastern United States, and Europe. The Yunnan-Asia alpine region, located in southern China and encompassing the Yunnan-Guizhou Plateau, the Qinghai-Tibetan Plateau, and the Hengduan Mountains, has unique natural geographic environments. Forest ecosystems in this region are crucial for soil and water conservation. Pinus yunnanensis Franch. forests, the most widely distributed forest type in southwest China, are the dominant afforestation species in the central Yunnan plateau region. The distribution area of the Pinus yunnanensis forest in Yunnan covers about 5 million hm2, accounting for 52% of the province’s forested area. Pinus yunnanensis are known for their rapid growth, high-quality timber, drought tolerance, and strong carbon sequestration capacity. The decomposition of its litter also holds significant scientific research value. Therefore, understanding how nitrogen deposition affects litter and its nutrient release is important for predicting the consequences of increased nitrogen levels on forest ecosystems. This study investigates the decomposition and nutrient release characteristics of leaf and twig litter of Pinus yunnanensis forests by simulating nitrogen deposition and conducting in situ decomposition tests using the litter decomposition bag method. We speculated that: (1) Nitrogen deposition affects the rate of remaining mass and decomposition in litters, with significant differences observed under different nitrogen additions; (2) Nitrogen deposition influences the release of nutrients from litters and alters their C, N, and P stoichiometric ratios, reflecting the process of litter decomposition; (3) Lignin and cellulose, which are more resistant to decomposition, play a significant role in litter decomposition, and their interaction corresponds to the inhibitory effects of nitrogen deposition on litter decomposition. This study aims to establish a theoretical foundation for understanding how nitrogen deposition influences litter decomposition. Thereby, it offers valuable references for the sustainable management and development of Pinus yunnanensis forests in the Central Yunnan Plateau.
2. Materials and Methods
2.1. Study Area Overview
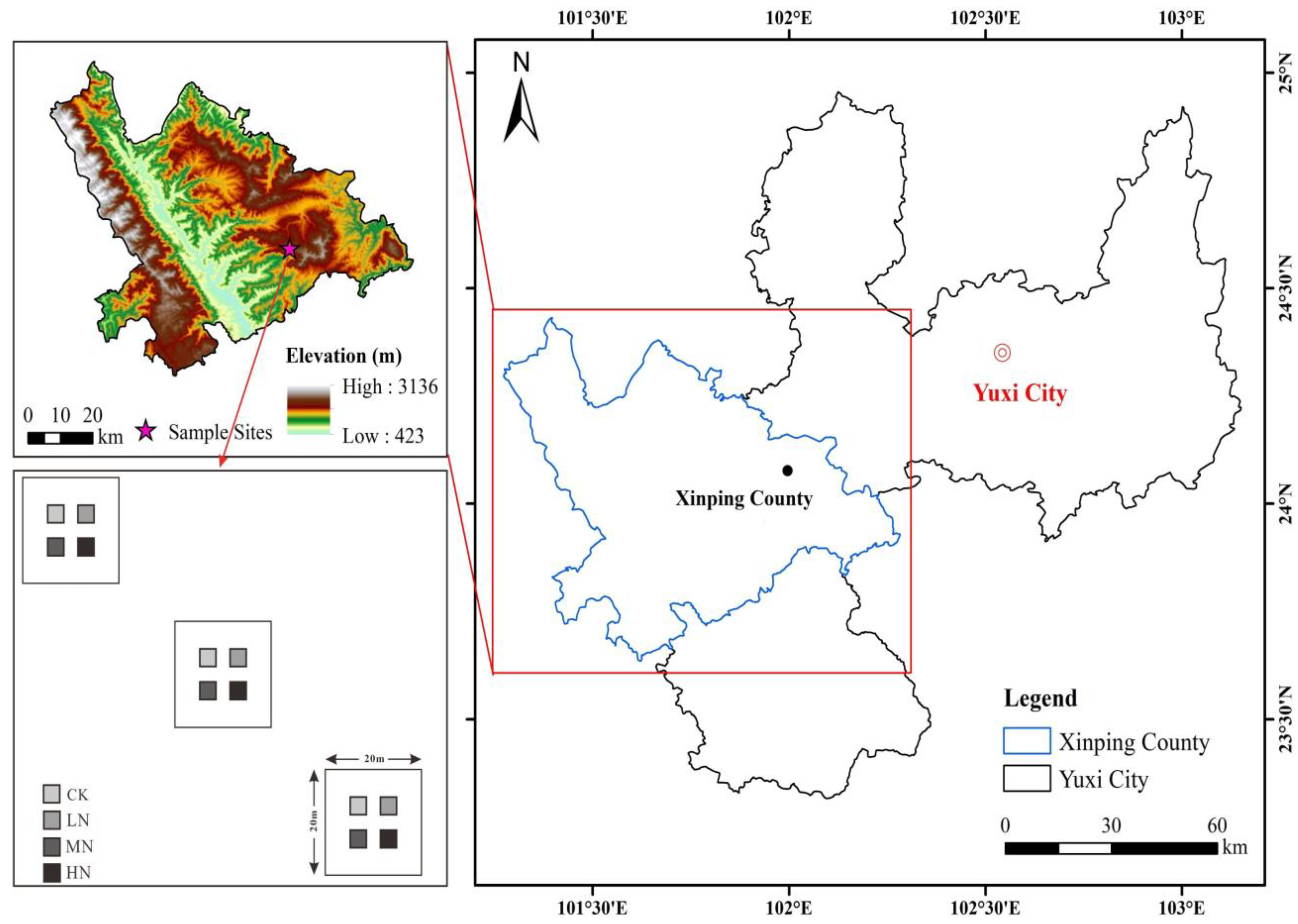
The research was conducted at the National Positioning Observatory of Forest Ecosystems, located in Yuxi City, Yunnan Province (23°46′18″–23°54′34″ N, 101°16′06″–101°16′12″ E). This area is situated at the boundary between the northern and southern subtropical climatic zones of Yunnan, featuring a diverse topography that results in a three-dimensional climate with distinct mountain-related weather patterns. Study area and sample plot locations are shown in Figure 1.
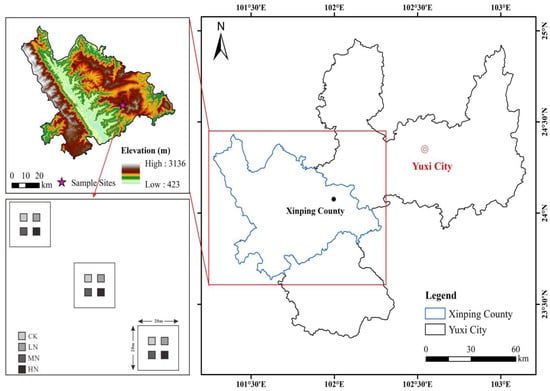
Figure 1.
Study area and sample plot locations.
The altitude in the study area ranges from 1260.0 m to 2614.4 m, with an average annual temperature of 14–16 °C and yearly rainfall between 1000 and 1100 mm. The region experiences clear dry and wet seasons. Soil types include mountain red soil and basalt red soil, with yellow-brown soil found at higher altitudes. This area boasts abundant forest resources, with a forest cover of 85.9%, and primary and secondary evergreen broad-leaved forests cover 64.3% of the region. Vegetation exhibits clear vertical distribution patterns as altitude increases. A total of 324 species from 137 genera in 98 families are present, including Pinus yunnanensis forests, Phoebe chinensis, and Arthoperis palisotii [30]. The information of the Pinus yunnanensis forest sample plots in this study is summarized in Table 1.

Table 1.
Information on Pinus yunnanensis Franch. forest plots.
2.2. Methodology
2.2.1. Plot Selection and Experimental Design
After field surveys, three 20 m × 20 m sample plots were established in the Pinus yunnanensis forest on Mopan Mountain, Central Yunnan. A buffer zone of at least 3 m was maintained between plots. Additionally, four smaller 3 m × 3 m subplots were randomly selected within each of the three sample plots, with a spacing of more than 10 m between each. Fresh, undecomposed leaf and twig litters were collected directly from the Pinus yunnanensis forest and returned to the laboratory. The samples were thoroughly mixed and naturally air-dried. Once air-drying was completed, 10 g portions of the dried leaf and twig litter were accurately weighed. These samples were placed in nylon decomposition bags (20 cm × 20 cm, with a 1 mm × 1 mm mesh aperture on both the upper and lower surfaces). To prevent interference between adjacent decomposition bags, a minimum spacing of 2 cm was maintained between them.
In October 2018, the litters from the soil surface of the small sample squares were first removed. The pre-treated litter bags were then evenly distributed in the sample squares, and 72 bags of leaf and twig litter were placed in each treatment sample square, resulting in a total of 288 bags across the different treatments. Subsequently, nitrogen was applied by spraying a CO(NH2)2 solution (simulating nitrogen deposition) onto the litter in each sample square. A review of relevant data indicated that the total nitrogen deposition in China was 19.6 ± 2.5 Tg N/a, with the wet-to-dry deposition ratio approximately equal to 1 [31]. Combining this with nitrogen deposition levels in southwest China (3.84 g·m−2·a−1) [32] and considering potential future changes in nitrogen deposition in the region, as well as experimental designs from the Qianzhong Karst region [33] and evergreen broad-leaved forests in the rain screen area of west China [23], a nitrogen deposition treatment scheme was developed. It was previously reported that evergreen broadleaf forests are approaching nitrogen saturation [34], suggesting that higher nitrogen treatments could accelerate litter responses.
