Reproductive Ecology of Lecythis Pisonis in Brazilian Agroforestry Systems: Implications for Conservation and Genetic Diversity
Abstract
1. Introduction
2. Materials and Methods
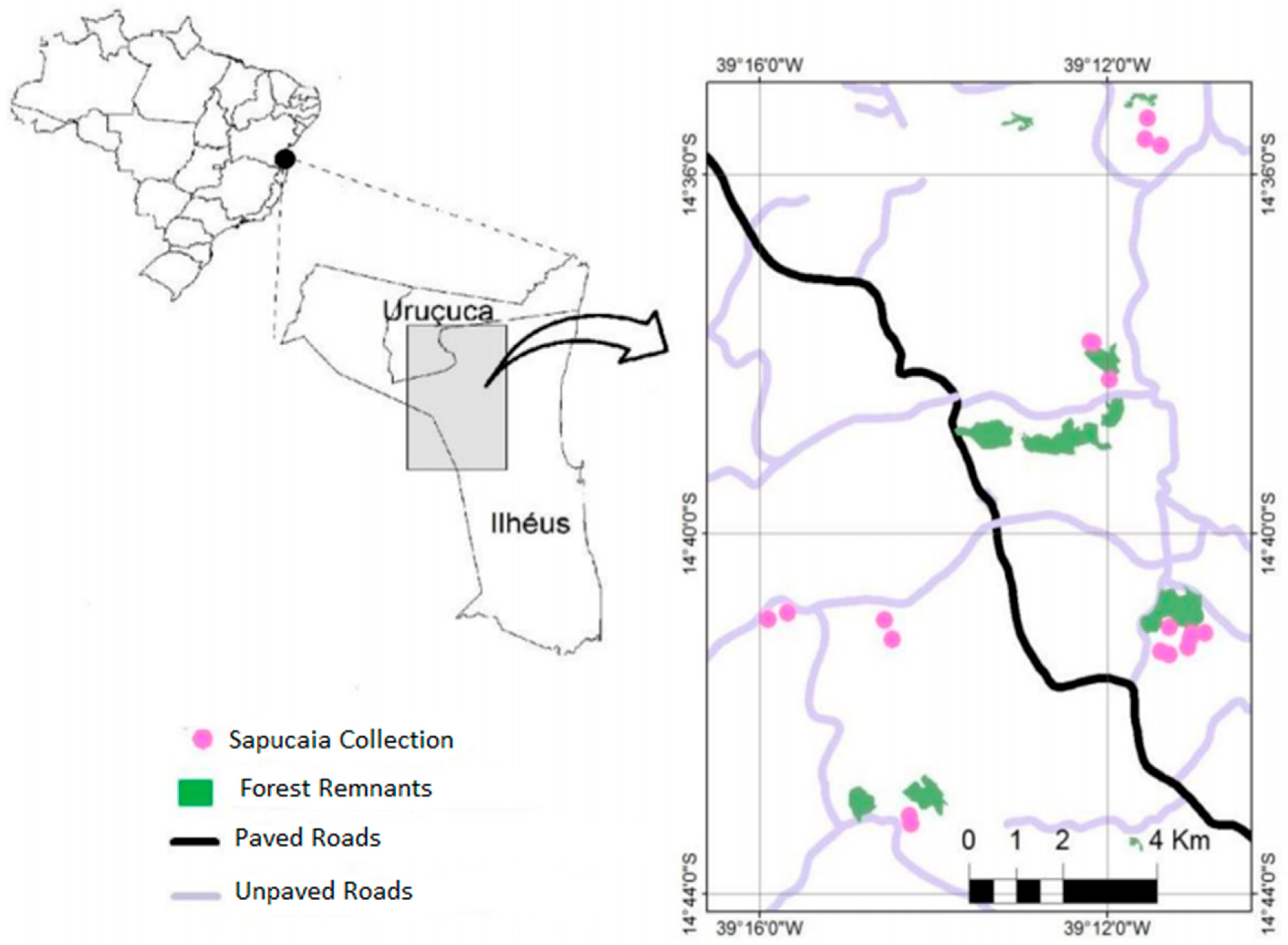
2.1. Study Area and Sampling Design
2.2. DNA Extraction and Quantification
2.3. Amplification of SSR Loci and Genotyping
2.4. Analysis of the Reproductive System
2.5. Paternity Analysis
2.6. Pollen Flow Distance Analysis
3. Results
3.1. Reproductive System
3.2. Paternity Analysis
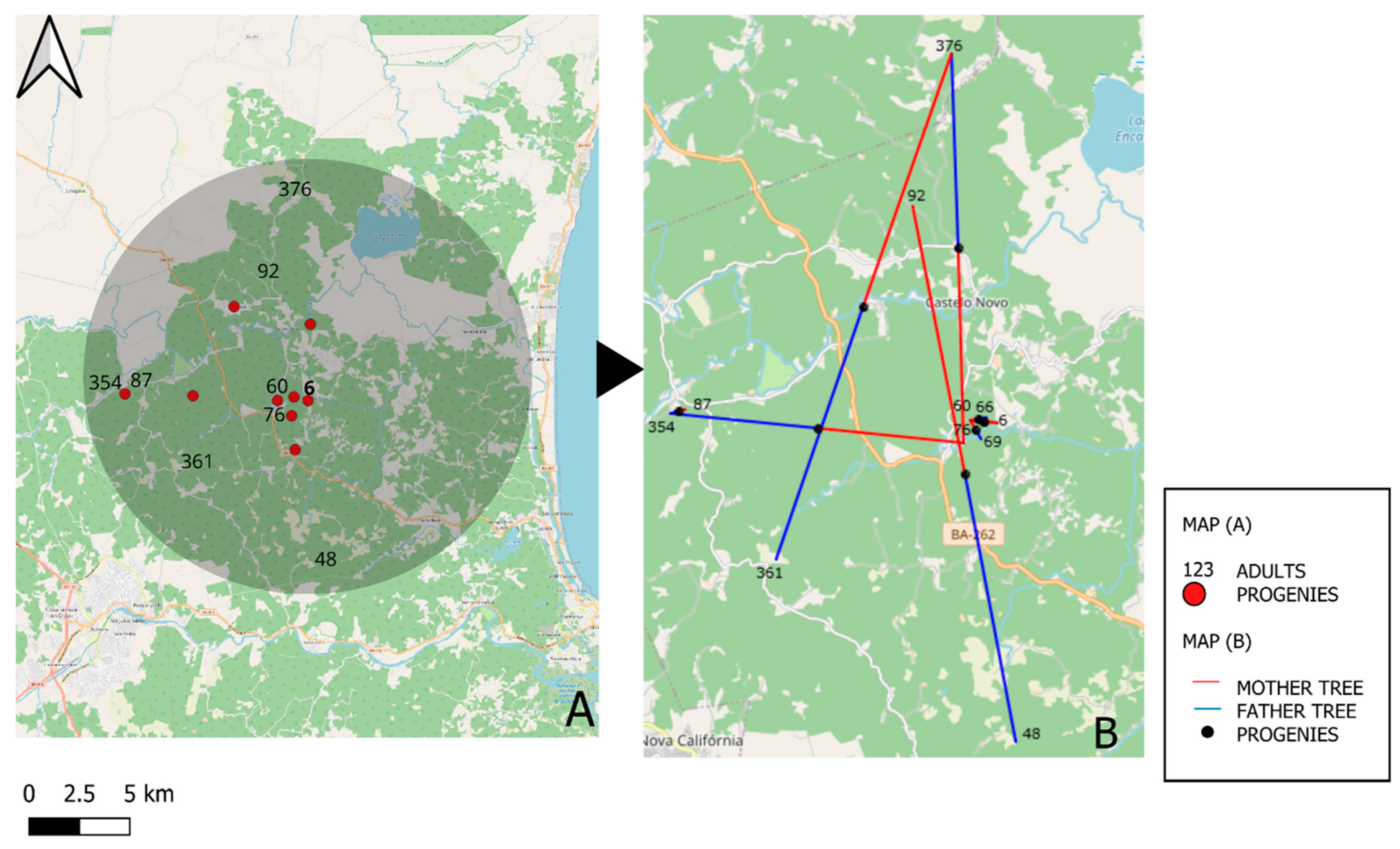
3.3. Pollen Flow Distance
4. Discussion
Reproduction System, Paternity, and Pollen Flow Distance
5. Conclusions and Conservation Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholes, R.; Montanarella, L.; Brainich, A.; Barger, N.; Brink, B.; Cantele, M.; Erasmus, B.; Fisher, J.; Gardner, T.; Holland, T.; et al. Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental Science Policy Platform on Biodiversity and Ecosystem Services; USDA: Washington, WA, USA, 2018. [Google Scholar]
- CBD. Zero Draft of the Post-2020 Global Biodiversity Framework Published by the Secretariat. In Proceedings of the Convention on Biological Diversity, Kunming, China, 24–29 February 2020. [Google Scholar]
- Leonardo Marques, U.; Joana Braun, B.; Davi, C. Policies to encourage agroforestry in the Southern Atlantic Forest. Land Use Policy 2022, 112, 105802. [Google Scholar]
- Nogué, S.; Tovar, C.; Bhagwat, S.A.; Finsinger, W.; Willis, K.J. Exploring the Ecological History of a Tropical Agroforestry Landscape Using Fossil Pollen and Charcoal Analysis from Four Sites in Western Ghats, India. Ecosystems 2018, 21, 45–55. [Google Scholar] [CrossRef]
- Santos, P.Z.F.; Crouzeilles, R.; Sansevero, J.B.B. Can agroforestry systems enhance biodiversity and ecosystem service provision in agricultural landscapes? A meta-analysis for the Brazilian Atlantic Forest. For. Ecol. Manag. 2019, 433, 140–145. [Google Scholar] [CrossRef]
- Kumar, V. Multifunctional Agroforestry Systems in Tropics Region. Nat. Environ. Pollut. Technol. 2016, 15, 365–376. [Google Scholar]
