Abstract
Phenology indicates the response of ecosystem dynamics to climate change. Shrubs are an important component of alpine forests, and play a key role in forest ecosystem function, especially in semiarid alpine regions. In 2015, we observed the dynamics of phenology in five shrub communities along an elevation gradient (2600–3300 m) in the Qilian Mountains. Our results showed that the length of the growing season decreased from 153 days for Caragana tangutica to 150 days for Berberis diaphana, 149 days for Potentilla fruticosa L., 144 days for Caragana jubata (Pall.) Poir., and 134 days for Salix gilashanica C. Wang et P. Y. Fu. The accumulated temperature of the five shrub communities during the growing season varied from 1735.4 °C for C. tangutica to 1051.3 °C for C. jubata. The beginning of the growing season was earlier at lower, than at higher, elevations, while the end of the growing season was later at lower, than at higher, elevations. Elevation and aspect were two important environmental factors that affected shrub phenology. In our study, low temperature, coinciding with the occurrence of early frost, particularly in higher elevations, was the key factor in promoting end-of-season shrub growth cessation.
1. Introduction
Vegetation phenology is one of the most sensitive indicators of climate change [1,2,3]. Temperate forests represent one of the major types of vegetation at middle to high latitudes in the northern hemisphere, and globally act as a large carbon sink [4,5]. Thus, a better understanding of the phenological variables in temperate forests will improve the accuracy of vegetation models and estimates of regional carbon fluxes [6,7,8,9]. Although phenological changes may vary with species and geographic locations, numerous observations have shown that the advances in spring are followed by delays in autumn phenology, with a corresponding lengthening of the plant growing season [10,11,12,13]. These shifts, which are correlated with changes in temperature, are ecological responses to anthropogenic global change [14,15,16,17].
Northwestern China has experienced a rapid temperature increase of 0.26 °C per decade during the past 50 years, higher than the national average increase of 0.14 °C per decade [18]. Climate anomalies in the area resulted in profound changes in subalpine shrubs, including alterations in the temporal niche of phenophases and the dynamic interaction with ambient conditions [19]. A study of the relationships between shrub phenology and meteorological factors will help us understand the characteristics and driving mechanisms of the responses of shrub phenology to climate change under different climatic conditions [20].
Studies concerning the response of plant phenology to global climate change can be divided into two categories. Some used the Normalized Difference Vegetation Index (NDVI) to distinguish different vegetation types for simulation, while others used averages of the annual, seasonal, and monthly temperatures, or the maximum and minimum temperatures, combined with observations of plant phenology [19,21,22,23,24,25,26]. However, phenological studies should also emphasize measurements of leaf area indices, or radiation above and below the canopy to determine the relationship between environmental factors and leaf phenology. In addition, litter fall and leaf fall observations should also be given priority [27]. Soil moisture may also affect the phenology of woody cover [3]. In water-limited areas, variations of precipitation are expected to modify phenological patterns of species, while higher temperatures may lead to an advanced spring phenology [28,29].