Based on the above considerations, four nitrogen deposition levels were established: control (CK, 0 g·m−2·a−1), low nitrogen (LN, 10 g·m−2·a−1), medium nitrogen (MN, 20 g·m−2·a−1), and high nitrogen (HN, 25 g·m−2·a−1), each with three replicates. The experiment ran from January to December 2019 (the first year) and from June 2020 to May 2021 (the second year, modified due to the COVID-19 pandemic). During these periods, the decomposition bags containing leaf and twig litter in each sample plot were treated monthly with the respective nitrogen levels. The CO(NH2)2 required for each nitrogen concentration was dissolved into 750 mL of deionized water (equivalent to 1.0 mm of new precipitation for the year). The solution was then sprayed uniformly from a height of 50 cm above the sample area using a sprayer. Specifically, the concentrations of CO(NH2)2 solution under various treatments were CK (0 g/L), LN (8.04 g/L), MN (16.08 g/L), and HN (20.09 g/L). The control group received an equal amount of deionized water, while all other experimental conditions were kept constant except for the varying nitrogen application levels. This process was repeated monthly until May 2021, resulting in 24 nitrogen applications. Therefore, after 24 nitrogen application treatments (for two years), N amounts of four nitrogen deposition levels were: CK (0 g), LN (2400 g), MN (4800 g), and HN (6000 g).
2.2.2. Sampling and Index Analysis
Over the course of two years of nitrogen treatment, leaf and twig litter samples were collected monthly from each treatment area within the Pinus yunnanensis forest (n = 9). Three bags of leaf and twig litter were collected from each treatment per month, resulting in 24 sampling events. The decomposition bags were sealed in self-sealing bags, labeled, and transported to the laboratory for further processing and analysis.
In the laboratory, the invasive roots, sediment, and other impurities were removed from the samples. The bags were then dried in envelopes at 65 °C until they reached a constant weight. This weight was used to calculate the mass remaining rate of the litter. Next, the dried samples were crushed, ground, and sieved (using a 0.25 mm pore size) for nutrient content analysis. The carbon content was determined utilizing the potassium dichromate-external sulfuric acid oxidation method; nitrogen content was measured using the semi-micro Kjeldahl method; phosphorus content was analyzed using the sulfuric acid-hydrogen peroxide decoction method followed by molybdenum antimony colorimetry [35]; and lignin and cellulose content were determined using the acidic wash fiber method. The carbon, nitrogen, and phosphorus contents were determined using the German-made analytik jena Multi N/C 3100 (Jena, Germany), the European-made FOSS Kjiltec 8400 from Hillerød, Denmark, and the Australian-made Agilent ICP-710 (Forest Hill, VIC, Australia).
2.3. Parameter Analysis
The remaining mass rates, as well as the lignin and cellulose contents in the litter, were measured by the following equations [32]:
where: Mt denotes the weight of the dried sample at time t of the litter (g); Mo indicates the weight of the starting air-dried sample of the litter (10.0 g); Ct denotes the lignin (or cellulose) content at time t (mg/g); Co indicates the initial lignin (cellulose) content (mg/g).
To determine the mass remaining rate (y, %) of the litter, an Olson negative exponential decay model was applied [33]:
where α denotes the fitting parameter, k denotes the coefficient of litter decomposition (kg·kg−1a−1), and t indicates the time (a).
The time required for 50% (T50%) and 95% (T95%) decomposition of the litter was calculated using the following equation [36]:
2.4. Data Analysis
Statistical analysis of the data was performed utilizing the Excel 2010 and SPSS 22.0 software. One-way ANOVA and the Least Significant Difference (LSD) method were applied to assess the significance of differences in the rates of remaining mass and substrate mass across different nitrogen application treatments, with an α value of 0.05. Data were plotted using Excel 2010 and Origin 2021. The initial chemical characteristics of the leaf and twig litter in the Pinus yunnanensis forest are shown in Table 2.

Table 2.
Initial mass percentages of chemical elements in leaf and twig litter.
3. Results
3.1. Effect of Nitrogen Deposition on the Decomposition Rate of Litter in Pinus yunnanensis Forests
3.1.1. Effect of Nitrogen Deposition on the Rate of Remaining Mass in Litters
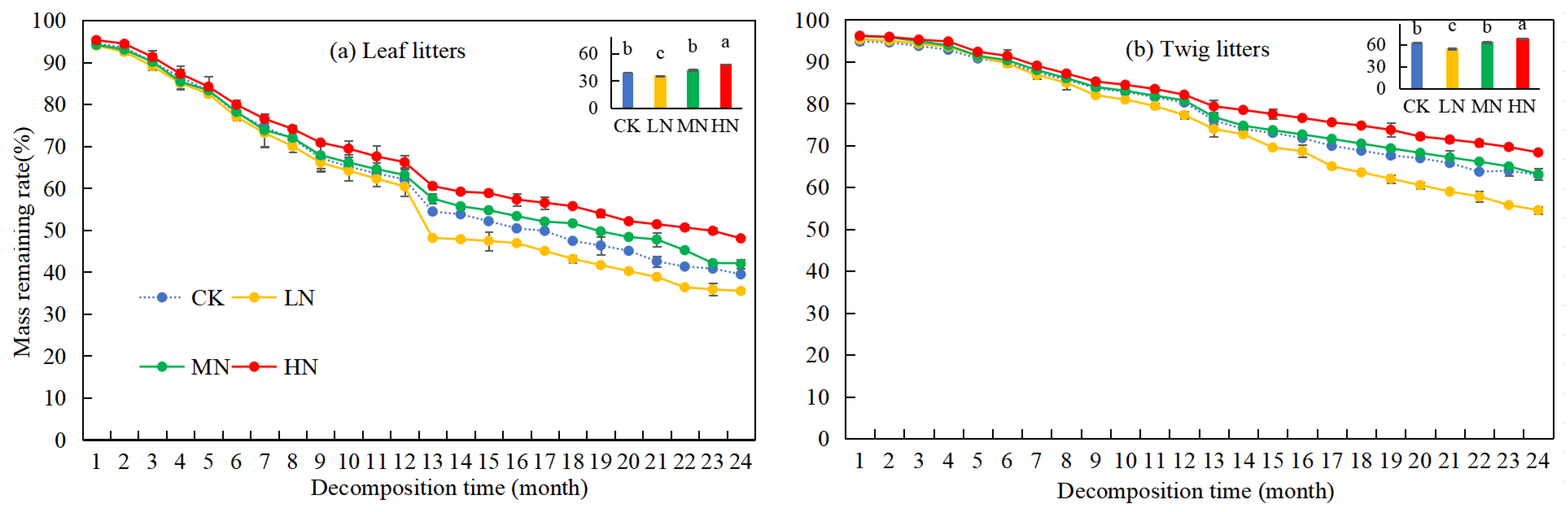
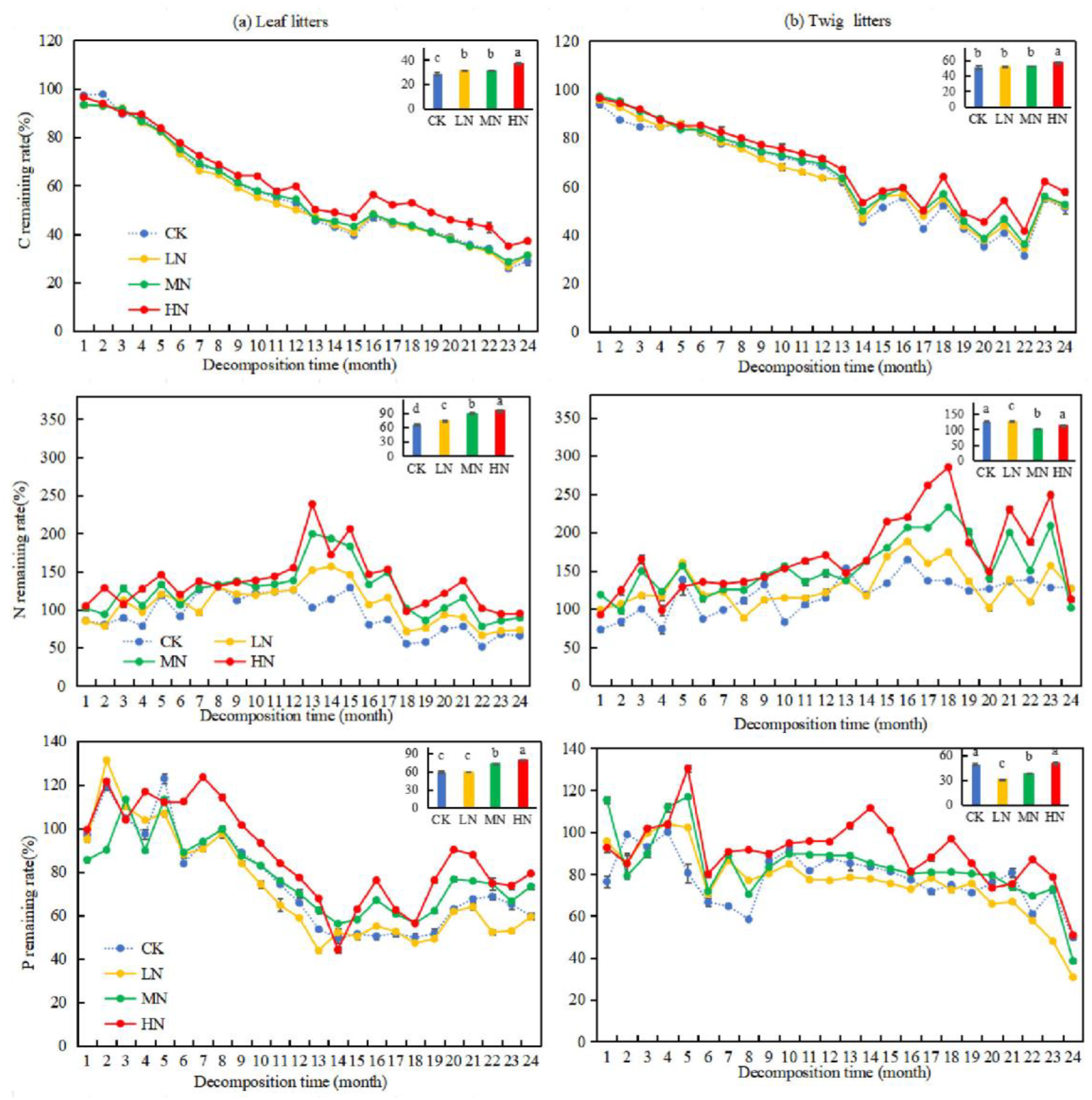
Over time, the rates of remaining mass from leaf and twig litters in Pinus yunnanensis forests showed a general decreasing trend (Figure 2). After two years of decomposition, the rates of remaining mass in leaf and twig litters were 39.43% and 62.93%, respectively. Under different nitrogen deposition treatments, the order of remaining mass rate was LN < CK = MN < HN for both leaf and twig litters. By the 24th month, the rates of remaining mass in leaf and twig litters under LN treatment were lower than those of the CK group, with reductions of 3.96% and 8.41%, respectively. Conversely, under HN treatment, the rates of remaining mass in leaf and twig litters were higher than in the CK group, with increases of 8.57% and 5.35%, respectively. Under MN treatment, the rates of remaining mass in litters did not show a significant difference compared to the CK group. These results suggest that LN treatment accelerated the decomposition of litter in Pinus yunnanensis forests, while HN treatments inhibited it. Additionally, the rate of remaining mass in leaf litters was significantly lower than that of twig litters (p < 0.05), indicating a faster decomposition rate for leaf litters. From the 13th month onward, the rates of remaining mass in both leaf and twig litters decreased more significantly, likely due to the sampling time.