- Mahmud, A.; Raj, D.; Jhariya, M. Agroforestry systems in the tropics: A critical review. Russ. Agric. Sci. 2020, 36, 83–87. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística. Agricultural Census; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2017.
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Heming, N.M.; Schroth, G.; Talora, D.C.; Faria, D. Cabruca agroforestry systems reduce vulnerability of cacao plantations to climate change in southern Bahia. Agron. Sustain. Dev. 2022, 42, 48. [Google Scholar] [CrossRef]
- De Souza, J.P.; de Carvalho Gonçalves, J.F.; Jaquetti, R.K.; Da Costa, K.C.P.; De Lima, R.M.B.; Fearnside, P.M.; da Rocha Nina Junior, A. Silvicultural interventions and agroforestry systems increase the economic and ecological value of Bertholletia excelsa plantations in the Amazon. Agrofor. Syst. 2023, 97, 197–207. [Google Scholar] [CrossRef]
- Evangelista de Oliveira, R.; Carvalhaes, M. Agroforestry as a tool for restoration in atlantic forest: Can we find multi-purpose species? Oecol. Aust. 2016, 20, 425–435. [Google Scholar] [CrossRef]
- Favreto, R.; Mello, R.S.P.; de Moura Baptista, L.R. Growth of Euterpe edulis Mart.(Arecaceae) under forest and agroforestry in southern Brazil. Agrofor. Syst. 2010, 80, 303–313. [Google Scholar] [CrossRef]
- Leal, J.; Santos, R.; Gaiotto, F. Effect of selective logging on genetic diversity and gene flow in Cariniana legalis sampled from a cacao agroforestry system. Genet. Mol. Res. 2014, 13, 626–635. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Shaded Perennial Agroforestry Systems. In An Introduction to Agroforestry: Four Decades of Scientific Developments; Springer International Publishing: Cham, Switzerland, 2021; pp. 137–168. [Google Scholar]
- Waqar, Z.; Fernandes, A.K.C.; Conceição, T.A.; Gaiotto, F.A. Genetic Diversity and Differentiation in Plathymenia reticulata Benth.: A Comparative Study of Forest and Cocoa Agroforest Systems in the Atlantic Forest Domain. Diversity 2025, 17, 129. [Google Scholar] [CrossRef]