Accurate estimates of canopy phenology are critical for quantifying carbon and water exchange between forests and the atmosphere, and predicting the response of forest ecosystems to climate change [26,30]. Previous studies of phenology in the Qilian Mountains in Northwestern China were largely based on remote sensing data, while field observations of phenology were scarce due to limited accessibility [18,19,31,32]. Although field measurements are time-consuming and laborious, their accuracy is unmatched by remote sensing observations. In this field study, we focused on the temperate subalpine shrublands in a semiarid alpine catchment in the Qilian Mountains. The phenophase variation in five typical shrubs along an elevation gradient was observed during the 2015 growing season. The objectives of this study were to (1) investigate the variability in phenology of shrubs along an elevation gradient; and (2) determine the relationships between temperature, topography, and phenophase changes.
2. Materials and Methods
2.1. Study Site
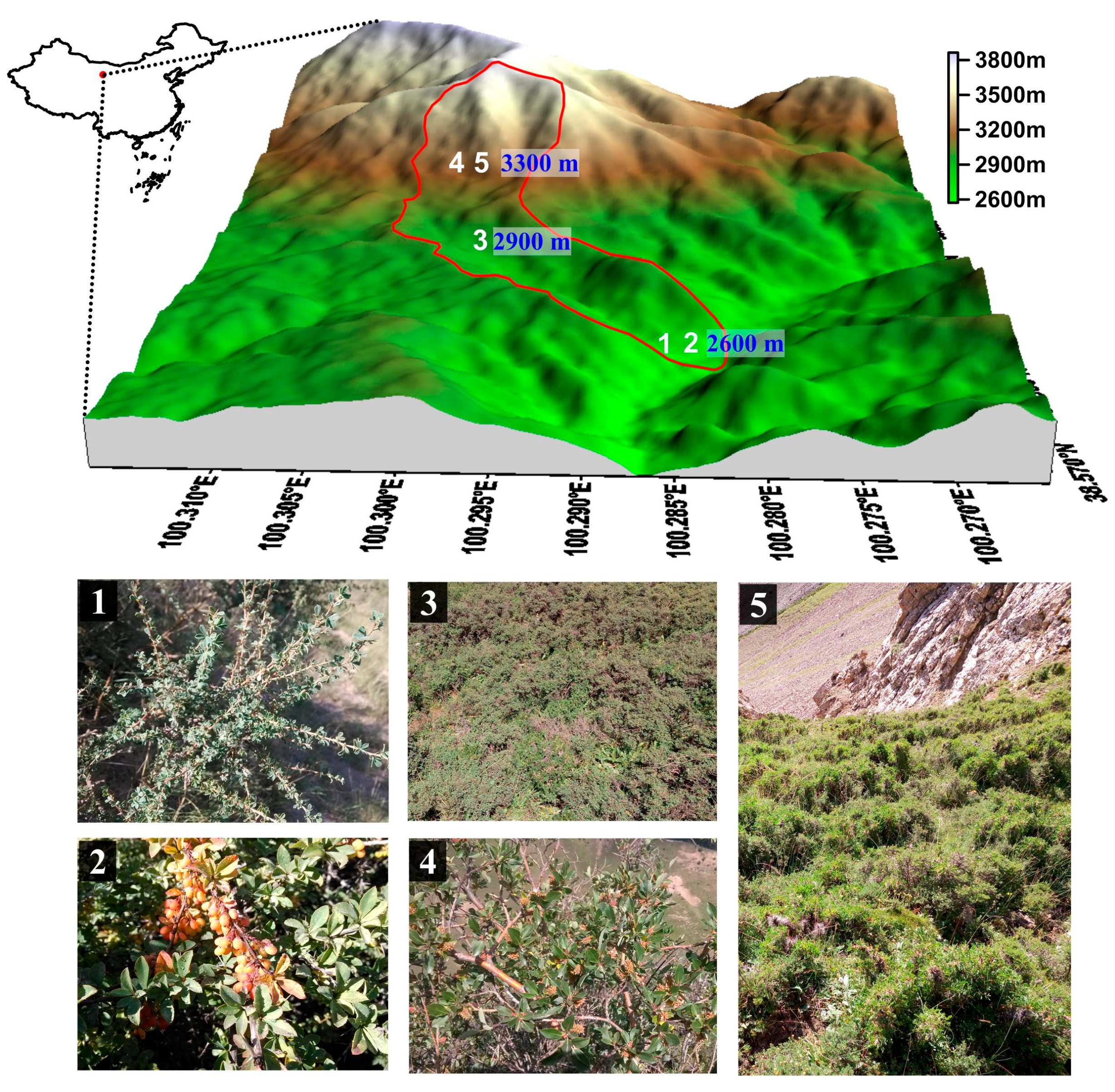
The study was conducted at five shrubland sites along an elevation gradient in a typical catchment of the Qilian Mountains (100°17′–100°18′ E, 38°32′–38°33′ N). The area of the catchment is 2.85 km2 at an elevation from 2500–3796 m. The catchment is characterized by a semiarid climate with an annual average temperature of 1.6 °C, and annual average precipitation in 2015 of 353.1 mm at the base of the mountains. From 2013–2015, seasonal precipitation was uneven, and precipitation from May to September accounted for almost 85% of that for the year. In 2015, the duration of annual sunshine reached 1892.6 h, and annual evaporation was 1081.7 mm. Spatial variability in topography, precipitation, and temperature led to significant differences in soil properties and vegetation communities. Along the elevation gradient, alpine meadow soil exhibited the highest accumulation of organic matter, followed by grey cinnamon soil, chestnut soil, and subalpine shrub meadow soil. Organic carbon and total nitrogen decreased with soil depth. From lower elevation to higher elevation, the vegetation types are mountain, forest grasslands, subalpine shrub meadows, and alpine meadows, respectively. The corresponding soil types are chestnut soil, grey cinnamon soil, subalpine shrub meadow soil, and alpine meadow soil, respectively [33]. Forests, dominated by Qinghai spruce (Picea crassifolia), are mainly distributed on the shady and semi-shady slopes at 2500–3300 m. Shrublands mainly occupy footslope positions of shady slopes and areas above the tree line. The main shrub species include Caragana tangutica, Berberis diaphana, and Potentilla fruticosa L. widely distributed at elevations <2900 m, and Caragana jubata (Pall.) Poir., and Salix gilashanica C. Wang et P. Y. Fu found above 3100 m. The five shrubs are woody perennials. The main herbaceous plants are Polygonum viviparum L., Carex atrata L., and Stipa capillata Linn. Study area with sample plots (numbers 1–5) and main shrub species was showed in Figure 1. Sample plots information was showed in Table 1.
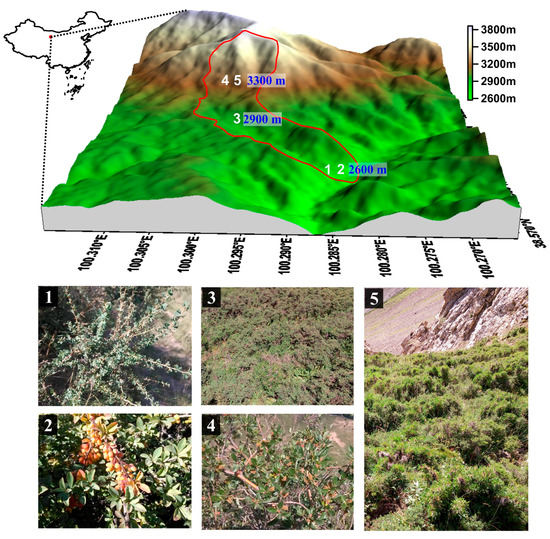
Figure 1.
Study area with sample plots (numbers 1–5) and main shrub species. (1) Caragana tangutica; (2) Berberis diaphana; (3) Potentilla Fruticusa L.; (4) Salix Gilashanica C. Wang et P. Y. Fu; and (5) Caragana Jubata (Pall.) Poir.

Table 1.
Sample plot information.
