A Multimodal Imaging-Supported Down Syndrome Mouse Model of RSV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Model
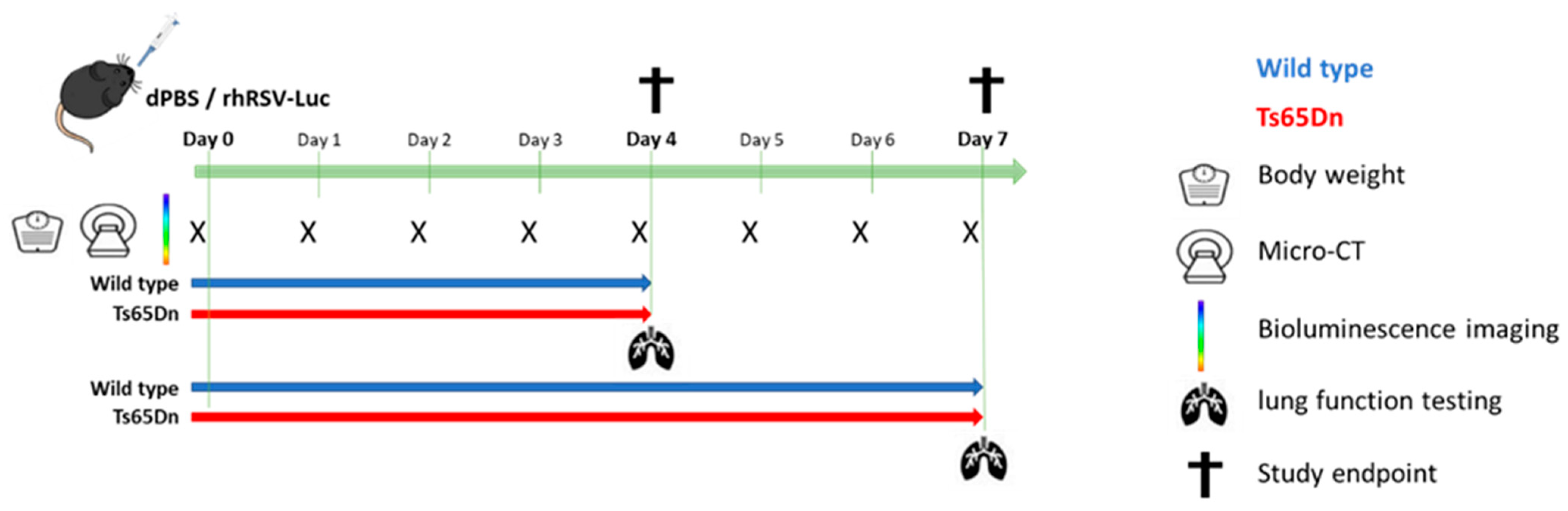
2.2. Experimental Design
2.3. Micro-Computed Tomography (Micro-CT)
2.4. Bioluminescence Imaging (BLI)
2.5. Lung Function and Airway Hyperreactivity Measurements
2.6. Bronchoalveolar Lavage
2.7. Differential Cell Counts
2.8. Viral Load Quantification
2.9. Spleen Cell Isolation
2.10. Lungs Cell Isolation
2.11. Flow Cytometric Immune Cell Analysis
2.12. Statistics
3. Results
3.1. Ts65Dn Mice Develop Upper Airway and Lung rhRSV-Luc Infection
3.2. Increased Airway Inflammation by hRSV-Luc Infection Does Not Affect Airway Reactivity in Ts65Dn Mice
3.3. Lung Immune Status Shows Limited Genotype-Specific Responses upon rhRSV-Luc Infection
3.4. A rhRSV-Luc Infection Results in Minor Splenic Innate Immune Activation
4. Discussion
4.1. In Vivo Imaging of a rhRSV-Luc DS Model Reveals Progressive Active Airway Infection without Severe Pneumonia
4.2. Differences in Viral Presence in Ts65Dn Mice May Be Linked to Genotype Related Immune Response
4.3. The Rationale behind Opting for the Ts65Dn Mouse Model, hRSV Strain and Instillation Route
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, N.M.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): A retrospective case series. Lancet Glob. Health 2017, 5, 984–991. [Google Scholar] [CrossRef]
- Reeves, R.M.; van Wijhe, M.; Tong, S.; Lehtonen, T.; Stona, L.; Teirlinck, A.C.; Fernandez, L.V.; Li, Y.; Giaquinto, C.; Fischer, T.K.; et al. Respiratory Syncytial Virus-Associated Hospital Admissions in Children Younger Than 5 Years in 7 European Countries Using Routinely Collected Datasets. J. Infect. Dis. 2020, 222, S599–S605. [Google Scholar] [CrossRef]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory Syncytial Virus—A Comprehensive Review. Clin. Rev. Allerg. Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Andeweg, S.P.; Schepp, R.M.; van de Kassteele, J.; Mollema, L.; Berbers, G.A.M.; van Boven, M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci. Rep. 2021, 11, 8953. [Google Scholar] [CrossRef]
- Lee, Y.-I.; Peng, C.-C.; Chiu, N.-C.; Huang, D.T.-N.; Huang, F.-Y.; Chi, H. Risk factors associated with death in patients with severe respiratory syncytial virus infection. J. Microbiol. Immunol. Infect. 2016, 49, 737–742. [Google Scholar] [CrossRef]
- Bloemers, B.L.P.; van Furth, A.M.; Weijerman, M.E.; Gemke, R.J.B.J.; Broers, C.J.M.; van den Ende, K.; Kimpen, J.L.L.; Strengers, J.L.M.; Bont, L.J. Down Syndrome: A Novel Risk Factor for Respiratory Syncytial Virus Bronchiolitis A Prospective Birth-Cohort Study. Pediatrics 2007, 120, 1076–1081. [Google Scholar] [CrossRef]
- Geoghegan, S.; Erviti, A.; Caballero, M.T.; Vallone, F.; Zanone, S.M.; Losada, J.V.; Bianchi, A.; Acosta, P.L.; Talarico, L.B.; Ferretti, A.; et al. Mortality due to Respiratory Syncytial Virus. Burden and Risk Factors. Am. J. Respir. Crit. Care Med. 2017, 195, 96–103. [Google Scholar] [CrossRef]