- Cabral, J.P.; Faria, D.; Morante-Filho, J.C. Landscape composition is more important than local vegetation structure for understory birds in cocoa agroforestry systems. For. Ecol. Manag. 2021, 481, 118704. [Google Scholar] [CrossRef]
- Kyndt, T.; Assogbadjo, A.E.; Hardy, O.J.; Glele Kakaï, R.; Sinsin, B.; Van Damme, P.; Gheysen, G. Spatial genetic structuring of baobab (Adansonia digitata, Malvaceae) in the traditional agroforestry systems of West Africa. Am. J. Bot. 2009, 96, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Danieli-Silva, A.; Varassin, I.G. Breeding system and thrips (Thysanoptera) pollination in the endangered tree Ocotea porosa (Lauraceae): Implications for conservation. Plant Species Biol. 2013, 28, 31–40. [Google Scholar] [CrossRef]
- Bortolanza Pereira, F.; Sebbenn, A.; Boshier, D.; Rossini, B.; Marino, C.; Freitas, M.L.; Rosa, J.R.; Vidal, E.; Tambarussi, E. Gene flow, mating patterns and inbreeding depression in Roupala montana var. brasiliensis, a neotropical timber species. New For. 2023, 55, 897–920. [Google Scholar] [CrossRef]
- Rogalski, J.M.; Reis, A.; Rogalski, M.; Montagna, T.; Dos Reis, M.S. Mating system and genetic structure across all known populations of Dyckia brevifolia: A clonal, endemic, and endangered rheophyte bromeliad. J. Hered. 2017, 108, 299–307. [Google Scholar] [CrossRef]
- Chybicki, I.J.; Oleksa, A. Seed and pollen gene dispersal in Taxus baccata, a dioecious conifer in the face of strong population fragmentation. Ann. Bot. 2018, 122, 409–421. [Google Scholar] [CrossRef]
- Browne, L.; Karubian, J. Habitat loss and fragmentation reduce effective gene flow by disrupting seed dispersal in a neotropical palm. Mol. Ecol. 2018, 27, 3055–3069. [Google Scholar] [CrossRef]
- Montagna, T.; Silva, J.; Pikart, T.; Reis, M. Reproductive ecology of Ocotea catharinensis, an endangered tree species. Plant Biol. 2018, 20, 926–935. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef]