2.2. Phenological Observations
This work was guided by the “Observation Methodology for Long-term Forest Ecosystem Research” developed by the National Standards of the People’s Republic of China (GB/T 33027-2016). We selected five species for our study: C. tangutica, B. diaphana, P. fruticusa, C. jubata, and S. gilashanica, growing along an elevation gradient at 2600 m to 3300 m in 2015. We chose a growing season as our aim to investigate the effects of topography on forests under climate change. Three quadrats (10 m × 10 m) were established for each shrub species. We randomly chose five individual shrubs exhibiting normal growth, flowering, and fruiting in each quadrat to observe changes in phenophases as per GB/T 33027-2016. A total of 15 individual shrubs of each species were tracked in the entire elevation gradient. Phenological observations began at budding date, and ended when the leaves started to fall, spanning a growing season. At the beginning and end of the growing season, phenological observations were made daily. During the growing season, we adjusted the observation intervals because of faster growth of shrubs in the middle growing season. In addition, air temperature data were obtained from the nearby automated meteorological stations at 2600, 2900, and 3300 m.
2.3. Sample Plot Investigation
Three quadrats per species were established to investigate shrub height, and basal and crown diameters. The elevation, latitude, and longitude of the quadrat were obtained using a global positioning system. We used a compass to measure the slope aspect, and a declinometer to measure the slope degree. The soil profile was excavated to determine soil type and depth. The line transect method [20] was used to measure percent cover of five shrub species in this study.
2.4. Data Processing
The methodology to calculate the duration of each phenological stage included transforming the date and time to Julian date (1 January was day 1; 31 December was day 365) [34], and we used Julian days for dates of budding, leaf opening, flowering, fruiting, leaf discoloration, and leaf falling. The length and Tacc of these phenophases were calculated based on the formulas below [13,34]:
where di was the length of phenophase i; a was the starting day of phenophase i; b was the end day of phenophase i; ati was the Tacc of phenophase i; and tj was the average temperature of phenophase i on day j. We used Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington, WA, USA) for data processing and mapping, and SPSS 21.0 software (SPSS Inc., IBM, Chicago, IL, USA) for statistical measures.
3. Results
3.1. Growth Parameters of Five Shrub Species
There were significant differences in height and canopy diameter of the five shrub species (p = 0.05) (Table 2). The branch diameter of P. fruticosa was significantly lower than that of the other four shrub species. Shrubs at lower elevations were taller than those at high elevations. In addition, the shrubs growing on the sunny slope were significantly taller than shrubs growing on semi-sunny slopes at the same elevation (Table 2).

Table 2.
Basic parameters of five shrubs species.
3.2. Duration of Phenological Stages of the Five Shrub Species
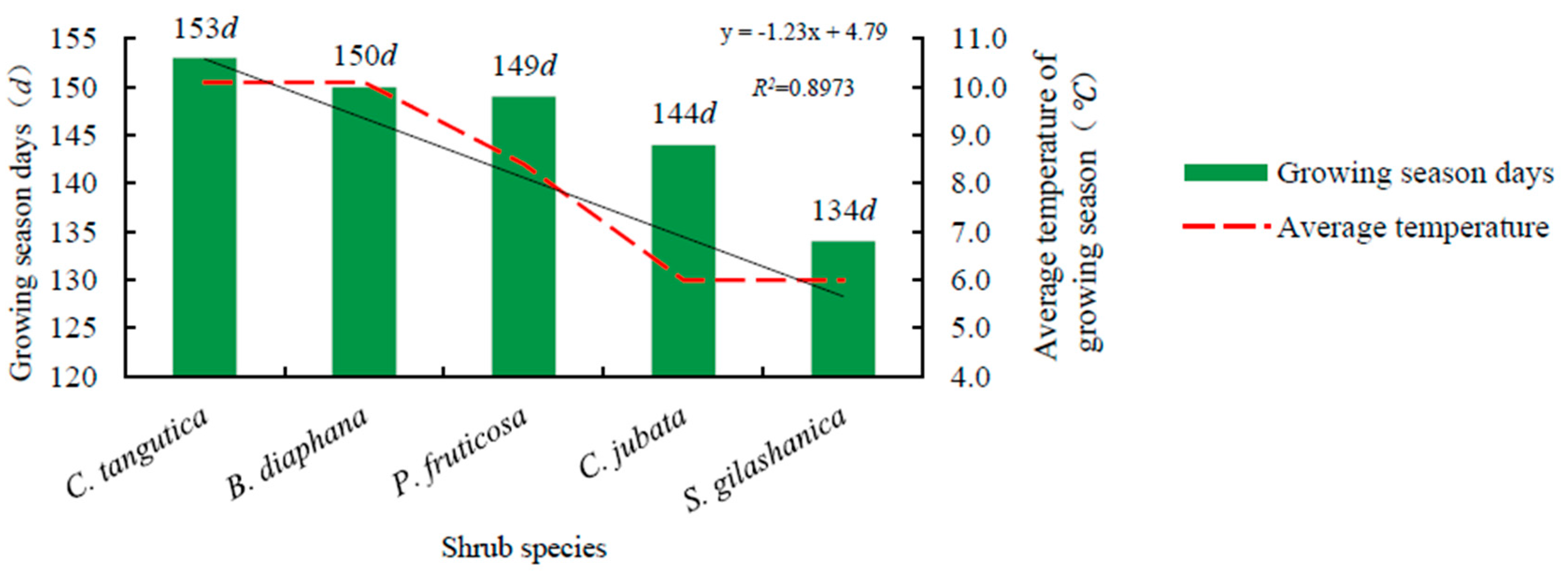
All observed shrub species generally started to bud from April to May (Table 3). C. tangutica at 2600 m budded about eight days earlier than C. jubata and S. gilashanica at 3300 m. B. diaphana at 2600 m was the earliest to open leaf, bear fruit, and change leaf color. The length of the growing season in the five shrub species decreased in the order of: C. tangutica (153 days), B. diaphana (150 days), P. fruticosa (149 days), C. jubata (144 days), and S. gilashanica (134 days). The average length of the growing season was 146 days, which was positively correlated to the average temperature of the growing season at the study site (R2 = 0.8973) (Figure 2).

Table 3.