- Sánchez-Luna, M.; Medrano, C.; Lirio, J.; The RISK-21 Study Group. Down syndrome as risk factor for respiratory syncytial virus hospitalization: A prospective multicenter epidemiological study. Influenza Other Respir. Viruses 2017, 11, 157–164. [Google Scholar] [PubMed]
- Löwensteyn, Y.N.; Phijffer, E.W.E.M.; Simons, J.V.L.; Scheltema, N.M.; Mazur, N.I.; Nair, H.; Bont, L.J.; on behalf of the RSV GOLD Study Group. Respiratory Syncytial Virus-related Death in Children with Down Syndrome: The RSV GOLD Study. Pediatr. Infect. Dis. J. 2020, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; El Azrak, M.; McCord, H.; Paes, B.A. Hospitalization for Respiratory Syncytial Virus in Children with Down Syndrome Less than 2 Years of Age: A Systematic Review and Meta-Analysis. J. Pediatr. 2018, 203, 92–100. [Google Scholar] [PubMed]
- Ram, G.; Chinen, J. Infections and immunodeficiency in Down syndrome: Immunodeficiency in Down syndrome. Clin. Exp. Immunol. 2011, 164, 9–16. [Google Scholar] [CrossRef]
- Alsubie, H.S.; Rosen, D. The evaluation and management of respiratory disease in children with Down syndrome (DS). Paediatr. Respir. Rev. 2018, 26, 49–54. [Google Scholar] [CrossRef]
- Arumugam, A.; Raja, K.; Venugopalan, M.; Chandrasekaran, B.; Kovanur Sampath, K.; Muthusamy, H.; Shanmugam, N. Down syndrome-A narrative review with a focus on anatomical features: Down Syndrome. Clin. Anat. 2016, 29, 568–577. [Google Scholar] [CrossRef]
- Pandit, C.; Fitzgerald, D.A. Respiratory problems in children with Down syndrome: Respiratory problems. J. Paediatr. Child. Health 2012, 48, 147–152. [Google Scholar] [CrossRef]
- Colvin, K.L.; Yeager, M.E. What people with Down Syndrome can teach us about cardiopulmonary disease. Eur. Respir. Rev. 2017, 26, 16. [Google Scholar] [CrossRef]
- Lam, D.J.; Jensen, C.C.; Mueller, B.A.; Starr, J.R.; Cunningham, M.L.; Weaver, E.M. Pediatric sleep apnea and craniofacial anomalies: A population-based case-control study. Laryngoscope 2010, 120, 2098–2105. [Google Scholar] [CrossRef]
- Danopoulos, S.; Deutsch, G.H.; Dumortier, C.; Mariani, T.J.; Al Alam, D. Lung disease manifestations in Down syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L892–L899. [Google Scholar] [CrossRef]
- Danopoulos, S.; Bhattacharya, S.; Deutsch, G.; Nih, L.R.; Slaunwhite, C.; Mariani, T.J.; Al Alam, D. Prenatal histological, cellular, and molecular anomalies in trisomy 21 lung. J. Pathol. 2021, 255, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.F.; Sullivan, K.D.; Pham, A.T.; Espinosa, J.M.; Pearson, C.G. Trisomy 21 Represses Cilia Formation and Function. Dev. Cell 2018, 46, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.B.; Milner, A.D.; Greenough, A. Respiratory syncytial virus infection and chronic respiratory morbidity—Is there a functional or genetic predisposition?: RSV and chronic respiratory morbidity. Acta Paediatr. 2012, 101, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.S.; Ivy, D.D. Pulmonary Hypertension in the Population with Down Syndrome. Cardiol. Ther. 2022, 11, 33–47. [Google Scholar] [CrossRef]