- Rosa, T.L.M.; de Araujo, C.P.; Kamke, C.; Ferreira, A.; da Silva Ferreira, M.F.; de Oliveira, J.P.B.; Schmildt, E.R.; Lopes, J.C.; Mengarda, L.H.G.; Otoni, W.C. Sapucaia nut: Morphophysiology, minerals content, methodological validation in image analysis, phenotypic and molecular diversity in Lecythis pisonis Cambess. Food Res. Int. 2020, 137, 109383. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.P.; Mori, S.A.; Law, W.; Ribeiro, M. Conservation assessment of Lecythidaceae from eastern Brazil. Kew Bull. 2016, 71, 1–19. [Google Scholar] [CrossRef]
- Khanduri, V.P. Pollen limitation failing reproductive success in selected animal pollinated trees of tropical moist deciduous forest of north-eastern hill region, India. Hacquetia 2023, 22, 117–129. [Google Scholar] [CrossRef]
- Mori, S.A.; Prance, G.T.; De Zeeuw, C.; Prance, G.T. Zygomorphic-Flowered New World genera (Couroupita, Corythophora, Bertholletia, Couratari, Eschweilera, & Lecythis); The New Your Botanical Garden: New York, NY, USA, 1990. [Google Scholar]
- Díaz-Hernández, B.G.; Colombo, C.A.; Morales-Marroquín, J.A.; Sanitá-Rodrigues, M.; Azevedo-Filho, J.A.; Zucchi, M.I. Assessing the genetic vulnerability of Macaúba palm [Acrocomia aculeata (Jacq.) Lodd. ex Mart.] through the mating system and genetic diversity of open-pollinated progenies. Ann. Appl. Biol. 2024, 184, 238–249. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; Florence, C.T.; Mariano-Neto, E.; Gaiotto, F.A. First microsatellite markers for Lecythispisonis (Lecythidaceae), an important resource for Brazilian fauna. Conserv. Genet. Resour. 2015, 7, 437–439. [Google Scholar] [CrossRef]
- Doyle, J.J. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Ritland, K.; Jain, S.K. A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity 1981, 47, 35–52. [Google Scholar] [CrossRef]
- Ritland, K. Extensions of models for the estimation of mating systems using n independent loci. Heredity 2002, 88, 221–228. [Google Scholar] [CrossRef]
- Ritland, K. A Series of FORTRAN Computer Programs for Estimating Plant Mating Systems. J. Hered. 1990, 81, 236–237. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Breed, M.F.; Stead, M.G.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Which provenance and where? Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Tambarussi, E.; Boshier, D.; Vencovsky, R.; Freitas, M.; Di-Dio, O.; Sebbenn, A. Several small: How inbreeding affects conservation of Cariniana legalis Mart. Kuntze (Lecythidaceae) the Brazilian Atlantic Forest’s largest tree. Int. For. Rev. 2016, 18, 502–510. [Google Scholar] [CrossRef]