Start and end dates of specific phenophases and their duration for five shrub species in 2015.
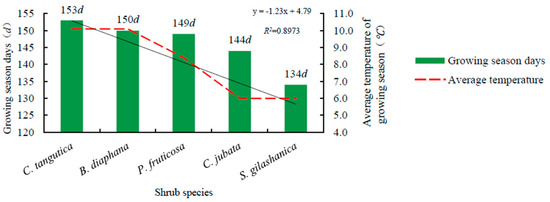
Figure 2.
Relationship between the growing season length and the average temperature.
Interspecific differences and slope aspect may have led to the shorter growing season in C. jubata than that in S. gilashanica. Similarly, the length of the growing season in B. diaphana was less than that in C. tangutica. Five shrub species began to change color in late August, and leaves started falling in the middle of September as the lowest temperature was a key driving factor leading to a dormant state based on our observations.
3.3. Variability in Temperature and Tacc
3.3.1. Changes in Temperature
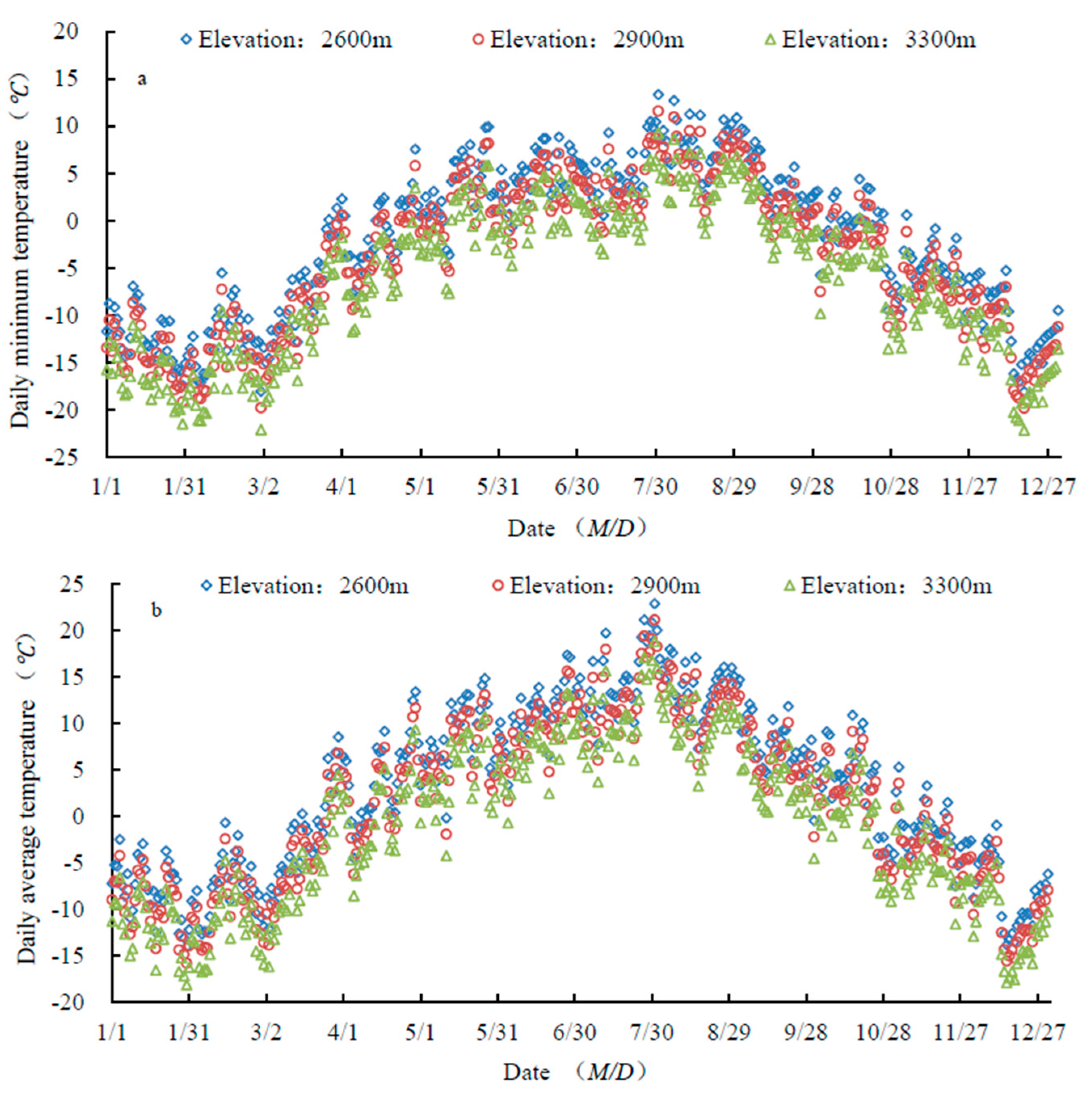
Daily average and minimum temperatures decreased with increasing elevation (Figure 3). Both temperatures peaked in late August, and started to drop in early September (Figure 3) (Supplementary Materials).
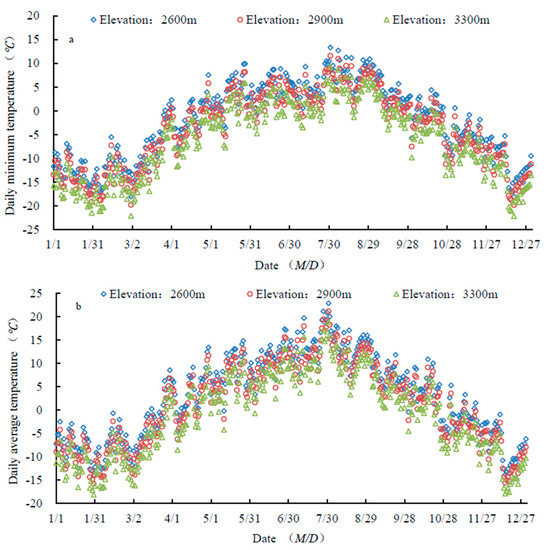
Figure 3.