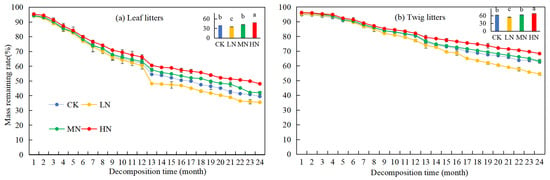
Figure 2.
Changes in mass remaining rate during the decomposition of leaf and twig litter under N deposition in the Pinus yunnanensis forest. Different histogram lowercase letters represent statistically significant differences between various treatments at the end of the experiment on the basis of three-way ANOVA (p < 0.05).
3.1.2. Fitting Model of the Decomposition Rate of Litters in Pinus yunnanensis Forest
Under different nitrogen deposition treatments, the mass remaining rate (y) of leaf and twig litter in the Pinus yunnanensis forest exhibited a significant negative exponential relationship with decomposition time (t), and the coefficients of determination (R2) for the fitted models were all greater than 0.900, as shown in Table 3. Among the treatments, the best-fitting results were obtained for leaf and twig litter under LN and MN treatments, respectively. The decomposition coefficients of leaf and twig litter were largest under LN treatment (0.533 and 0.251), with the shortest times required for 50% (1.30 and 2.76 years) and 95% (5.62 and 11.94 years) decomposition. In contrast, the decomposition coefficients of litters were smallest under HN treatment (0.443 and 0.194), with the longest times required for 50% (1.56 and 3.57 years) and 95% (6.76 and 15.44 years) decomposition. These results indicate that litters decomposed the fastest under LN treatment and the slowest under HN treatment.

Table 3.
Exponential regression equation of the remaining rate of leaf and twig litter quality with decomposition time under different N deposition treatments.
3.2. Impact of Nitrogen Deposition on the Remaining Lignin and Cellulose Rates in Pinus yunnanensis Forest
3.2.1. Effect of Nitrogen Deposition on the Lignin Remaining Rate of Litters in Pinus yunnanensis Forest
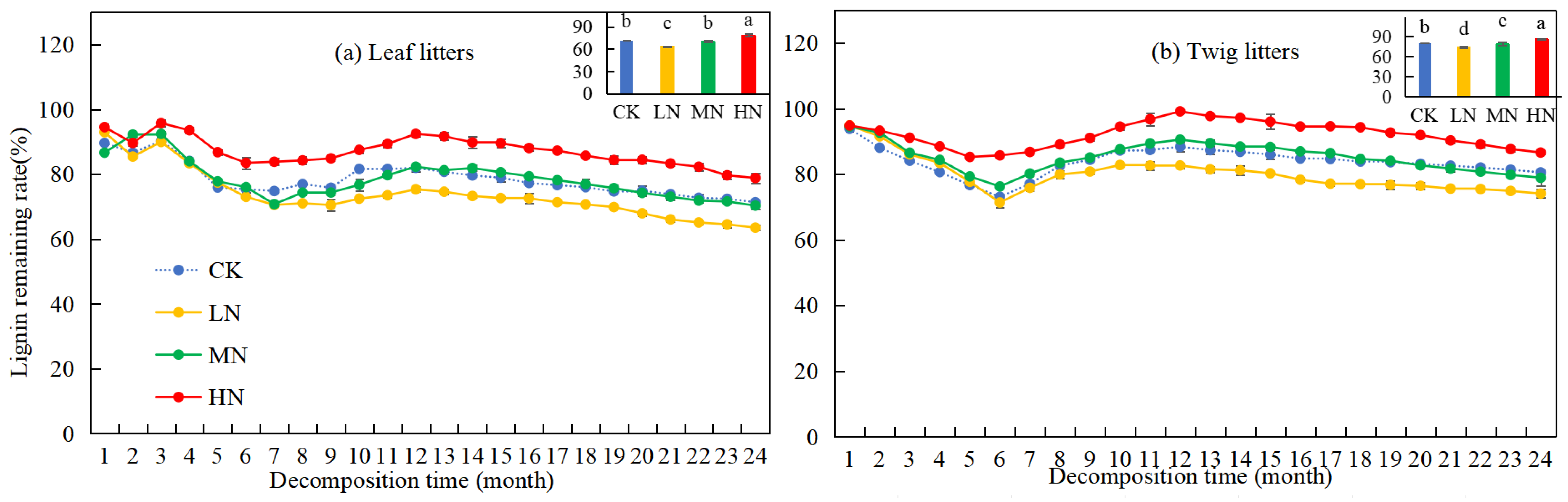
The lignin remaining rate of leaf and twig litter in the Pinus yunnanensis forest exhibited an initial decline, followed by an increase and a slight decrease toward the end of the study (Figure 3). After two years of decomposition, the lignin remaining rates of leaf and twig litter were 71.36% and 80.52%, respectively. Specifically, MN treatment showed no significant influence on the lignin remaining rate of leaf litters. However, under HN treatment, the lignin remaining rate of both leaf and twig litter was significantly higher (7.47% and 6.04%) compared to CK, while under LN treatment, the lignin remaining rate was significantly lower (7.86% and 6.46%) than CK. Under different nitrogen application levels, the lignin remaining rates of leaf and twig litter were observed to follow the pattern: LN < CK = MN < HN and LN < CK < MN < HN, respectively. The results indicate that HN treatment inhibited the decomposition of lignin, while LN treatment promoted lignin decomposition in leaf and twig litter.
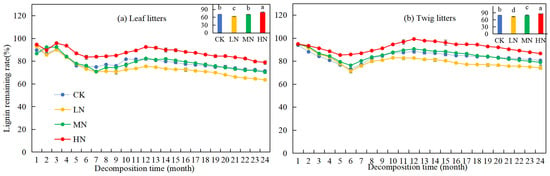
Figure 3.
Variation in lignin remaining rates during leaf and twig litter decomposition under N deposition in Pinus yunnanensis forests. Different histogram lowercase letters represent statistically significant differences between various treatments at the end of the experiment on the basis of three-way ANOVA (p < 0.05).
3.2.2. Effects of Nitrogen Deposition on Cellulose Remaining Rate of Leaf and Twig Litter of Pinus yunnanensis Forests
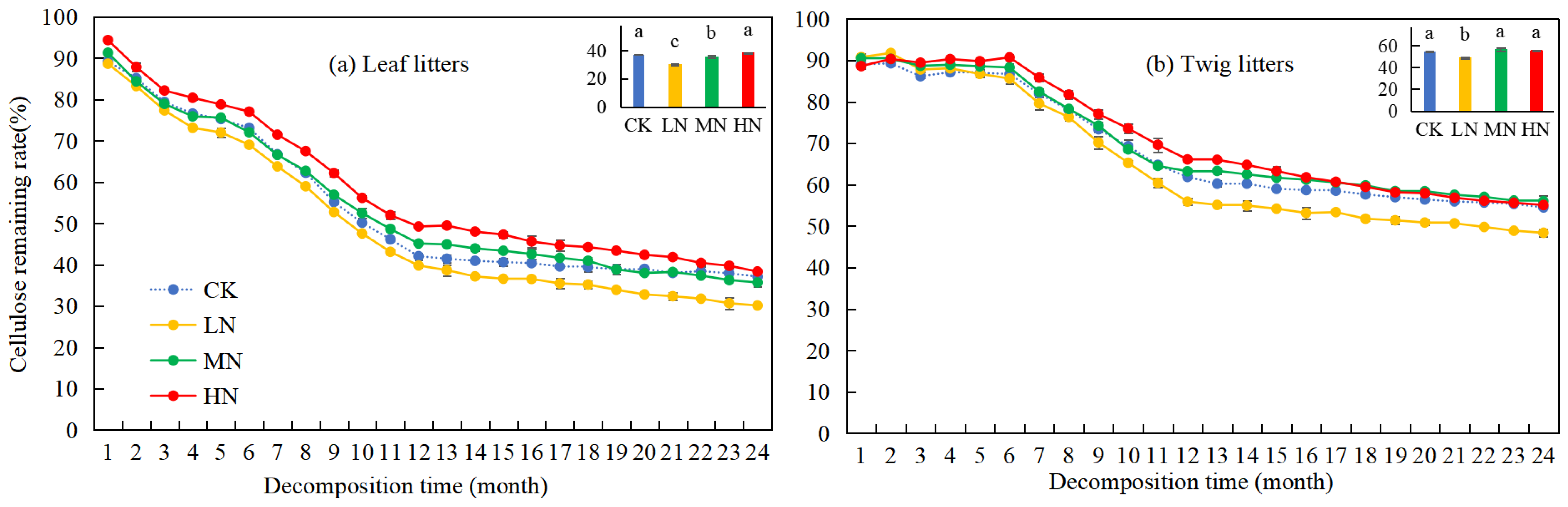
Over time, the cellulose remaining rates of leaf and twig litter in the Pinus yunnanensis forests showed a general decline (Figure 4). At the beginning of the experiment, the cellulose remaining rates were high, at 89.2% for leaf litters and 88.98% for twig litters, but after two years of decomposition, they decreased by 52.1% and 34.49%, respectively. Under different nitrogen application treatments, the cellulose remaining rate of leaf litters exhibited a significant decrease in the first year, while the cellulose remaining rate of twig litters declined significantly only after the seventh month. During the second year, both leaf and twig litter showed a more gradual decline in cellulose remaining rates. Additionally, the cellulose remaining rates of leaf and twig litter under LN treatment were significantly lower (p < 0.05) compared with CK, with reductions of 6.99% and 6.12% (37.10% and 54.49%, respectively). MN and HN treatments did not affect the cellulose remaining rates of leaf and twig litter, suggesting that LN treatment contributed to the degradation of cellulose in leaf and twig litter.