- Zhang, Z.; Gale, S.W.; Li, J.-H.; Fischer, G.A.; Ren, M.-X.; Song, X.-Q. Pollen-mediated gene flow ensures connectivity among spatially discrete sub-populations of Phalaenopsis pulcherrima, a tropical food-deceptive orchid. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Bawa, K.S. Plant-pollinator interactions in tropical rain forests. Annu. Rev. Ecol. Syst. 1990, 21, 399–422. [Google Scholar] [CrossRef]
- Eckert, C.G.; Kalisz, S.; Geber, M.A.; Sargent, R.; Elle, E.; Cheptou, P.-O.; Goodwillie, C.; Johnston, M.O.; Kelly, J.K.; Moeller, D.A. Plant mating systems in a changing world. Trends Ecol. Evol. 2010, 25, 35–43. [Google Scholar] [CrossRef]
- Sujii, P.S.; Tambarussi, E.V.; Grando, C.; de Aguiar Silvestre, E.; Viana, J.P.G.; Brancalion, P.H.; Zucchi, M.I. High gene flow through pollen partially compensates spatial limited gene flow by seeds for a Neotropical tree in forest conservation and restoration areas. Conserv. Genet. 2021, 22, 383–396. [Google Scholar] [CrossRef]
- Perrut-Lima, P.; Sebbenn, A.M.; Francisconi, A.F.; Picanço-Rodrigues, D.; Clement, C.R. Genetic diversity and mating system of in three localities along the lower Solimões River in Central Amazonia. Silvae Genet. 2023, 72, 81–91. [Google Scholar] [CrossRef]
- Mori, S.A.; Prance, G.T. The “sapucaia” group of Lecythis (Lecythidaceae). Brittonia 1981, 33, 70–80. [Google Scholar] [CrossRef]
- Mori, S.A. Biologia da polinização em Lecythidaceae. Acta Bot. Bras. 1987, 1, 121–124. [Google Scholar] [CrossRef]
- Mori, S.A.; Orchard, J.E.; Prance, G.T. Intrafloral Pollen Differentiation in the New World Lecythidaceae, Subfamily Lecythidoideae. Science 1980, 209, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.A.; Prance, G.T. Lecitidaceas: Familia da Castanha-Do-Para; Ministério da Agricultura, Pecuária e Abastecimento (MAPA): Rio de Janeiro, Brazil, 1983.