Variability in daily minimum temperature (a) and daily average temperature (b).
C. tangutica and B. diaphana began to change leaf color when the minimum temperature reached 9.5 °C, and their leaves began to fall when the minimum was 4.4 °C. P. fruticosa began to change leaf color when the minimum was 5.6 °C, and its leaf fall started when the minimum was 2.7 °C. C. jubata and S. gilashanica began to change leaf color when the minimum was 5.5 °C. Leaf fall in C. jubata began when minimum T was 2.3 °C, and in S. gilashanica, when it was −2.9 °C. The first frost occurred on 18 September. At this time, the minimum temperatures were 2.8 °C, 1.1 °C, and −1.2 °C at 2600 m, 2900 m, and 3300 m, respectively.
3.3.2. Variability in Tacc in 2015
The start of growing season varied from the 26 April in C. tangutica, to the 5 May for C. jubata and S. gilashanica. The end of growing season varied from 15 September in C. jubata, to 26 September for P. fruticosa (Table 3).
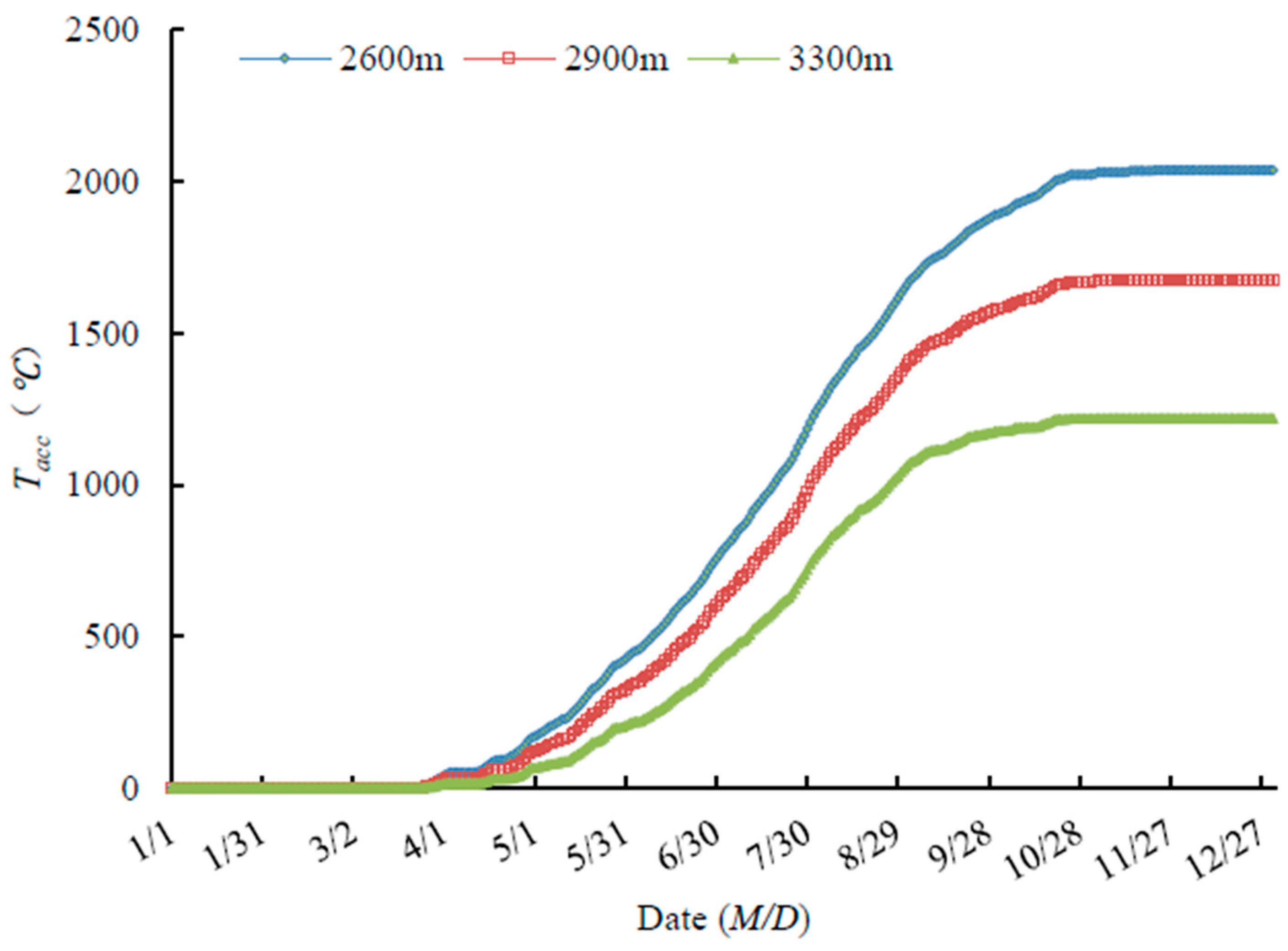
Tacc began to increase at the end of April, when the shrubs began to bud break. Therefore, Tacc is an important factor promoting shrub growth. In late September, Tacc no longer increased, and growth cessation began and shrubs started to become dormant. At this time, low temperature was an important factor promoting shrub dormancy. Fruit of all five species started to ripen in early August as the Tacc reached the maximum (Figure 4).