- Bush, D.; Galambos, C.; Ivy, D.D. Pulmonary hypertension in children with Down syndrome. Pediatr. Pulmonol. 2020, 1–9. [Google Scholar] [CrossRef]
- Lozano-Espinosa, D.A.; Huertas-Quiñones, V.M.; Rodríguez-Martínez, C.E. Impact of pulmonary hypertension and congenital heart disease with hemodynamic repercussion on the severity of acute respiratory infections in children under 5 years of age at a pediatric referral center in Colombia, South America. Cardiol. Young 2020, 30, 1866–1873. [Google Scholar] [CrossRef]
- Illouz, T.; Biragyn, A.; Iulita, M.F.; Flores-Aguilar, L.; Dierssen, M.; De Toma, I.; Antonarakis, S.E.; Yu, E.; Herault, Y.; Potier, M.-C.; et al. Immune Dysregulation and the Increased Risk of Complications and Mortality Following Respiratory Tract Infections in Adults with Down Syndrome. Front. Immunol. 2021, 12, 621440. [Google Scholar] [CrossRef]
- Huggard, D.; Doherty, D.G.; Molloy, E.J. Immune Dysregulation in Children with Down Syndrome. Front. Pediatr. 2020, 8, 73. [Google Scholar] [CrossRef]
- Liggett, L.A.; Galbraith, M.D.; Smith, K.P.; Sullivan, K.D.; Granrath, R.E.; Enriquez-Estrada, B.; Kinning, K.T.; Shaw, J.R.; Rachubinski, A.L.; Espinosa, J.M.; et al. Precocious clonal hematopoiesis in Down syndrome is accompanied by immune dysregulation. Blood Adv. 2021, 5, 1791–1796. [Google Scholar] [CrossRef]
- Huggard, D.; Kelly, L.; Ryan, E.; McGrane, F.; Lagan, N.; Roche, E.; Balfe, J.; Leahy, T.R.; Franklin, O.; Doherty, D.G.; et al. Increased systemic inflammation in children with Down syndrome. Cytokine 2020, 127, 154938. [Google Scholar] [CrossRef]
- Waugh, K.A.; Araya, P.; Pandey, A.; Jordan, K.R.; Smith, K.P.; Granrath, R.E.; Khanal, S.; Butcher, E.T.; Estrada, B.E.; Rachubinski, A.L.; et al. Mass Cytometry Reveals Global Immune Remodeling with Multi-lineage Hypersensitivity to Type I Interferon in Down Syndrome. Cell Rep. 2019, 29, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Morris, J.K. Trends in maternal age distribution and the live birth prevalence of Down’s syndrome in England and Wales: 1938–2010. Eur. J. Hum. Genet. 2013, 21, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Tsou, A.Y.; Bulova, P.; Capone, G.; Chicoine, B.; Gelaro, B.; Harville, T.O.; Martin, B.A.; McGuire, D.E.; McKelvey, K.D.; Peterson, M.; et al. Medical Care of Adults with Down Syndrome: A Clinical Guideline. JAMA 2020, 324, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Krilov, L.R. Respiratory syncytial virus vaccine: Where are we now and what comes next? Expert Opin. Biol. Ther. 2018, 18, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.A.F.; Bont, L.; Manzoni, P.; Fauroux, B.; Paes, B.; Figueras-Aloy, J.; Checchia, P.A.; Carbonell-Estrany, X. Past, Present and Future Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children. Infect. Dis. Ther. 2018, 7, 87–120. [Google Scholar] [CrossRef]