- Thornhill, N.W. The Natural History of Inbreeding and Outbreeding: Theoretical and Empirical Perspectives; University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Ismail, S.A.; Kokko, H. An analysis of mating biases in trees. Mol. Ecol. 2020, 29, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Winston, M.L. Bee Biology: Ecology and Natural History of Tropical Bees. David W. Roubik. Cambridge University Press, New York, 1989. x, 514 pp., illus. $69.50. Cambridge Tropical Biology Series. Science 1990, 248, 1026–1027. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.H.; Freitas, B.M. Colonização e biologia reprodutiva de mamangavas (Xylocopa frontalis) em um modelo de ninho racional. Ciênc. Rural 2003, 33, 693–697. [Google Scholar] [CrossRef]
- Haring, V.; Gray, J.E.; McClure, B.A.; Anderson, M.A.; Clarke, A.E. Self-incompatibility: A self-recognition system in plants. Science 1990, 250, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.S. Breeding systems of trees in a tropical wet forest. N. Z. J. Bot. 1979, 17, 521–524. [Google Scholar] [CrossRef]
- Fujii, S.; Kubo, K.-I.; Takayama, S. Non-self-and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Wadt, L.d.O.; Baldoni, A.; Silva, V.; Campos, T.d.; Martins, K.; Azevedo, V.; Mata, L.; Botin, A.; Hoogerheide, E.; Tonini, H. Mating system variation among populations, individuals and within and among fruits in Bertholletia excelsa. Silvae Genet. 2015, 64, 248–259. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Ramos, S.L.F.; Ferreira, M.J.; Lopes, R.; Meneses, C.H.S.G.; Valente, M.S.F.; da Silva, R.F.; Batista, J.d.S.; Muniz, A.W.; Lopes, M.T.G. Mating System Analysis and Genetic Diversity of Parkia multijuga Benth. One Native Tree Species of the Amazon. Forests 2024, 15, 172. [Google Scholar] [CrossRef]
- O’Malley, D.; Buckley, D.; Prance, G.; Bawa, K. Genetics of Brazil nut (Bertholletia excelsa Humb. & Bonpl.: Lecythidaceae) 2. Mating system. Theor. Appl. Genet. 1988, 76, 929–932. [Google Scholar]
- Gusson, E.; Sebbenn, A.M.; Kageyama, P.Y. Mating system in Eschweilera ovata (Cambess.) Miers populations. Rev. Árvore 2006, 30, 491–502. [Google Scholar] [CrossRef]
- Tambarussi, E.V. Contemporary Gene Flow, Mating System, and Spatial Genetic Structure in a Jequitibá-Rosa (Cariniana legalis Mart. Kuntze) Fragmented Population by Microsatellite Markers. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2013. [Google Scholar]
- Guidugli, M.; Nazareno, A.; Feres, J.; Contel, E.; Mestriner, M.; Alzate-Marin, A. Small but not isolated: A population genetic survey of the tropical tree Cariniana estrellensis (Lecythidaceae) in a highly fragmented habitat. Heredity 2016, 116, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Feres, J.M.; Nazareno, A.G.; Borges, L.M.; Guidugli, M.C.; Bonifacio-Anacleto, F.; Alzate-Marin, A.L. Depicting the mating system and patterns of contemporary pollen flow in trees of the genus Anadenanthera (Fabaceae). PeerJ 2021, 9, e10579. [Google Scholar] [CrossRef]