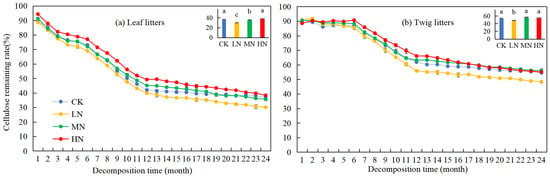
Figure 4.
Variation in cellulose remaining rates during leaf and twig litter decomposition under N deposition in the Pinus yunnanensis forests. Different histogram lowercase letters represent statistically significant differences between various treatments at the end of the experiment on the basis of three-way ANOVA (p < 0.05).
3.3. Effect of Nitrogen Sedimentation on the Remaining C, N, and P Rates in Litter of Pinus yunnanensis Forests
After two years of decomposition, the remaining C, N, and P rates in leaf litters, along with the C and P retention rates in twig litters, generally decreased over time (Figure 5). Under HN treatment, the nutrient remaining rate of C, N, and P in the leaf litters and the nutrient remaining rate in the twig litters were significantly higher than those in CK, LN, and MN (p < 0.05). However, HN treatment did not significantly affect the release of N and P from the twig litters. In the 24th month of decomposition, the C and N nutrient remaining rate in the leaf litters under LN, MN, and HN treatments was significantly higher than those in CK (p < 0.05). In contrast, the N nutrient remaining rate in the twig litters under LN, MN, and HN treatments decreased by 0.25%, 13.98%, and 25.16%, respectively, compared to CK. Additionally, MN and HN treatments showed significant differences in the P nutrient remaining rate of leaf litters compared to CK (p < 0.05), with increases of 13.56% and 19.57%, respectively. The effect of LN treatment on the P nutrient remaining rate in leaf litters was insignificant. Significant differences were observed in the P nutrient remaining rate in twig litters under LN and MN treatments compared to CK (p < 0.05), with decreases of 18.96% and 11.17%, respectively. These findings suggest that MN and HN treatments hindered the release of P in leaf litters, while LN and MN treatments promoted the release of P in twig litters.
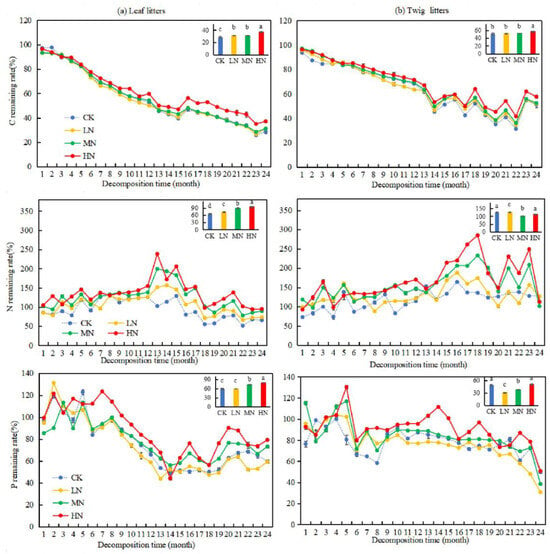
Figure 5.
Changes of the C, N, and P remaining rates during the decomposition of leaf and twig litters under N deposition in the Pinus yunnanensis forests. Different histogram lowercase letters represent statistically significant differences between various treatments at the end of the experiment on the basis of three-way ANOVA (p < 0.05).
3.4. Stoichiometric Ratio of Litter in Pinus yunnanensis Forests
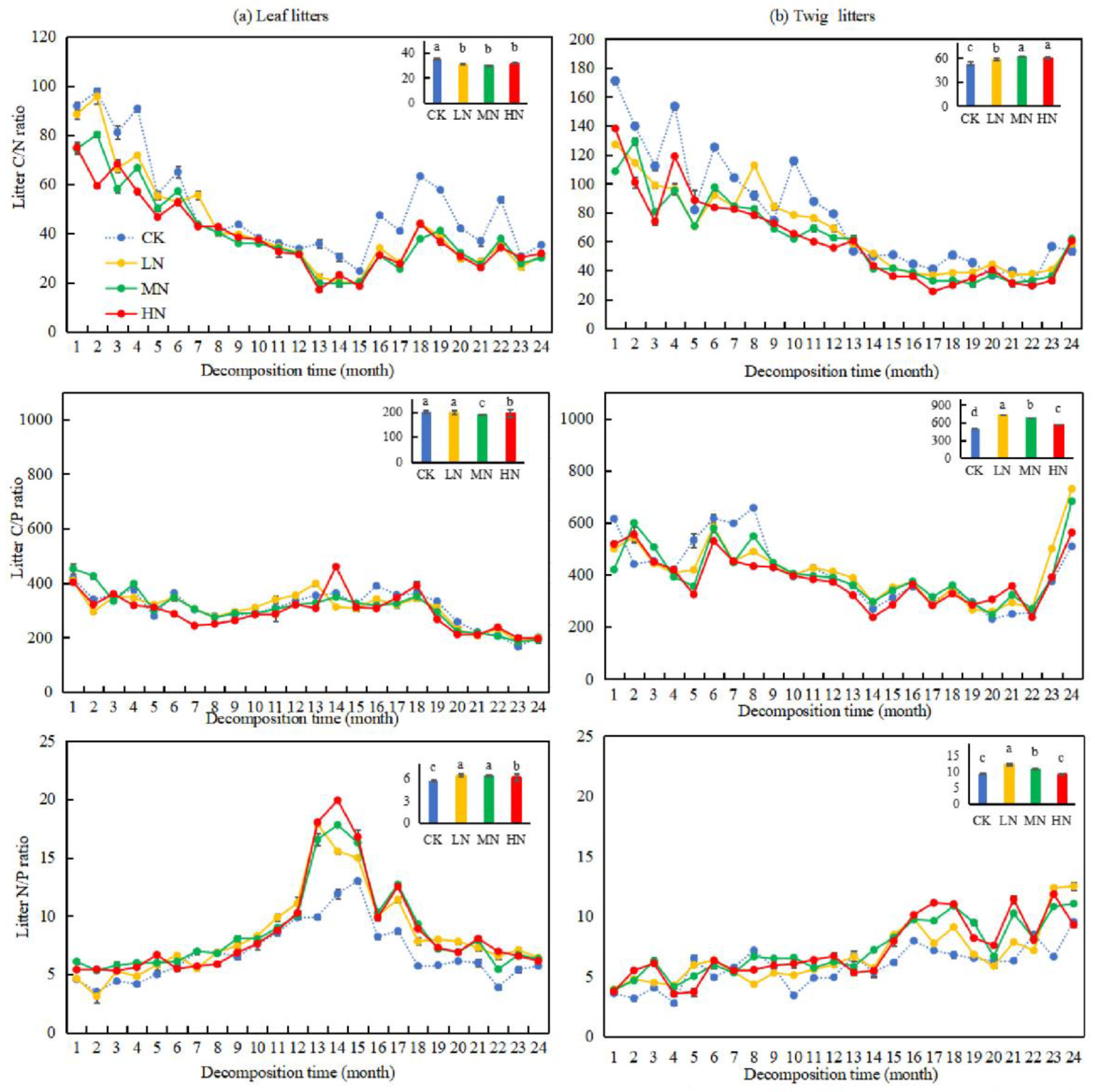
As shown in Figure 6, notable variations (p < 0.05) were found in the C/N and C/P of leaf and twig litters under nitrogen treatments compared to CK. The C/P of twig litters increased by 220.28%, 73.32%, and 52.94% under LN, MN, and HN treatments, respectively, compared to CK. Conversely, the C/P of leaf litters decreased significantly by 11.21% and 5.87% under MN and HN treatments. Additionally, the N/P of leaf litters was significantly higher (p < 0.05) under all nitrogen treatments (LN, MN, and HN) compared to CK, while the N/P ratio of twig litters showed a significant increase (p < 0.05) under LN and MN treatments. After two years of decomposition, the C/N, C/P, and N/P of leaf litters were smaller than those of twig litters under different N application treatments.
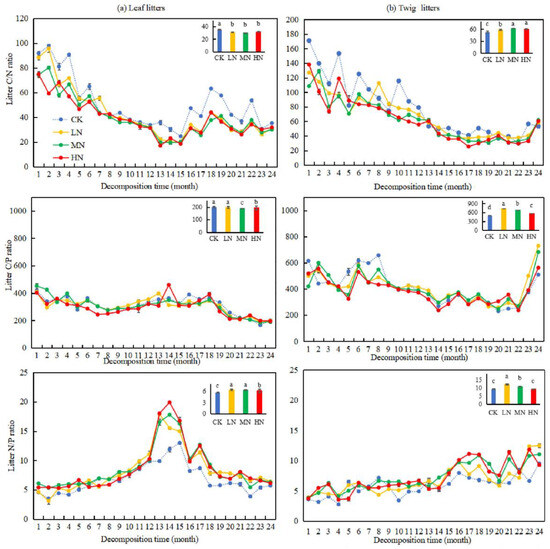
Figure 6.
