The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus, Plants, and Growth Conditions
2.2. Protein Extraction, Trypsin Digestion and Isobaric Tag for Relative and Absolute Quantitation (Itraq) Analysis
2.3. LC-MS/MS Analysis and Protein Identification and Quantification
2.4. Bioinformatic Analysis
2.5. RNA Extraction and Real Time Quantitative RT-PCR (RT-qPCR)
2.6. Analysis of MTC-Related Metabolites
2.7. Statistics
3. Results
3.1. Protein Profiles of PVY-Infected Gala Plants at Normal and High Temperature
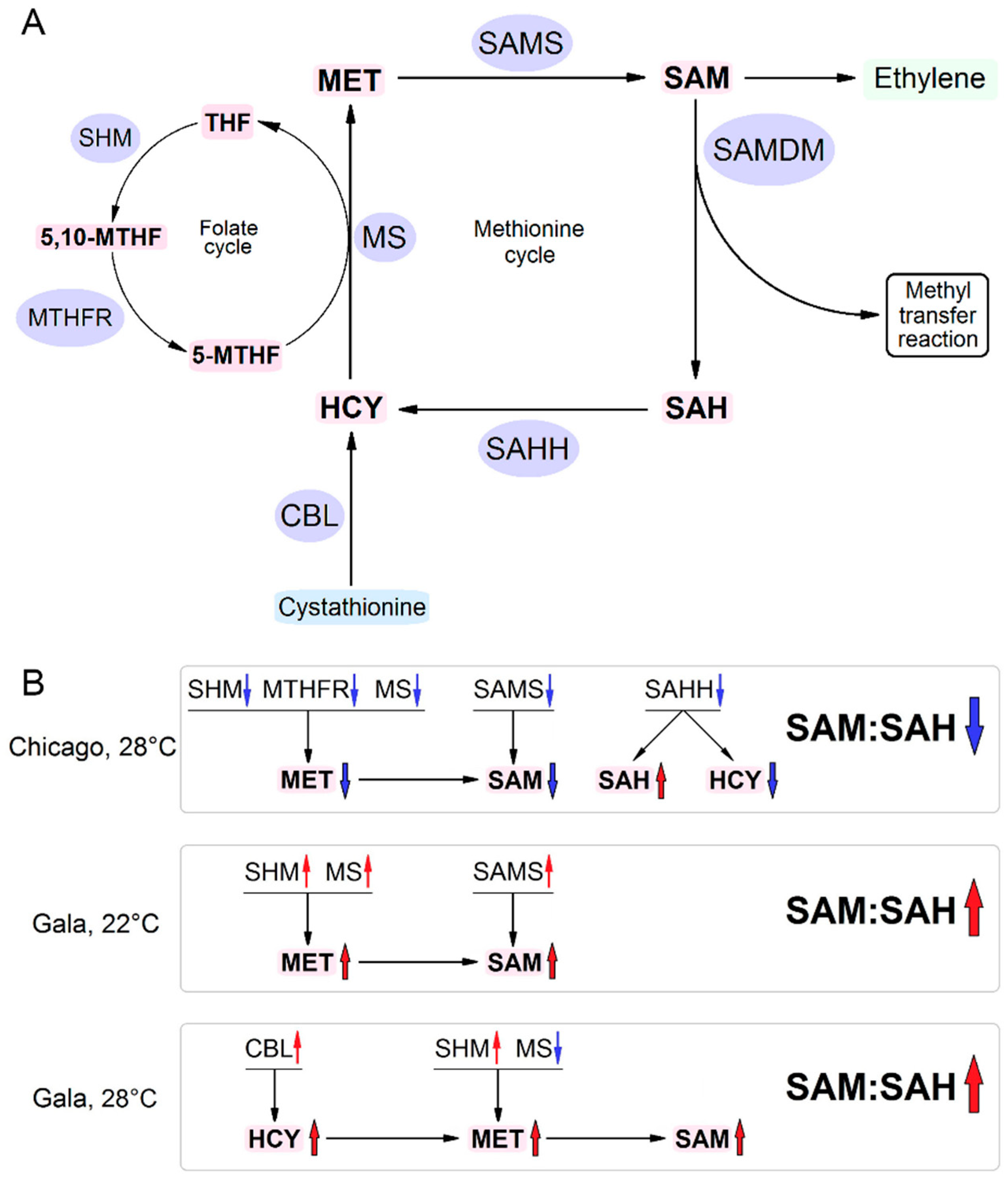
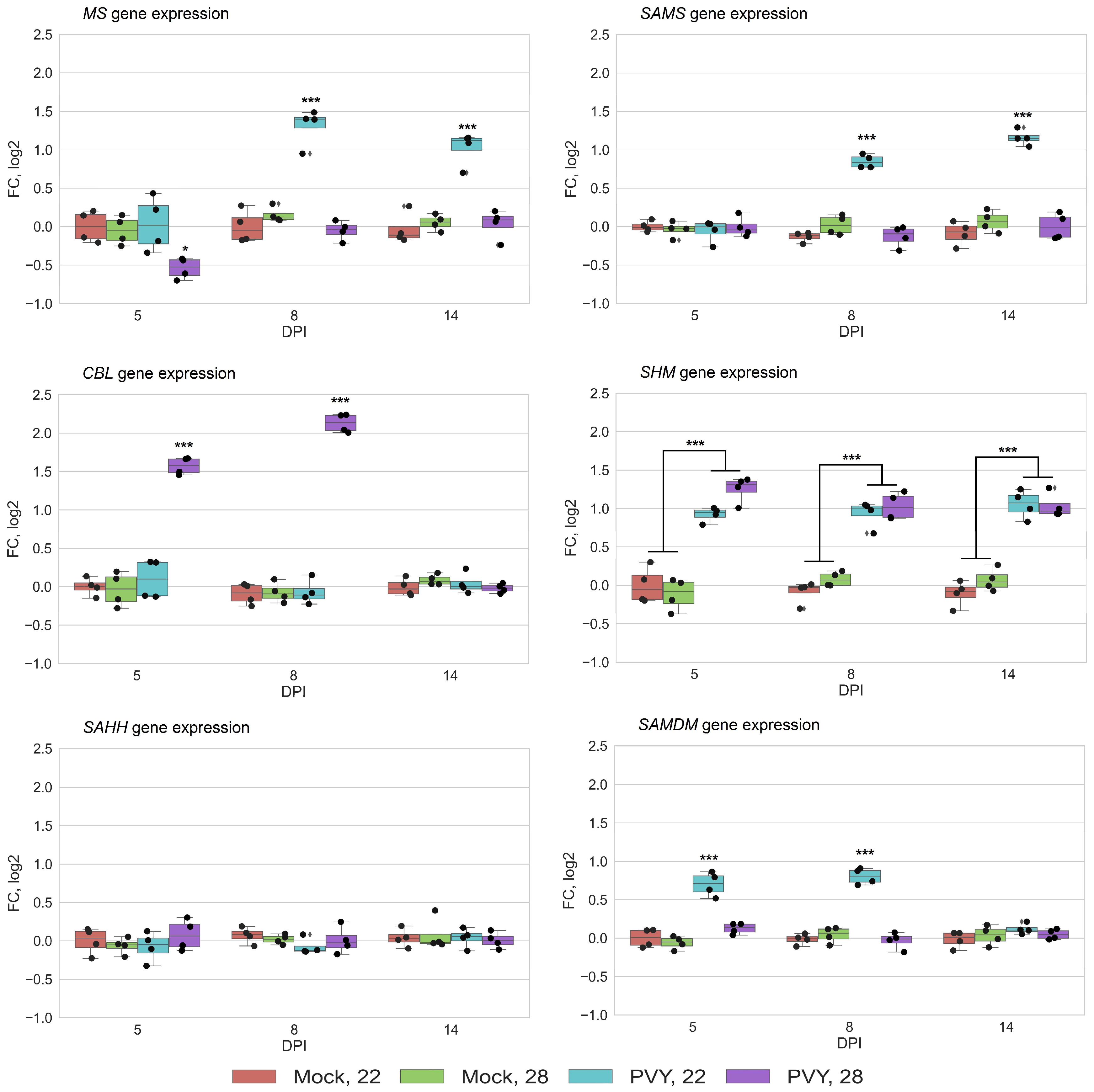
3.2. RNA Expression Levels of Key MTC-Related Genes
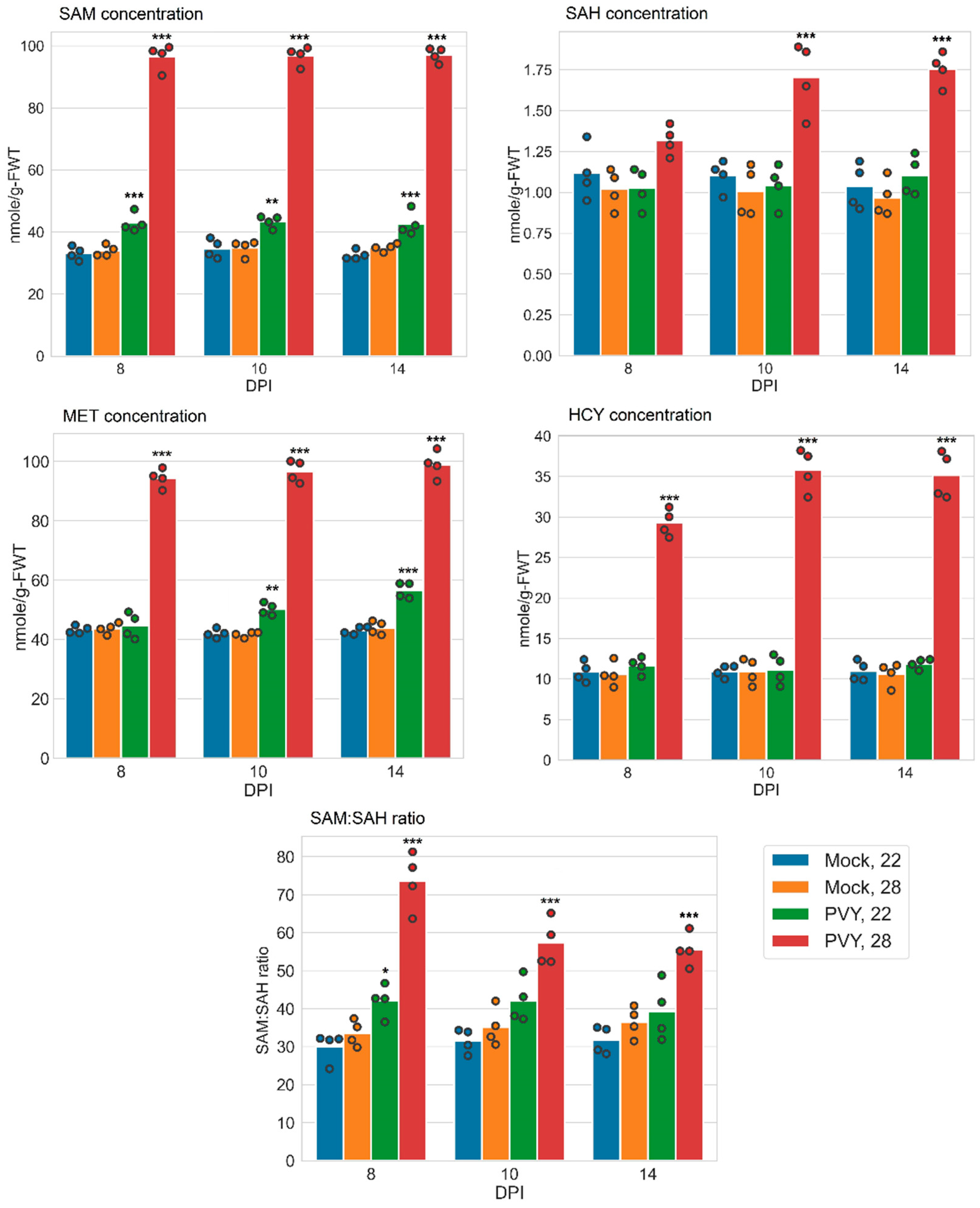
3.3. Accumulation of MTC Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elena, S.F.; Fraile, A.; García-Arenal, F. Chapter Three—Evolution and Emergence of Plant Viruses. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 88, pp. 161–191. [Google Scholar] [CrossRef] [Green Version]
- Syller, J.; Grupa, A. Antagonistic within-host interactions between plant viruses: Molecular basis and impact on viral and host fitness. Mol. Plant Pathol. 2016, 17, 769–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llave, C. Dynamic cross-talk between host primary metabolism and viruses during infections in plants. Curr. Opin. Virol. 2016, 19, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkinen, K.; De, S. The significance of methionine cycle enzymes in plant virus infections. Curr. Opin. Plant Biol. 2019, 50, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, I.; Spechenkova, N.; Mamaeva, A.; Makhotenko, A.V.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Role of the methionine cycle in the temperature-sensitive responses of potato plants to potato virus Y. Mol. Plant Pathol. 2021, 22, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. RNA silencing. Trends Biochem. Sci. 2005, 30, 290–293. [Google Scholar] [CrossRef]
- Ding, S.-W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Ding, S.-W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019, 19, 31–44. [Google Scholar] [CrossRef]