- Baldoni, A.; Wadt, L.; Campos, T.; Silva, V.; Azevedo, V.; Mata, L.; Botin, A.; Mendes, N.; Tardin, F.; Tonini, H. Contemporary pollen and seed dispersal in natural populations of Bertholletia excelsa (Bonpl.). Genet. Mol. Res. 2017, 16, gmr16039756. [Google Scholar] [CrossRef] [PubMed]
- Lôbo, R.N.B.; Villela, L.; Facó, O. Programas de Melhoramento Genético de Caprinos e Ovinos: Importância Prática. In Simpósio de Caprinos e Ovinos da Escola de Veterinária da UFMG; Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 2005. [Google Scholar]
- Yu, D.; Gu, X.; Zhang, S.; Dong, S.; Miao, H.; Gebretsadik, K.; Bo, K. Molecular basis of heterosis and related breeding strategies reveal its importance in vegetable breeding. Hortic. Res. 2021, 8, 120. [Google Scholar] [CrossRef]
- Holsinger, K.E. Reproductive systems and evolution in vascular plants. Proc. Natl. Acad. Sci. USA 2000, 97, 7037–7042. [Google Scholar] [CrossRef] [PubMed]
- Sabadin, F.; DoVale, J.C.; Platten, J.D.; Fritsche-Neto, R. Optimizing self-pollinated crop breeding employing genomic selection: From schemes to updating training sets. Front. Plant Sci. 2022, 13, 935885. [Google Scholar] [CrossRef]
- Perfecto, I.; Vandermeer, J. Biodiversity conservation in tropical agroecosystems: A new conservation paradigm. Ann. New York Acad. Sci. 2008, 1134, 173–200. [Google Scholar] [CrossRef]
- Faria, D.; Laps, R.R.; Baumgarten, J.E.; Cetra, M. Bat and Bird Assemblages from Forests and Shade Cacao Plantations in Two Contrasting Landscapes in the Atlantic Forest of Southern Bahia, Brazil. Biodivers. Conserv. 2006, 15, 587–612. [Google Scholar] [CrossRef]
- Faria, D.; Paciencia, M.; Dixo, M.; Laps, R.; Baumgarten, J. Ferns, frogs, lizards, birds and bats in forest fragments and shade cacao plantations in two contrasting landscapes in the Atlantic forest, Brazil. Biodivers. Conserv. 2007, 16, 2335–2357. [Google Scholar] [CrossRef]
- Dawson, I.K.; Guariguata, M.R.; Loo, J.; Weber, J.C.; Lengkeek, A.G.; Bush, D.; Cornelius, J.P.; Guarino, L.; Kindt, R.; Orwa, C.; et al. What is the relevance of smallholders’ agroforestry systems for conserving tropical tree species and genetic diversity in circa situm, in situ and ex situ settings? A review. Biodivers. Conserv. 2013, 22, 301–324. [Google Scholar] [CrossRef]
- Sambuichi, R.H.R. Fitossociologia e diversidade de espécies arbóreas em cabruca (mata atlântica raleada sobre plantação de cacau) na Região Sul da Bahia, Brasil. Acta Bot. Bras. 2002, 16, 89–101. [Google Scholar] [CrossRef]
- Sambuichi, R.H.R. Estrutura e dinâmica do componente arbóreo em área de cabruca na região cacaueira do sul da Bahia, Brasil. Acta Bot. Bras. 2006, 20, 943–954. [Google Scholar] [CrossRef]
- Uezu, A.; Beyer, D.; Metzger, J.P. Can agroforest woodlots work as stepping stones for birds in the Atlantic forest region? Biodivers. Conserv. 2008, 17, 1907–1922. [Google Scholar] [CrossRef]
- Sambuichi, R.H.R.; Mielke, M.S.; Pereira, C.E. Nossas Árvores: Conservação, Uso e Manejo de Árvores Nativas no Sul da Bahia; Editus: Luxembourg, 2009. [Google Scholar]
- Sambuichi, R.H.R.; Vidal, D.B.; Piasentin, F.B.; Jardim, J.G.; Viana, T.G.; Menezes, A.A.; Mello, D.L.N.; Ahnert, D.; Baligar, V.C. Cabruca agroforests in southern Bahia, Brazil: Tree component, management practices and tree species conservation. Biodivers. Conserv. 2012, 21, 1055–1077. [Google Scholar] [CrossRef]