Changes in the C/N, C/P, and N/P during the decomposition of leaf and twig litters under N deposition in the Pinus yunnanensis forest. Different histogram lowercase letters represent statistically significant differences between various treatments at the end of the experiment on the basis of three-way ANOVA (p < 0.05).
3.5. Nutrient Release from Litters and Lignin and Cellulose Remaining Rates
The analysis of variance (Table 4) for the remaining mass rate, lignin retention rate, cellulose retention rate, nutrient retention rates (C, N, P), and their stoichiometric ratios in the leaf and twig litter of the Pinus yunnanensis forest revealed that both decomposition time and nitrogen deposition significantly affected these parameters (p < 0.05). The interaction of decomposition time and nitrogen deposition did not significantly affect the remaining mass rate, lignin retention rate, cellulose retention rate, and C nutrient remaining rate of leaf and twig litter. Furthermore, decomposition time did not influence the retention rate of the leaf and twig litter.

Table 4.
Results of the analysis of variance (ANOVA) on the impact of decomposition duration and nitrogen treatment on the remaining mass rate, lignin, cellulose, and the elements C, N, and P, along with their stoichiometric ratios in leaf and twig litter.
3.5.1. Correlation Between Lignin Remaining Rate and Nutrient Remaining Rate in Leaf and Twig Litter
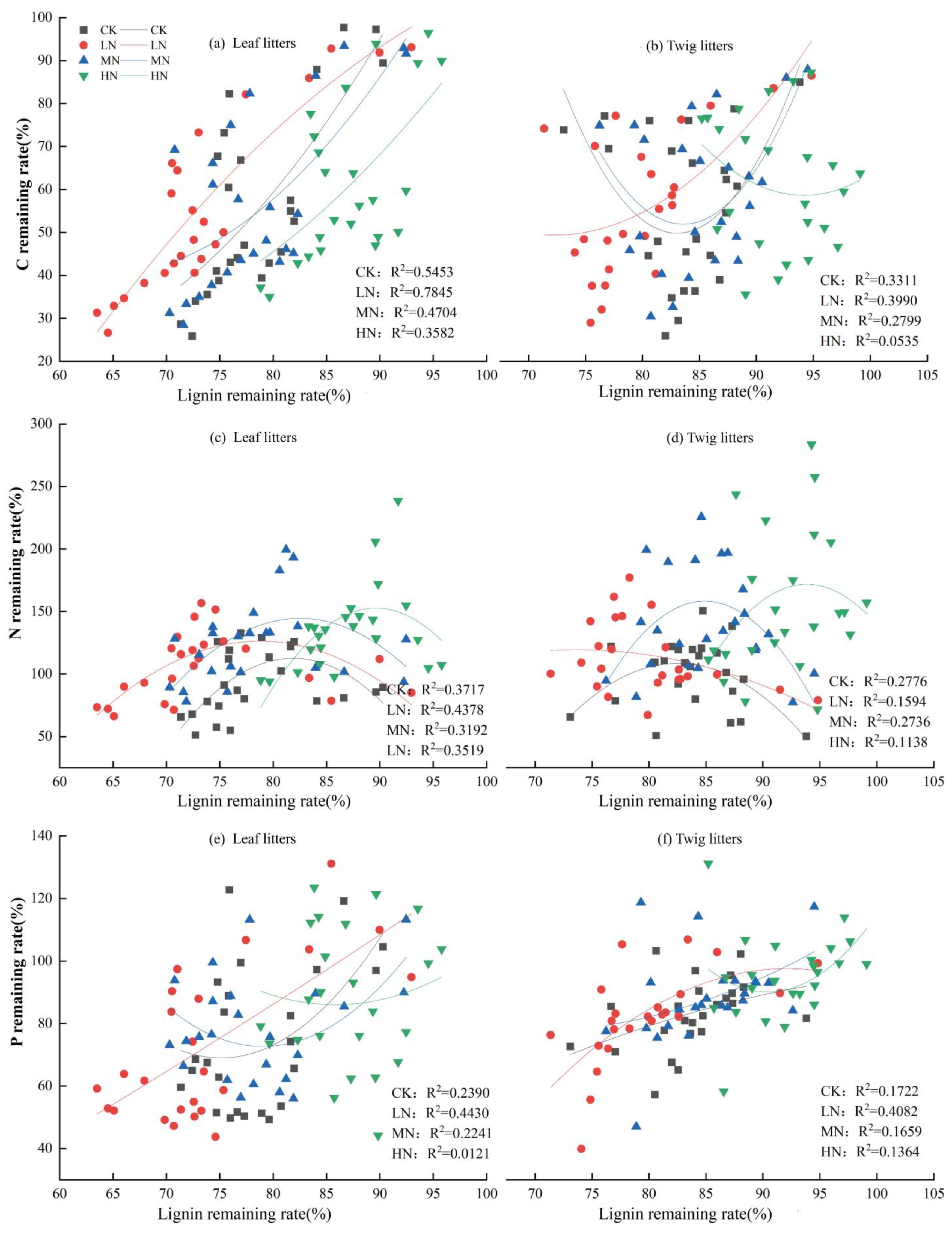
Correlation analysis of the C, N, and P nutrient remaining rates with their corresponding lignin remaining rates in the leaf and twig litter (Figure 7) showed significant correlations between lignin remaining rates and C nutrient remaining rates in leaf litters (R2 = 0.3582–0.7845; p < 0.01; Figure 7a), as well as between lignin remaining rates and N nutrient remaining rates (R2 = 0.3192–0.4378; p < 0.05; Figure 7c). In contrast, no significant correlation was found between the lignin remaining rate of leaf litters and the P nutrient remaining rate under CK, MN, and HN treatments (Figure 7e). For twig litters, the lignin remaining rate significantly correlated with both C and N nutrient remaining rates under CK and MN treatments (p < 0.05). However, under LN treatment, the lignin remaining rate of twig litters had a highly significant link with the P nutrient remaining rate (p < 0.01). No significant correlations were found between the lignin remaining rate of twig litters and nutrient remaining rates (C, N, P) under HN treatment.
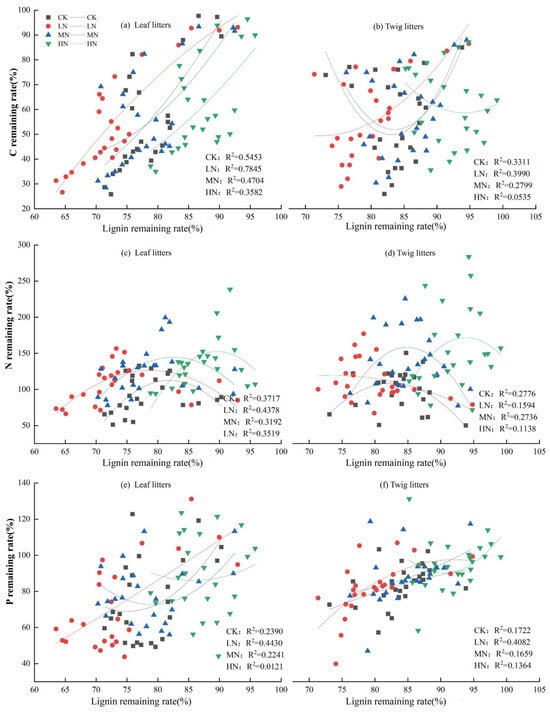
Figure 7.
Correlation between lignin and C, N, and P nutrient remaining rates of leaf and twig litters.
3.5.2. Correlation of Cellulose Remaining Rate of Leaf and Twig Litters with Their Nutrient Remaining Rates
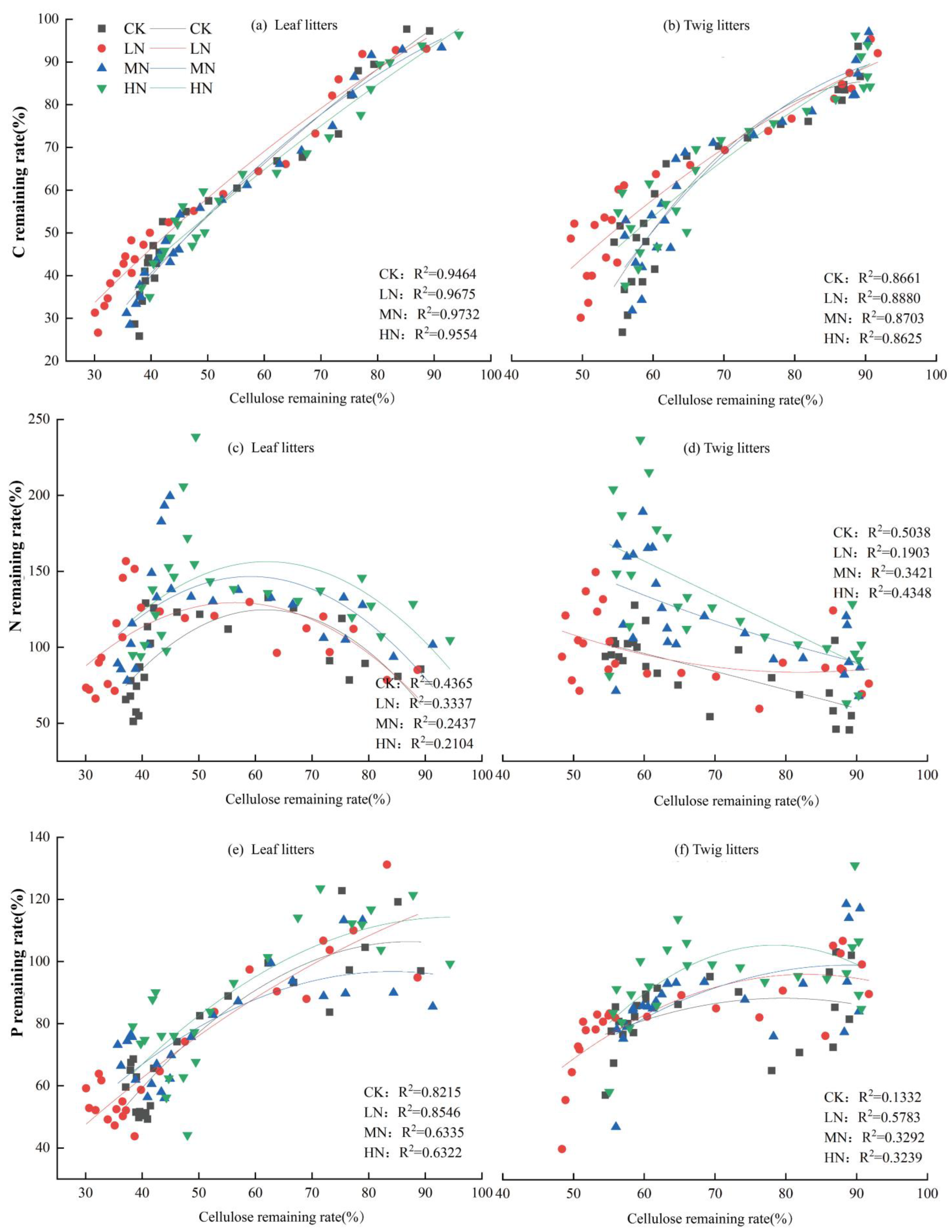
The cellulose remaining rate in leaf litters showed a highly significant correlation with C nutrient remaining rates (R2 = 0.9464–0.9732; p < 0.01; Figure 8a) and P nutrient remaining rates (R2 = 0.6322–0.8546; p < 0.01; Figure 8e). No significant correlation was found between the cellulose remaining rate of leaf litters and the N nutrient remaining rate under MN and HN treatments. For twig litters, the cellulose remaining rate had a highly significant correlation with the C nutrient remaining rate (R2 = 0.8625–0.8880; p < 0.01; Figure 8b). Except for the LN treatment, the cellulose remaining rate of twig litters showed a significant link with the N nutrient remaining rate (R2 = 0.3421–0.5038; p < 0.05; Figure 7d). The cellulose remaining rate of twig litters showed a significant correlation with the P nutrient remaining rate (R2 = 0.3239–0.5783; p < 0.05; Figure 8f), except under CK treatment. Overall, cellulose in the litter of the Pinus yunnanensis forest exhibited a stronger influence on nutrient remaining rates than lignin.