- Mlotshwa, S.; Pruss, G.J.; Vance, V. Small RNAs in viral infection and host defense. Trends Plant Sci. 2008, 13, 375–382. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y. Dissection of RNAi-based antiviral immunity in plants. Curr. Opin. Virol. 2018, 32, 88–99. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Yu, B.; Liu, J.; Chen, X. Methylation Protects miRNAs and siRNAs from a 3′-End Uridylation Activity in Arabidopsis. Curr. Biol. 2005, 15, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, K.I.; Eskelin, K.; Bašić, M.; De, S.; Lõhmus, A.; Varjosalo, M.; Mäkinen, K. Molecular insights into the function of the viral RNA silencing suppressor HCPro. Plant J. 2016, 85, 30–45. [Google Scholar] [CrossRef]
- Cañizares, M.C.; Lozano-Durán, R.; Canto, T.; Bejarano, E.R.; Bisaro, D.M.; Navas-Castillo, J.; Moriones, E. Effects of the Crinivirus Coat Protein–Interacting Plant Protein SAHH on Post-Transcriptional RNA Silencing and Its Suppression. Mol. Plant-Microbe Interact. 2013, 26, 1004–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrance, L.; Talianksy, M.E. Potato Virus Y Emergence and Evolution from the Andes of South America to Become a Major Destructive Pathogen of Potato and Other Solanaceous Crops Worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection with Potato Virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.W.; Pehu, E.; Woods, R.D.; Jones, M.G.K. Resistance to potato virus Y and potato virus X in Solanurn brevidens. Ann. Appl. Biol. 1990, 116, 151–156. [Google Scholar] [CrossRef]
- Faurobert, M.; Pelpoir, E.; Chaïb, J. Phenol Extraction of Proteins for Proteomic Studies of Recalcitrant Plant Tissues. In Plant Proteomics: Methods and Protocols; Thiellement, H., Zivy, M., Damerval, C., Méchin, V., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2007; pp. 9–14. [Google Scholar] [CrossRef]
- Ow, S.Y.; Salim, M.; Noirel, J.; Evans, C.; Rehman, I.; Wright, P.C. iTRAQ Underestimation in Simple and Complex Mixtures: “The Good, the Bad and the Ugly”. J. Proteome Res. 2009, 8, 5347–5355. [Google Scholar] [CrossRef]