- Tabarelli, M.; Aguiar, A.V.; Ribeiro, M.C.; Metzger, J.P.; Peres, C.A. Prospects for biodiversity conservation in the Atlantic Forest: Lessons from aging human-modified landscapes. Biol. Conserv. 2010, 143, 2328–2340. [Google Scholar] [CrossRef]
| Primer | Repeat Motif | Primer Sequences (5′–3′) | Range (bp) | Ta (°C) | GenBank No. |
|---|---|---|---|---|---|
| Lec31 | (GA)n | F: AGCCTGACATGAGTTCAG AAG R: TGAGCACCATAAGTTACGTC | 180–186 | 58 | KM92086 |
| Lec41 | (GA)n | F: TGAGTGAGCGAGTAAGGAATG R: CACGTTTTGGTTTTGTTGAG | 224–230 | 58 | KM92089 |
| Lec50 | (GA)n | F: TGACATTACAAAGAGTATGG R: TTGAAGATGTTGTTGTTGAG | 149–151 | 58 | KM92092 |
| Lec144 | (GA)n | F: TTGAGTTGGTAAGTGGAAATG R: AGGTTGTTTGAGTGGGAG | 224–230 | 58 | KM92098 |
| Lec272 | (GA)n | F: CAGTTTTGTTGTTGTTGAG R: GACCTTTGTTTTGGAGGT | 142–146 | 60 | KM92099 |
| Lec166 | (TCA)n | F: AGGCTGACCTGAGATGG R: AGTTAGAGGTTGTTGAGAG | 237–253 | 58 | KM92090 |
| Lec172 | (GA)n | F: TGAGTGTTGTTGGTTGAG R: AGGTTGAGTTGTTGTTGA | 195–205 | 58 | KM92095 |
| Lec195 | (GA)n | F: TGAGTTGTTGTTGTTGAG R: GACGTTTTGGTTTTGTTGAG | 149–159 | 58 | KM92096 |
| Parameters | Estimated | Standard Deviation |
|---|---|---|
| Fm | 0.431 | 0.103 |
| Tm | 1.000 | 0.000 |
| Ts | 0.960 | 0.011 |
| Tm–Ts | 0.040 | 0.010 |
| Rt | 0.000 | 0.003 |
| Rpm | 0.306 | 0.064 |
| Progeny | Mother Plant | Father Plant | LOD | Distance of Pollen Flow (km) |
|---|---|---|---|---|
| P11 | F6 | F60 | 1.86 × 10¹⁴ | 1.16 |
| P20 | F6 | F60 | 4.94 × 10¹⁴ | 1.16 |
| P28 | F60 | F69 | 7.41 × 10¹³ | 0.25 |
| P31 | F60 | F66 | 2.18 × 10¹⁴ | 0.29 |
| P101 | F76 | F376 | 1.06 × 10¹⁴ | 10.81 |
| P104 | F76 | F354 | 1.08 × 10¹⁴ | 7.83 |
| P144 | F87 | F354 | 2.46 × 10¹⁴ | 0.25 |
| P147 | F92 | F48 | 1.01 × 10¹⁴ | 15.12 |
| P264 | F376 | F361 | 5.22 × 10¹⁴ | 15.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqar, Z.; Rodrigues, A.B.; Florence, C.T.; Mariano Neto, E.; Gaiotto, F.A. Reproductive Ecology of Lecythis Pisonis in Brazilian Agroforestry Systems: Implications for Conservation and Genetic Diversity. Forests 2025, 16, 718. https://doi.org/10.3390/f16050718
Waqar Z, Rodrigues AB, Florence CT, Mariano Neto E, Gaiotto FA. Reproductive Ecology of Lecythis Pisonis in Brazilian Agroforestry Systems: Implications for Conservation and Genetic Diversity. Forests. 2025; 16(5):718. https://doi.org/10.3390/f16050718
Chicago/Turabian StyleWaqar, Zubaria, Acácia Brasil Rodrigues, Ciro Tavares Florence, Eduardo Mariano Neto, and Fernanda Amato Gaiotto. 2025. "Reproductive Ecology of Lecythis Pisonis in Brazilian Agroforestry Systems: Implications for Conservation and Genetic Diversity" Forests 16, no. 5: 718. https://doi.org/10.3390/f16050718
APA StyleWaqar, Z., Rodrigues, A. B., Florence, C. T., Mariano Neto, E., & Gaiotto, F. A. (2025). Reproductive Ecology of Lecythis Pisonis in Brazilian Agroforestry Systems: Implications for Conservation and Genetic Diversity. Forests, 16(5), 718. https://doi.org/10.3390/f16050718