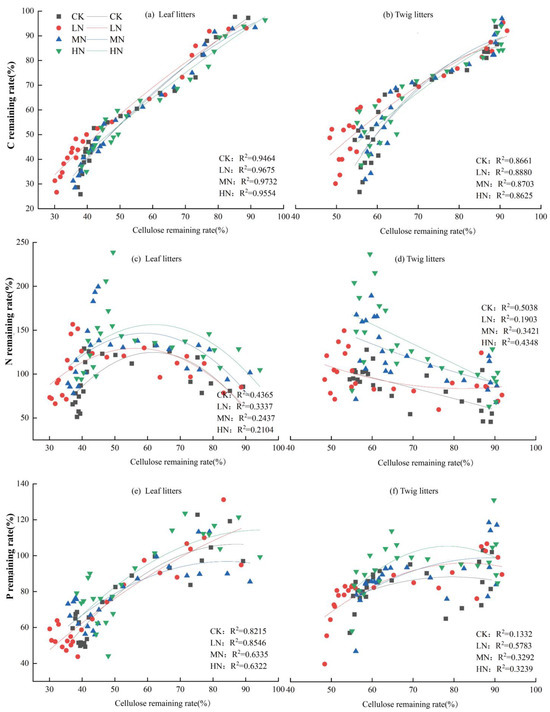
Figure 8.
Correlation between cellulose and C, N, and P nutrient remaining rates of leaf and twig litters.
4. Discussion
4.1. Decomposition Characteristics of Leaf and Twig Litter in Pinus yunnanensis Forest in Response to Simulated Nitrogen Deposition
The decomposition of forest litter is affected by various factors, such as the physicochemical properties of the litter, the plant growth stage [15], the stand type [26,37], and external environmental variables such as soil fauna [14], climate, soil temperature, and the moisture content [8]. As a result, the response of the litter to nitrogen deposition can vary. Previous studies have indicated that nitrogen deposition can accelerate [14,21] or inhibit [23] litter decomposition. These results are likely influenced by factors such as the nitrogen type, form, concentration, and duration of addition [38,39], and the quality of soil and litter [40,41].
In a two-year nitrogen application tracking experiment conducted on the litter of the Pinus yunnanensis forest, the results demonstrated that the mass remaining rates of leaf and twig litters under different nitrogen application levels followed the trend: LN < CK = MN < HN. These results were further confirmed by the decomposition rate fitting, which showed that litters decomposed fastest under the LN treatment and slowest under the HN treatment. These results show that low N promoted litter decomposition, while both medium and high N treatments inhibited litter decomposition. This finding aligns with Hypothesis 1, which posits that nitrogen deposition levels significantly influence the mass remaining rate and decomposition rate of litters. Low nitrogen levels enhance litter decomposition for two main reasons. First, nitrogen addition increases the nitrogen and phosphorus concentrations in litters, while carbon and lignin concentrations remain relatively unchanged, leading to improved litter quality. This improvement in litter quality likely outweighs the negative effect of reduced microbial activity, thus promoting higher decomposition rates. Second, nitrogen addition supplies essential nitrogen for microorganisms and alters the soil microbial community, particularly by lowering the C/N ratio in the soil. This shift in the microbial community facilitates carbon and other nutrient cycling, thereby enhancing litter decomposition rates through interactions with the soil environment and the litter [40,42]. Furthermore, since high-nutrient litter decomposes faster than low-nutrient litter, increased nitrogen deposition can influence litter decomposition by enhancing the availability of soil nitrogen or other elements, as well as the quantity and quality of litter input [43].
However, when nitrogen application exceeds the soil’s nitrogen saturation, excess nitrogen at a high level may bind to lignin and its degradation intermediates, inhibiting lignin-degrading enzyme activity, producing difficult-to-degrade substances, and slowing down litter decomposition. Additionally, a high nitrogen level may reduce the diversity, number of taxa, and individual counts of soil arthropods involved in decomposition, thus inhibiting litter decomposition [6,26,41]. These findings are consistent with Kong et al. [41], who researched the effect of nitrogen addition to Pinus thunbergii Parl litter in the Yimeng Mountains. In the Pinus yunnanensis forest, the mass remaining rates of twig litters were consistently higher than those of leaf litters, likely due to the higher lignin content of the twig litters. Lignin, a significant organic component of forest litter, is challenging to degrade during the decomposition process due to its complex and stable chemical properties, which render it the rate-limiting factor in litter decomposition. After nitrogen was added, the remaining lignin content in the litter followed a similar pattern, initially decreasing and then increasing. This response of litter to nitrogen addition reflects the impact of lignin content. When external nitrogen was introduced into the system, it could interact with lignin and its breakdown products, slowing down the decomposition of lignin. Nitrogen addition altered the C/N of litters, thus promoting the decomposition of litters. Conversely, when the lignin content of litters was high, litter decomposition was inhibited by nitrogen addition [41,44]. Because the lignin content in twig litters is greater than that in leaf litters, and their C/N is also higher than that of leaf litters. Consequently, the decomposition of twig litters is slower, and their remaining rate is higher than that of leaf litters. The carbon content from the litter of Pinus yunnanensis forests was relatively high, with values of 351.37–579.25 mg/g for leaf litters and 282.54–570.30 mg/g for twig litters, while the corresponding nitrogen content was only 5.94−17.02 mg/g and 3.34–9.96 mg/g; the range of C/N was 24.65–97.80 and 29.98–139.68, respectively. In the 24th month, the C/N of twig litters under LN, MN, and HN treatments was significantly higher than those under CK. The variation and extent of the C/N ratio in litter determine the order of its decomposition [21]. Thus, nitrogen addition influences the rate of litter decomposition by altering C/N. Ultimately, while the effect of nitrogen deposition on litter decomposition is influenced by the external nitrogen input, it primarily depends on the inherent quality of the litter itself [45].
4.2. Response of Nutrient Release from Leaf and Twig Litters to Simulated Nitrogen Deposition in Pinus yunnanensis Forest
Nitrogen deposition enhances litter quality by raising its nitrogen content, thereby lowering the carbon-to-nitrogen ratio. Litter with a low C/N ratio, considered high quality, is more readily decomposed by decomposers, leading to greater nutrient release [21]. Previous research has indicated that nitrogen from decomposing litter is released when the C/N ratio falls below 40 [28]. In this experiment, the initial C/N of leaf and twig litters in the Pinus yunnanensis forest was 80.9 and 132.05, respectively. In the middle and late stages of decomposition, nitrogen release from leaf litters was higher than from twig litters, which delayed the release of nitrogen from the twig litters. This difference is likely due to the significantly lower C/N of leaf litters, which promoted nitrogen release [46]. Our results indicated significant differences in the nitrogen remaining rates of leaf litters under different nitrogen application treatments (p < 0.05), with the order CK < LN < MN < HN. This finding suggests that nitrogen deposition inhibited nitrogen release from leaf litters, possibly by reducing microbial nitrogen demand [41], altering the microbial community from bacterial-dominated to fungal-dominated, and thereby lowering microbial nitrogen demand in leaf litters [47].
Phosphorus release from decomposing litter is primarily regulated by the C/P. When the C/P exceeds 700, phosphorus is fixed [28]. The initial C/P of leaf and twig litters in the Pinus yunnanensis forest was 417.22 and 495.17, respectively, and phosphorus release occurred throughout the experimental period under all nitrogen application treatments. The MN and HN treatments showed significant increases in the phosphorus remaining rate of leaf litters (p < 0.05), with increases of 13.56% and 19.57%, respectively, compared to CK. This is consistent with findings from another study [48], which suggests that nitrogen enrichment slows phosphorus release and favors phosphorus accumulation, leading to net phosphorus fixation in the litter. This effect became more pronounced with increasing nitrogen application and experimental duration. In contrast, the phosphorus remaining rate of twig litters was significantly lower (p < 0.05) under LN and MN treatments, with decreases of 18.96% and 11.17%, respectively, compared to CK. These results indicate that MN and HN treatments inhibited phosphorus release from leaf litters, while LN and MN treatments promoted phosphorus release from twig litters. The above results demonstrate that leaf and twig litters respond differently to varying nitrogen deposition levels. The results align with Hypothesis 2 and can be attributed to the quality of the substrate litter, the amount of nitrogen applied, the duration of the experiment, and the environmental conditions [48].