- STRING: Functional Protein Association Networks. Available online: https://string-db.org/cgi/input?sessionId=bqGWh48lnqym&input_page_show_search=on (accessed on 12 April 2021).
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- g:Profiler—A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists. Available online: https://biit.cs.ut.ee/gprofiler/gost (accessed on 12 April 2021).
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
- Phytozome v12.1: Home. Available online: https://phytozome.jgi.doe.gov/pz/portal.html (accessed on 12 April 2021).
- Nicot, N.; Hausman, J.-F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Baebler, Š.; Stare, K.; Kovač, M.; Blejec, A.; Prezelj, N.; Stare, T.; Kogovšek, P.; Pompe-Novak, M.; Rosahl, S.; Ravnikar, M.; et al. Dynamics of Responses in Compatible Potato—Potato virus Y Interaction Are Modulated by Salicylic Acid. PLoS ONE 2011, 6, e29009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rossum, G. Python Tutorial, Technical Report CS-R9526; Centrum voor Wiskunde en Informatica: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant–virus interaction. Mol. Plant Pathol. 2018, 19, 504–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Pandey, A.; Pandey, G.K. β-catenin in plants and animals: Common players but different pathways. Front. Plant Sci. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Li, S.; Ren, H. Profilin as a regulator of the membrane-actin cytoskeleton interface in plant cells. Front. Plant Sci. 2013, 4, 512. [Google Scholar] [CrossRef] [Green Version]
- Pitzalis, N.; Heinlein, M. The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement. J. Exp. Bot. 2018, 69, 117–132. [Google Scholar] [CrossRef]
- Bozhkov, P.V.; Suarez, M.F.; Filonova, L.H.; Daniel, G.; Zamyatnin, A.A.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Smertenko, A. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 14463–14468. [Google Scholar] [CrossRef] [Green Version]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Droux, M.; Ravanel, S.; Douce, R. Methionine Biosynthesis in Higher Plants.II. Purification and Characterization of Cystathionine β-Lyase from Spinach Chloroplasts. Arch. Biochem. Biophys. 1995, 316, 585–595. [Google Scholar] [CrossRef]