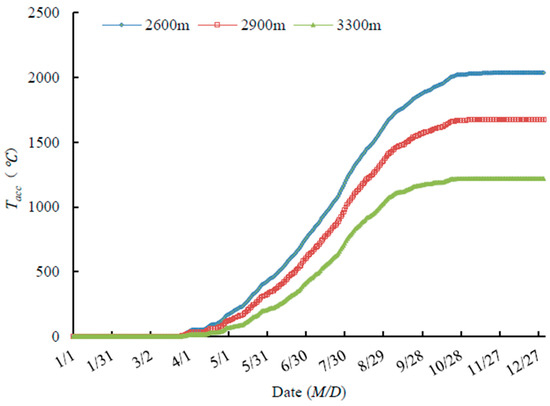
Figure 4.
A Tacc at different elevations.
3.3.3. Tacc of Different Phenophases
Shrub phenophases changed from one to the next only when temperature accumulated to specific levels (Table 4). C. jubata and S. gilashanica began to bud at lower Tacc than C. tangutica and B. diaphana. Specifically, budbreak in C. jubata and S. gilashanica occurred at 75.5 °C, while that in B. diaphana occurred at 144.7 °C. C. tangutica began to leaf fall at higher Tacc than C. jubata. Specifically, leaf fall in C. tangutica occurred at 1858.6 °C, while that in C. jubata occurred at 1126.8 °C.

Table 4.
Tacc of individual phenophases and their duration for each shrub.
Tacc for both, the beginning and end of each phenophase in high-elevation shrubs was lower than that in low-elevation shrubs. Tacc on the sunny slope was higher than that on the semi-sunny slope. During the growing period at 2600 m, Tacc within each phenophase increased before fruit ripening, and decreased after that. A similar pattern was observed in shrubs at 2900 m and 3300 m (Table 4) (Supplementary Materials).
4. Discussion
4.1. Phenophases of Shrub Species
The beginning of the growing season in the Qilian Mountains fell between the 120th and 160th day of the year, that is, between early May and early June [31]. In general, vegetation in the Qilian Mountains showed a trend of greening from the southeast to the northwest [32]. This trend may be associated with a decrease in precipitation from the southeast to the northwest. The results of our study showed that the average growing season of typical shrubs in the Qilian Mountains started on the 122nd day, and ended on the 267th day, with the average length of the growing season of 146 days. Zhao et al. (2015) showed that the growing season in the Qilian Mountains started on the 139th day, and ended on the 250th day, with the average length of the growing season spanning 112 days [31]. Temperature and species differences mainly determined the differences in growth and phenology of the five shrub species in our study.
4.2. Effects of Temperature on Shrub Phenology
Tacc is one of the most important factors affecting plant growth and, therefore, is of great significance in ecology and forest management [34]. Temperature is the main driver of phenological dynamics for many plants; in many cases, higher temperature is likely to accelerate plant development and lead to earlier switching to the next ontogenetic stage [8]. During our field observation, we found snow and frost at the beginning and end of the growing season, respectively. When snowfall occurred, vegetation growth slowed. Frost occurred in early September, and was followed by the onset of leaf drop. We speculated that the low temperature had a profound effect on phenological dynamics. A similar conclusion was drawn by Du et al. (2014) and He et al. (2015), who demonstrated the primary role of minimum temperature in controlling the start and the end of the growing season [18,19]. Gough et al. (2010) suggested that the end of season phenology may be less responsive to environmental variability than to genetics [5]. Furthermore, precipitation was coupled to a shift in maximum NDVI [28]. The restrictive role of minimum temperatures in the growth of subalpine plants requires further study as it is expected to be closely associated with the frequency of frost risk in spring and autumn [35,36].
Our study showed that the Tacc within each of the five phenophases in the five shrub communities first increased, then declined. The variability in Tacc was consistent with the length of the phenophase. Chang et al. (2012) also indicated that the variation in Tacc was largely caused by the length of the phenophase, while the temperature-induced variation was comparatively less. Chang et al. (2016) found that the length of phenophase could not be shortened or extended indefinitely [34], which was consistent with our study.
4.3. Effects of Topography on Shrub Phenology
4.3.1. Elevation
In this study, the growing season for C. tangutica and B. diaphana at 2600 m started earlier and ended later than that for C. jubata and S. gilashanica at 3300 m. As the elevation decreased, the beginning and end of the growing season showed a trend of advance and delay, respectively, and the length of the growing season increased. This was possibly associated with the limited heat at higher elevations.
The spatial patterns of vegetation phenology in the Dan Jiang Kou reservoir area indicated that the relatively longer length of season in the south resulted from an early start and late end of season. Regression models and correlation analysis indicated that elevation was moderately related to vegetation [32]. Further, a close connection was established between the growth of the main vegetation types and precipitation in the mid-Qilian Mountains, while temperature was the vital climatic factor for some special vegetation types, such as alpine meadows and coniferous forests, located in regions with high elevation or sufficient water resources [37]. Elevation may also be a key factor affecting vegetation phenology due to the different thermal conditions at different elevations.
4.3.2. Slope Aspect
In a mountainous ecosystem, slope aspect is also important topographic factors affecting water and heat dynamics [32]. Vegetation growth on shady slopes may be restricted by heat conditions, while increases in precipitation and warming may enhance vegetation productivity in such areas; the duration and flux intensity of solar radiation vary from sunny to shady slopes [38]. However, on sunny slopes in mountainous areas, increased evapotranspiration and an effect of soil water stress may be more significant than on shady slopes [3,39]. Yin et al. (2016) found that topographic variables, such as slope gradient and growing-season direct solar radiation may have minor influences on the growth-climate relationships [38]. Our study indicated that the growing season of C. tangutica on the sunny slope started earlier than that of B. diaphana on the semi-sunny slope. This result was consistent with previous results; however, the growing season of C. jubata on the sunny slope was shorter than that of S. gilashanica on the semi-sunny slope, which may be caused by interspecific differences.