The impact of nitrogen deposition on the N and P nutrient remaining rates of litter is influenced by the type of litter, particularly its stoichiometric characteristics during decomposition [45]. After two years of decomposition of leaf and twig litters in the Pinus yunnanensis forest, the N/P ratio increased under all nitrogen application treatments. This finding aligns with a global meta-analysis by Xu Hongwei et al., which showed a significant increase in the N/P of litters after nitrogen application. This suggests that nitrogen addition alleviated nitrogen limitation in litter while exacerbating phosphorus limitation. This effect may be attributed to reduced N and P release during decomposition, leading to higher C/N and C/P, which in turn lowers microbial biomass and alters the microbial community. Additionally, nitrogen deposition may inhibit nutrient release from litter by suppressing the synthesis of decomposition enzymes [38,39].
4.3. Effects of Lignin and Cellulose Degradation on Nutrient Release from Leaf and Twig Litters of Pinus yunnanensis Forest
After two years of litter decomposition tracking in the Pinus yunnanensis forest, lignin and cellulose decomposition stabilized after the 12th month under all nitrogen application levels. In the early stages of decomposition, more carbon sources are readily decomposed, facilitating the breakdown of lignin and cellulose. However, as decomposition progresses to the middle stage, lignin and cellulose degradation slows due to the depletion of fast-acting nutrients [49]. The overall trend in lignin and cellulose decomposition showed a decrease in their remaining rates. Due to varying degrees of degradation, the lignin remaining rate remained relatively stable, fluctuating within a narrow range (65%–95%), while cellulose degradation was more pronounced (remaining rates of leaf and twig litters decreased from 89.2 and 88.98 to 37.10 and 54.49, respectively). Furthermore, the C/N of the litter in the Pinus yunnanensis forest exhibited an overall decreasing trend during decomposition. These findings align with previous research [21,42], which indicates that increased nitrogen and carbon concentrations reduce the C/N of litters. This reduction, along with the increase in soluble matter and cellulose decomposition, typically accelerates the decomposition rate of litter. As nitrogen concentration increased, ligninase activity was inhibited, further promoting litter decomposition through higher nitrogen content and lower lignin levels [50]. Chen Bo et al.’s study on litter in subtropical forests also found a significant relationship between the rates of cellulose and lignin breakdown and the C/N ratio [51]. In this study, LN treatment facilitated the degradation of lignin and cellulose in leaf and twig litter. Similarly, when analyzing the effect of nitrogen deposition on the litter mass remaining rate in Pinus yunnanensis forest, the LN treatment promoted decomposition, with the mass remaining rates following the order LN < CK < MN < HN. This result is consistent with research conducted in Rhododendron delavayi forests at Bailey’s Rhododendron National Forest Park in southwestern China, which showed that low nitrogen levels enhance litter decomposition by promoting the release of lignin and cellulose, reducing litter remaining. A small increase in nitrogen may affect enzyme activity in litter, altering the content of metabolites [49]. Lignin and cellulose showed similar reactions to nitrogen deposition, which supports Hypothesis 3. This is mainly due to the fact that the decomposition of litter lignin is influenced by the presence of cellulose, hemicellulose, and pectin, which are shielded by lignin and hemicellulose, thereby hindering the breakdown of both lignin and cellulose [32].
Phosphorus is a critical nutrient for plant development and influences litter decomposition by modifying microbial biomass and community structure [27]. In this experiment, nitrogen application increased the litter N/P during the decomposition of Pinus yunnanensis forest, suggesting that nitrogen addition exacerbated phosphorus limitation for microorganisms, affecting cellulose and lignin degradation [48].
Overall, after two years of decomposition, the nutrient remaining rates for C, N, and P in leaf litters and the C and P remaining rates in twig litters decreased over time. By the 24th month of decomposition, the lignin remaining rates in leaf and twig litters reached 71.36% and 80.52%, respectively. The gradual accumulation of lignin in the litters under all treatments provided physical protection for cellulose and other components, potentially hindering the decomposition rate and indirectly inhibiting nutrient release [21]. Nitrogen deposition did not lead to significant changes in decomposition. However, nitrogen addition stimulated decomposition in ecosystems with low background nitrogen deposition and high litter mass. In contrast, nitrogen deposition inhibited decomposition in ecosystems with higher nitrogen deposition and lower litter mass. The direction, magnitude, and persistence of nitrogen effects on decomposition largely depend on the specific mechanisms driving these effects. In this study, the nutrient stoichiometry of leaf and twig litter in the Pinus yunnanensis forest provided a useful indicator for characterizing the mechanisms of litter decomposition and nutrient release. Soil microorganisms, community composition (including soil fauna), and physicochemical factors also play a crucial role in the decomposition of litter. Previous research has indicated that reduced pH levels promote microbial attachment, and when combined with optimal temperature and sufficient moisture, they can enhance microbial activity and accelerate litter breakdown [51]. Therefore, integrating soil biology and properties is essential for understanding how long-term nitrogen deposition influences forest litter decomposition, providing a theoretical foundation for nitrogen deposition’s impact on ecosystem nutrient cycling and function.
5. Conclusions
In the study, it was found that nitrogen addition affected the process of litter decomposition and nutrient release to varying degrees, and the different components of the litters showed different responses to nitrogen addition. This reflected the fact that while the impact of nitrogen deposition on litter decomposition is influenced by the nitrogen addition level, it is also closely tied to the characteristics of the litter itself. The nutrient remaining rates of litter C, N, and P throughout the decomposition process fluctuated across different decomposition stages. After nitrogen application treatments, the C/N of the litters significantly decreased (by 57.53%–64.96% in leaf litters and 43.07%–68.83% in twig litters), while the N/P increased (by 3.78%–38.53% in leaf litters and 148%–218.32% in twig litters). Due to nitrogen enrichment, the release of phosphorus slowed down. Consequently, nitrogen addition alleviated nitrogen limitation in the litters while exacerbating phosphorus limitation. Moreover, the nutrient remaining rates of C, N, and P in leaf litters and C and P in twig litters were highest under high nitrogen treatments. Under short-term or even long-term conditions, high nitrogen deposition can delay the decomposition of litters and the release of C, N, and P nutrients, thereby influencing the nutrient cycling and overall function of the ecosystem in southwest China. Therefore, the best balance point suitable for the coordinated development of ecosystems should be found by taking into account external factors and internal conditions in accordance with the different characteristics of ecosystems and the degree of their response, thereby effectively reducing the negative impacts of high nitrogen deposition on ecosystems.
Author Contributions
Conceptualization, visualization, methodology, data curation, and writing—original draft, Y.N.; investigation, methodology, data curation, W.C.; investigation, validation, software, Z.H.; resources, supervision, project administration Y.Z.; investigation, validation, L.Z.; investigation, validation, X.L.; project administration, supervision, funding acquisition, writing—review and editing, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Yunnan Fundamental Research Projects (202401AT070262), Agricultural Joint Special Project of Yunnan Province (202301BD070001-059), and the First-Class Discipline in Soil and Water Conservation and Desertification Prevention in Yunnan Province (SBK20240017).