- Ramirez-Prado, J.S.; Piquerez, S.J.M.; Bendahmane, A.; Hirt, H.; Raynaud, C.; Benhamed, M. Modify the Histone to Win the Battle: Chromatin Dynamics in Plant–Pathogen Interactions. Front. Plant Sci. 2018, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, M.V.; Chen, H.; Gladon, R.J.; Braun, E.; Hannapel, D.J. A Leaf Lipoxygenase of Potato Induced Specifically by Pathogen Infection. Plant Physiol. 2000, 124, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffatt, B.A.; Weretilnyk, E.A. SustainingS-adenosyl-l-methionine-dependent methyltransferase activity in plant cells. Physiol. Plant. 2001, 113, 435–442. [Google Scholar] [CrossRef]
- Bernardo, P.; Charles-Dominique, T.; Barakat, M.; Ortet, P.; Fernandez, E.; Filloux, D.; Hartnady, P.; Rebelo, T.A.; Cousins, S.R.; Mesleard, F.; et al. Geometagenomics illuminates the impact of agriculture on the distribution and prevalence of plant viruses at the ecosystem scale. ISME J. 2018, 12, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkonen, J.P.T. Chapter 28—Viruses: Economical Losses and Biotechnological Potential. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Mackerron, D.K.L., Taylor, M.A., Ross, H.A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 619–641. [Google Scholar] [CrossRef]
- Whitworth, J.L.; Nolte, P.; McIntosh, C.; Davidson, R. Effect of Potato virus Y on Yield of Three Potato Cultivars Grown under Different Nitrogen Levels. Plant Dis. 2006, 90, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Solomon-Blackburn, R.M.; Bradshaw, J.E. Resistance to Potato virus Y in a Multitrait Potato Breeding Scheme without Direct Selection in Each Generation. Potato Res. 2007, 50, 87–95. [Google Scholar] [CrossRef]
- Foong, S.-L.; Paek, K.-H. Capsicum annum Hsp26.5 promotes defense responses against RNA viruses via ATAF2 but is hijacked as a chaperone for tobamovirus movement protein. J. Exp. Bot. 2020, 71, 6142–6158. [Google Scholar] [CrossRef]