Many other climatic factors need to be considered in any future studies; these may include potential changes in soil moisture and temperature, duration of insolation, effective solar radiation, and the depth of frozen soil. Moreover, identifying and quantifying climatic constraints on phenological dynamics in forests is a complex task that needs to be addressed at appropriate scales. This study is more or less descriptive in nature because of the limited sample sizes. Thus, we will choose greater sample sizes to study the relationship between phonology and climatic change in future.
5. Conclusions
In our study, the lowest temperature may be the environmental factor precipitating leaf discoloration and fall in shrubs, and the highest temperature may be the environmental factor leading to leaf expansion and fruit ripening. Shrub phenology will progress from the previous to the next phenophase as long as the Tacc reaches a certain level. The start date of high-elevation growth is relatively late, while the end date is relatively early. Low temperature at the beginning of the growing season may not be conducive to shrub bud break. Lower temperatures at the end of the growing season also promote shrub growth cessation, which may lead to a shortening of the growing season at high elevations. Frostbite may affect the growing season of shrubs and result in a shorter growing season at higher elevations. In addition, the slope aspect may be an important factor affecting plant phenology due to the difference in insolation and illumination among aspects.
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4907/9/2/58/s1.
Acknowledgments
The research was founded by the National Natural Science (91425301). This work was also supported by the CFERN AND BEIJING TECHNO SOLUTIONS Award Funds for excellent academic achievements. We thank Ming Jin, Wenmao Jing, and Hui Zhang for supporting our field observations. We also thank Meng Zhu of the University of the Chinese Academy of Sciences for revising grammar and content of this paper.
Author Contributions
X.L., G.L., and S.W. conceived and designed the experiments; Y.Z., W.Z., and J.M. performed the experiments; Y.Z. analyzed the data; and Y.Z. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontaillerc, J.Y.; Louveta, J.M.; Kremera, A.; Michaleta, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Luo, Z.K.; Osbert, J.S.; Ge, Q.S.; Xu, W.T.; Zheng, J.Y. Phenological responses of plants to climate change in an urban environment. Ecol. Res. 2007, 22, 507–514. [Google Scholar] [CrossRef]
- Josiane, S.; Aude, V.; Karine, P.; Remy, S.; Marc, A.; Nicolas, B.; Patricia, D.R.; Sylvie, G.; Marielle, G.; Abakar, H.M.; et al. Relationships between climate, soil moisture and phenology of the woody cover in two sites located along the West African latitudinal gradient. J. Hydrol. 2009, 375, 78–89. [Google Scholar]
- Frank, M.C.; Thomas, R. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar]
- Gough, C.M.; Flower, C.E.; Vogel, C.S.; Curtis, P.S. Phenological and Temperature Controls on the Temporal Non-Structural Carbohydrate Dynamics of Populus grandidentata and Quercus rubra. Forests 2010, 1, 65–81. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.L.; Sun, X.M.; Zhang, L.; Yu, G.R.; Ren, X.L.; Wang, J.Y.; Zhang, J.H. Species- and Community-Scale Simulation of the Phenology of a Temperate Forest in Changbai Mountain Based on Digital Camera Images. J. Resour. Ecol. 2013, 4, 317–326. [Google Scholar]
- Michael, A.W.; Peter, E.T.; Steven, W.R. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Glob. Biogeochem. Cycles 1997, 11, 217–234. [Google Scholar]
- Franz, W.B.; Alberte, B.; Kristin, B.; Daniel, D.; Wolfgang, L.; Jörg, S.; Stephen, S. Responses of spring phenology to climate change. New Phytol. 2004, 162, 295–309. [Google Scholar]
- Valdez, H.M.; Andrade, J.L.; Jackson, P.C.; Rebolledo, V.M. Phenology of five tree species of a tropical dry forest in Yucatan, Mexico: effects of environmental and physiological factors. Plant Soil 2010, 329, 155–171. [Google Scholar] [CrossRef]
- Richardson, A.D.; Bailey, A.S.; Denny, E.G.; Martin, C.W.; Keefe, J.O. Phenology of a northern hardwood forest canopy. Glob. Chang. Biol. 2006, 12, 1174–1188. [Google Scholar] [CrossRef]
- De Medeiros, D.P.W.; Lopes, A.V.; Zickel, C.S. Phenology of woody species in tropical coastal vegetation, northeastern Brazil. Funct. Ecol. Plants 2007, 202, 513–520. [Google Scholar] [CrossRef]
- Yuan, W.P.; Zhou, G.S.; Wang, Y.H.; Han, X.; Wang, Y.S. Simulating phenological characteristics of two dominant grass species in a semi-arid steppe ecosystem. Ecol. Res. 2007, 22, 784–791. [Google Scholar] [CrossRef]
- Chang, Z.F.; Zhu, S.J.; Han, F.G.; Zhong, S.N. Differences in response of desert plants of different ecotypes to climate warming: a case study in Minqin, Northwest China. J. Arid Land 2012, 4, 140–150. [Google Scholar] [CrossRef]
- Wolfe, D.W.; Schwartz, M.D.; Lakso, A.N.; Otsuki, Y.; Pool, R.M.; Shaulis, N.J. Climate change and shifts in spring phenology of three horticultural woody perennials in northeastern USA. Int. J. Biometeorol. 2005, 49, 303–309. [Google Scholar] [CrossRef] [PubMed]