Data Availability Statement
Data are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank Chenggong Song, Jinmei Xing, Xiaodong Li, and Qian Wang for their assistance in the field, and Meiting Li for her help with the illustrations in this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Xiao, C.; Hu, Q.; Chen, X.; Zhao, T.; Guo, X.; Chen, F. Research Progress of nitrogen deposition based on bibliometrics. Acta Ecol. Sin 2023, 43, 1294–1307. [Google Scholar]
- Lin, Y.; Zheng, S.; Su, J.; Rong, J.; He, T.; Zheng, Y.; Chen, L. Analyzing the impact of simulated nitrogen deposition on stoichiometric properties and yield of Ma bamboo (Dendrocalamus latiflorus Munro) shoots, leaves, and soil substrate. Forests 2024, 15, 151. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, H.; Zhao, M.; Monaco, T.A.; Rong, Y.; Huang, D.; Song, Q.; Zhao, K.; Wang, D. Soil extracellular enzyme activities and the abundance of nitrogen-cycling functional genes responded more to N addition than P addition in an Inner Mongolian meadow steppe. Sci. Total Environ. 2021, 759, 143541. [Google Scholar] [CrossRef]
- Tie, L.; Wei, S.; Peñuelas, J.; Sardans, J.; Peguero, G.; Zhou, S.; Liu, X.; Hu, J.; Huang, C. Phosphorus addition reverses the negative effect of nitrogen addition on soil arthropods during litter decomposition in a subtropical forest. Sci. Total Environ. 2021, 781, 146786. [Google Scholar] [CrossRef]
- Borer, E.T.; Stevens, C.J. Nitrogen deposition and climate: An integrated synthesis. Trends Ecol. Evol. 2022, 37, 541–552. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.E.J.; Mathe, L.O.J.; van Rooyen, I.L.; Brink, H.G.; Nicol, W. Optimal growth conditions for Azolla pinnata R. Brown: Impacts of light intensity, nitrogen addition, pH control, and humidity. Plants 2022, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jia, S.; Wang, X.; Shao, M.; Liao, W.; Guenther, A.; Flechard, C.; Yu, P.; Zhong, B.; Chang, M. Precipitation trend increases the contribution of dry reduced nitrogen deposition. npj Clim. Atmos. Sci. 2023, 6, 62. [Google Scholar] [CrossRef]
- Meng, Y.-n.; Li, T.; Liu, H.; Li, S.-p.; Xu, Z.; Jiang, Y. Legacy effects of nitrogen deposition and increased precipitation on plant productivity in a semi-arid grassland. Plant Soil 2023, 491, 69–84. [Google Scholar] [CrossRef]
- Song, X.; Jiang, H.; Zhang, Z.; Zhou, G.; Zhang, S.; Peng, C. Interactive effects of elevated UV-B radiation and N deposition on decomposition of Moso bamboo litter. Soil Biol. Biochem. 2014, 69, 11–16. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H.; Mo, Q.; Zhang, Z.; Huang, G.; Kong, F.; Liu, Y.; Wang, G.G. Effects of elevated UV-B radiation and N deposition on the decomposition of coarse woody debris. Sci. Total Environ. 2019, 663, 170–176. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Behm, J.E.; Wan, S.; Yan, J.; Ye, Q.; Zhang, W.; Yang, X.; Fu, S. Effects of canopy nitrogen addition on soil fauna and litter decomposition rate in a temperate forest and a subtropical forest. Geoderma 2021, 382, 114703. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, S.; Cheng, X.; Wei, X. Alternating processes of dry and wet nitrogen deposition have different effects on the function of canopy leaves: Implications for leaf photosynthesis. Front. Plant Sci. 2023, 13, 1105075. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhang, H.; Zhang, H.; Tang, Z. Responses of litter decomposition and nutrient dynamics to nitrogen addition in temperate shrublands of North China. Front. Plant Sci. 2021, 11, 618675. [Google Scholar] [CrossRef] [PubMed]
- Zi, H.; Hu, L.; Wang, C. Differentiate responses of soil microbial community and enzyme activities to nitrogen and phosphorus addition rates in an alpine meadow. Front. Plant Sci. 2022, 13, 829381. [Google Scholar] [CrossRef]
- Xie, D.; Si, G.; Zhang, T.; Mulder, J.; Duan, L. Nitrogen deposition increases N2O emission from an N-saturated subtropical forest in southwest China. Environ. Pollut. 2018, 243, 1818–1824. [Google Scholar] [CrossRef]
- Gundale, M.J. The impact of anthropogenic nitrogen deposition on global forests: Negative impacts far exceed the carbon benefits. Glob. Change Biol. 2022, 28, 690–692. [Google Scholar] [CrossRef]
- Fang, H.; Mo, J. Effects of nitrogen deposition on forest litter decomposition. Acta Ecol. Sin. 2006, 26, 3127–3136. [Google Scholar]
- Wang, M.; Liu, G.; Xing, Y.; Yan, G.; Wang, Q. Long-Term Nitrogen Addition Accelerates Litter Decomposition in a Larix gmelinii Forest. Forests 2024, 15, 372. [Google Scholar] [CrossRef]
- Trentini, C.P.; Villagra, M.; Pámies, D.G.; Laborde, V.B.; Bedano, J.C.; Campanello, P.I. Effect of nitrogen addition and litter removal on understory vegetation, soil mesofauna, and litter decomposition in loblolly pine plantations in subtropical Argentina. For. Ecol. Manag. 2018, 429, 133–142. [Google Scholar] [CrossRef]
- Zhou, S.-x.; Huang, C.-d.; Han, B.-h.; Xiao, Y.-x.; Tang, J.-d.; Xiang, Y.-b.; Luo, C. Simulated nitrogen deposition significantly suppresses the decomposition of forest litter in a natural evergreen broad-leaved forest in the Rainy Area of Western China. Plant Soil 2017, 420, 135–145. [Google Scholar] [CrossRef]
- Gill, A.L.; Schilling, J.; Hobbie, S.E. Experimental nitrogen fertilisation globally accelerates, then slows decomposition of leaf litter. Ecol. Lett. 2021, 24, 802–811. [Google Scholar] [CrossRef]
- Lo Cascio, M.; Morillas, L.; Ochoa-Hueso, R.; Delgado-Baquerizo, M.; Munzi, S.; Roales, J.; Spano, D.; Cruz, C.; Gallardo, A.; Manrique, E. Nitrogen deposition effects on soil properties, microbial abundance, and litter decomposition across three shrublands ecosystems from the Mediterranean Basin. Front. Environ. Sci. 2021, 9, 709391. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.Y.; Ruan, H. Responses of litter decomposition and nutrient release to N addition: A meta-analysis of terrestrial ecosystems. Appl. Soil Ecol. 2018, 128, 35–42. [Google Scholar]
- Zhang, J.; Li, J.; Fan, Y.; Mo, Q.; Li, Y.; Li, Y.; Li, Z.; Wang, F. Effect of nitrogen and phosphorus addition on litter decomposition and nutrients release in a tropical forest. Plant Soil 2020, 454, 139–153. [Google Scholar] [CrossRef]
- Zhuang, L.; Liu, Q.; Liang, Z.; You, C.; Tan, B.; Zhang, L.; Yin, R.; Yang, K.; Bol, R.; Xu, Z. Nitrogen additions retard nutrient release from two contrasting foliar litters in a subtropical forest, southwest China. Forests 2020, 11, 377. [Google Scholar] [CrossRef]
- Schwede, D.B.; Simpson, D.; Tan, J.; Fu, J.S.; Dentener, F.; Du, E.; deVries, W. Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environ. Pollut. 2018, 243, 1287–1301. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Song, Y.-L.; Wang, K.-Q.; Yang, X.-Y.; Xing, J.-M.; Zhang, Z.-M. Responses of litter decomposition in two subalpine plantations to simulated nitrogen deposition in central Yunnan, China. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2020, 31, 2523–2532. [Google Scholar]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Xing, J.; Hu, C.; Song, C.; Wang, K.; Song, Y. Nitrogen Deposition Modulates Litter Decomposition and Enhances Water Retention in Subtropical Forests. Forests 2024, 15, 522. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, J.; Liao, X.; Liu, J.; Wang, D.; Li, J.; Yan, Q. Ecological stoichiometry of Cinnamomum migao leaf litter and soil nutrients under nitrogen deposition in a karst region. Ecosphere 2021, 12, e03738. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Roeder, M.; Dossa, G.G.; Cornelissen, J.H.C.; Yang, X.; Nakamura, A.; Tomlinson, K.W. Liana litter decomposes faster than tree litter in a multispecies and multisite experiment. J. Ecol. 2022, 110, 2433–2447. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Qiao, Y.; Cao, Y.; Jiao, Y.; Yang, A.; Liu, M.; Ma, L.; Song, M.; Fu, S. Atmospheric nitrogen deposition affects forest plant and soil system carbon: Nitrogen: Phosphorus stoichiometric flexibility: A meta-analysis. For. Ecosyst. 2024, 11, 100192. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Cheng, X.; Liu, G. Nitrogen addition stimulates litter decomposition rate: From the perspective of the combined effect of soil environment and litter quality. Soil Biol. Biochem. 2023, 179, 108992. [Google Scholar] [CrossRef]
- Kong, B.; Zhou, J.; Qi, L.; Jiao, S.; Ma, L.; Geng, W.; Zhao, Y.; Gao, T.; Gong, J.; Li, K. Effects of nitrogen deposition on leaf litter decomposition and soil organic carbon density in arid and barren rocky mountainous regions: A case study of Yimeng mountain. Forests 2023, 14, 1351. [Google Scholar] [CrossRef]
- Hou, S.L.; Hättenschwiler, S.; Yang, J.J.; Sistla, S.; Wei, H.W.; Zhang, Z.W.; Hu, Y.Y.; Wang, R.Z.; Cui, S.Y.; Lü, X.T. Increasing rates of long-term nitrogen deposition consistently increased litter decomposition in a semi-arid grassland. New Phytol. 2021, 229, 296–307. [Google Scholar] [CrossRef]
- Osborne, B.B.; Soper, F.M.; Nasto, M.K.; Bru, D.; Hwang, S.; Machmuller, M.B.; Morales, M.L.; Philippot, L.; Sullivan, B.W.; Asner, G.P. Litter inputs drive patterns of soil nitrogen heterogeneity in a diverse tropical forest: Results from a litter manipulation experiment. Soil Biol. Biochem. 2021, 158, 108247. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Chen, F.-S.; Wang, G.G.; Fang, X.-M.; Wan, S.-Z.; Zhang, Y.; Liang, C. Nitrogen deposition effect on forest litter decomposition is interactively regulated by endogenous litter quality and exogenous resource supply. Plant Soil 2019, 437, 413–426. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Y.; Xu, J.; He, H.; Tao, Y.; Yang, Z.; Bai, Y. Effects of exogenous N and endogenous nutrients on alpine tundra litter decomposition in an area of high nitrogen deposition. Sci. Total Environ. 2022, 805, 150388. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; DesRochers, A.; Baldy, V. Effect of mixing herbaceous litter with tree litters on decomposition and N release in boreal plantations. Plant Soil 2016, 398, 229–241. [Google Scholar] [CrossRef]
- Song, Y.; Xing, J.; Hu, C.; Song, C.; Wang, Q.; Wang, S. Decomposition and carbon and nitrogen releases of twig and leaf litter were inhibited by increased level of nitrogen deposition in a subtropical evergreen broad-leaved forest in Southwest China. Forests 2024, 15, 492. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, J.; Hao, J.; Li, C.; Quan, W. Decomposition Characteristics of Lignocellulosic Biomass in Subtropical Rhododendron Litters under Artificial Regulation. Metabolites 2023, 13, 279. [Google Scholar] [CrossRef]
- Yue, K.; Peng, C.; Yang, W.; Peng, Y.; Zhang, C.; Huang, C.; Wu, F. Degradation of lignin and cellulose during foliar litter decomposition in an alpine forest river. Ecosphere 2016, 7, e01523. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Chen, L.; Jiang, L.; Hong, Y.; Zhu, J.; Liu, J.; Xu, D.; Kuang, K.; He, Z. Microclimate along an elevational gradient controls foliar litter cellulose and lignin degradation in a subtropical forest. Front. For. Glob. Change 2023, 6, 1134598. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).