- Corrêa, R.L.; Sanz-Carbonell, A.; Kogej, Z.; Müller, S.Y.; Ambrós, S.; López-Gomollón, S.; Gómez, G.; Baulcombe, D.C.; Elena, S.F. Viral Fitness Determines the Magnitude of Transcriptomic and Epigenomic Reprograming of Defense Responses in Plants. Mol. Biol. Evol. 2020, 37, 1866–1881. [Google Scholar] [CrossRef]
- Kuźnicki, D.; Meller, B.; Arasimowicz-Jelonek, M.; Braszewska-Zalewska, A.; Drozda, A.; Floryszak-Wieczorek, J. BABA-Induced DNA Methylome Adjustment to Intergenerational Defense Priming in Potato to Phytophthora infestans. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef]

| Identifications | PVY 22 °C 8 dpi | PVY 28 °C 8 dpi | PVY 22 °C 14 dpi | PVY 28 °C 14 dpi |
|---|---|---|---|---|
| Peptides | 11,353 | 14,106 | 12,893 | 13,703 |
| Protein groups | 2386 | 2693 | 2722 | 2619 |
| Samples | SAM (nmol/g-FWT) | SAM:SAH | ||
|---|---|---|---|---|
| Gala | Chicago | Gala | Chicago | |
| Mock 22 °C | 33.15 ± 1.07 | 27.94 ± 0.36 | 30.13 ± 1.98 | 33.32 ± 0.63 |
| Mock 28 °C | 33.95 ± 0.89 | 27.16 ± 0.61 | 33.56 ± 1.70 | 29.78 ± 0.82 |
| PVY 22 °C | 42.99 ± 1.49 ** | 28.02 ± 0.34 | 42.16 ± 2.10 * | 30.98 ± 0.61 |
| PVY 28 °C | 96.349 ± 2.05 *** | 10.96 ± 0.51 | 73.61 ± 3.78 *** | 3.62 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spechenkova, N.; Fesenko, I.A.; Mamaeva, A.; Suprunova, T.P.; Kalinina, N.O.; Love, A.J.; Taliansky, M. The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index. Viruses 2021, 13, 955. https://doi.org/10.3390/v13060955
Spechenkova N, Fesenko IA, Mamaeva A, Suprunova TP, Kalinina NO, Love AJ, Taliansky M. The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index. Viruses. 2021; 13(6):955. https://doi.org/10.3390/v13060955
Chicago/Turabian StyleSpechenkova, Nadezhda, Igor A. Fesenko, Anna Mamaeva, Tatyana P. Suprunova, Natalia O. Kalinina, Andrew J. Love, and Michael Taliansky. 2021. "The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index" Viruses 13, no. 6: 955. https://doi.org/10.3390/v13060955
APA StyleSpechenkova, N., Fesenko, I. A., Mamaeva, A., Suprunova, T. P., Kalinina, N. O., Love, A. J., & Taliansky, M. (2021). The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index. Viruses, 13(6), 955. https://doi.org/10.3390/v13060955