- McEwan, R.W.; Brecha, R.J.; Geiger, D.R.; John, G.P. Flowering phenology change and climate warming in southwestern Ohio. Plant Ecol. 2011, 212, 55–61. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, C.H. Climate-associated changes in spring plant phenology in China. Int. J. Biometeorol. 2012, 56, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; He, Z.B.; Yang, J.J.; Chen, L.F.; Zhu, X. Detecting the effects of climate change on canopy phenology in coniferous forests in semi-arid mountain regions of China. Int. J. Remote Sens. 2014, 35, 6490–6507. [Google Scholar] [CrossRef]
- He, Z.B.; Du, J.; Zhao, W.Z.; Yang, J.J.; Chen, L.F.; Zhu, X.; Chang, X.X.; Liu, H. Assessing temperature sensitivity of subalpine shrub phenology in semi-arid mountain regions of China. Agric. For. Meteorol. 2015, 213, 42–52. [Google Scholar] [CrossRef]
- Li, C.H.; Pei, S.X.; Guo, Q.S.; Xin, X.B.; Hong, M. Phenological responses of three shrub species to climate change in Harbin, Heilongjiang province. J. Northeast For. Univ. 2011, 39, 58–61. (In Chinese) [Google Scholar]
- Pei, S.X.; Guo, Q.S.; Xin, X.B.; Hong, M.; Kang, Y. Research on plant phenological responses to climate change abroad. World For. Res. 2009, 22, 31–37. (In Chinese) [Google Scholar]
- Xie, B.N.; Qin, Z.F.; Wang, Y.; Chang, Q.R. Monitoring vegetation phenology and their response to climate change on Chinese Loess Plateau based on remote sensing. Trans. Chin. Soc. Agric. Eng. 2015, 31, 153–160. (In Chinese) [Google Scholar]
- Chen, X.Q.; Tan, Z.J.; Schwartz, M.D.; Xu, C.X. Determining the growing season of land vegetation on the basis of plant phenology and satellite data in Northern China. Int. J. Biometeorol. 2000, 44, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Oscar, G.; Juan, J.S. Long-term temporal changes of plant phenology in the Western Mediterranean. Glob. Chang. Biol. 2009, 15, 1930–1948. [Google Scholar]
- Gunderson, A.C.; Edwards, N.T.; Walker, A.V.; O’Hara, K.H.; Campion, C.M.; Hanson, P.J. Forest phenology and a warmer climate—Growing season extension in relation to climatic provenance. Glob. Chang. Biol. 2012, 18, 2008–2025. [Google Scholar] [CrossRef]
- Seyed, A.H.; Sasan, B.K.; Asadollah, M. Evaluating Beech Tree Phenology in a Deciduous Broadleaf Forest in Northern Iran Using Ground Observation and MODIS Images. J. Sustain. For. 2011, 30, 697–712. [Google Scholar]
- Menzel, A. Phenology: Its importance to the global change community. Clim. Chang. 2002, 54, 379–385. [Google Scholar] [CrossRef]
- Jolly, W.M.; Running, S.W. Effects of precipitation and soil water potential on drought deciduous phenology in the Kalahari. Glob. Chang. Biol. 2004, 10, 303–308. [Google Scholar] [CrossRef]
- Xie, S.C.; Sheng, C.Y.; Li, S.C. A phonological study on main tree species of montane humid evergreen broad-leaved forest in Ailao Mountains. Acta Ecol. Sin. 1997, 17, 53–55. (In Chinese) [Google Scholar]
- Gough, C.M.; Flower, C.E.; Vogel, C.S.; Dragoni, D.O.; Curtis, P.S. Whole ecosystem labile carbon production in a north temperate deciduous forest. Agric. For. Meteorol. 2009, 149, 1531–1540. [Google Scholar] [CrossRef]
- Zhao, Z.; Jia, W.X.; Zhang, Y.S.; Liu, Y.R.; Chen, J.H. Monitoring and change trends of phonological phase in the Qilian Mountains based on remote sensing. J. Desert Res. 2015, 35, 1388–1395. (In Chinese) [Google Scholar]
- Deng, S.F. Impacts of Climate Change on Vegetation in Qilian Mountains from 2000 to 2011; Lanzhou University: Lanzhou, China, 2013. [Google Scholar]
- Jiang, L. Research on Soil Genetic Characteristics and Taxonomic Classification of Typical Soil Types in the Xi-shui Forest Zone of the Qilian Mountains; Northwest Agricultural and Forestry University: Yangling, China, 2012. [Google Scholar]
- Chang, Z.F.; Zhao, M.; Han, F.G.; Zhong, S.N.; Li, F.M. Phenological characteristics of desert plant in Minqin Desert area. Sci. Silvae Sin. 2008, 44, 58–64. (In Chinese) [Google Scholar]
- Annie, D.A.; Sergio, R.; Tommaso, A.; Antonio, S. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar]
- Han, F.; Zhang, Q.; Buyantuev, A.; Niu, J.M.; Liu, P.T.; Li, X.H.; Kang, S.; Zhang, J.; Chang, C.M.; Li, Y.P. Effects of climate change on phenology and primary productivity in the desert steppe of Inner Mongolia. J. Arid Land 2015, 7, 251–263. [Google Scholar] [CrossRef]
- Peng, H.H.; Li, C.K.; Tang, Z.G.; Liang, J. Spatial patterns in the terrestrial vegetation phenology of Danjiangkou reservoir area and its relation with elevation. Resour. Environ. Yangtze Basin 2016, 25, 1626–1634. (In Chinese) [Google Scholar]
- Yin, Z.Y.; Li, M.; Zhang, Y.; Shao, X. Growth–climate relationships along an elevation gradient on a southeast-facing mountain slope in the semi-arid eastern Qaidam Basin, northeastern Tibetan Plateau. Trees 2016, 30, 1095–1109. [Google Scholar] [CrossRef]
- Crimmins, T.M.; Crimmins, M.A.; Bertelsen, C.D. Spring and summer patterns in flowering onset, duration, and constancy across a water-limited gradient. Am. J. Bot. 2013, 100, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).