- Powell, K. The new wave of vaccines for a killer respiratory virus. Nature 2021, 600, 379–380. [Google Scholar] [CrossRef]
- Taylor, G. Animal models of respiratory syncytial virus infection. Vaccine 2017, 35, 469–480. [Google Scholar] [CrossRef]
- Gupta, M.; Dhanasekaran, A.R.; Gardiner, K.J. Mouse models of Down syndrome: Gene content and consequences. Mamm. Genome 2016, 27, 538–555. [Google Scholar] [CrossRef]
- Choong, X.Y.; Tosh, J.L.; Pulford, L.J.; Fisher, E.M.C. Dissecting Alzheimer disease in Down syndrome using mouse models. Front. Behav. Neurosci. 2015, 9, 268. [Google Scholar] [CrossRef]
- Rameix-Welti, M.-A.; Le Goffic, R.; Hervé, P.-L.; Sourimant, J.; Rémot, A.; Riffault, S.; Yu, Q.; Galloux, M.; Gault, E.; Eléouët, J.-F. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nat. Commun. 2014, 5, 5104. [Google Scholar] [CrossRef] [PubMed]
- Seldeslachts, L.; Vanderbeke, L.; Fremau, A.; Reséndiz-Sharpe, A.; Jacobs, C.; Laeveren, B.; Ostyn, T.; Naesens, L.; Brock, M.; Van De Veerdonk, F.L.; et al. Early oseltamivir reduces risk for influenza-associated aspergillosis in a double-hit murine model. Virulence 2021, 12, 2493–2508. [Google Scholar] [CrossRef] [PubMed]
- Dekoster, K.; Decaesteker, T.; Berghen, N.; Van den Broucke, S.; Jonckheere, A.-C.; Wouters, J.; Krouglov, A.; Lories, R.; De Langhe, E.; Hoet, P.; et al. Longitudinal micro-computed tomography-derived biomarkers quantify non-resolving lung fibrosis in a silicosis mouse model. Sci. Rep. 2020, 10, 16181. [Google Scholar] [CrossRef] [PubMed]
- Vande Velde, G.; Poelmans, J.; De Langhe, E.; Hillen, A.; Vanoirbeek, J.; Himmelreich, U.; Lories, R.J. Longitudinal micro-CT provides biomarkers of lung disease that can be used to assess the effect of therapy in preclinical mouse models, and reveal compensatory changes in lung volume. Dis. Model. Mech. 2016, 9, 91–98. [Google Scholar] [PubMed]
- Resendiz-Sharpe, A.; da Silva, R.P.; Geib, E.; Vanderbeke, L.; Seldeslachts, L.; Hupko, C.; Brock, M.; Lagrou, K.; Vande Velde, G. Longitudinal multimodal imaging-compatible mouse model of triazole-sensitive and -resistant invasive pulmonary aspergillosis. Dis. Model. Mech. 2022, 15, dmm049165. [Google Scholar] [CrossRef]
- Devos, F.C.; Maaske, A.; Robichaud, A.; Pollaris, L.; Seys, S.; Lopez, C.A.; Verbeken, E.; Tenbusch, M.; Lories, R.; Nemery, B.; et al. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir. Res. 2017, 18, 123–137. [Google Scholar] [CrossRef]
- Jonckheere, A.-C.; Seys, S.F.; Steelant, B.; Decaesteker, T.; Dekoster, K.; Cremer, J.; Dilissen, E.; Schols, D.; Iwakura, Y.; Vande Velde, G.; et al. Innate Lymphoid Cells Are Required to Induce Airway Hyperreactivity in a Murine Neutrophilic Asthma Model. Front. Immunol. 2022, 13, 849155. [Google Scholar] [CrossRef]
- Pollenus, E.; Pham, T.-T.; Vandermosten, L.; Robalo, Q.; Possemiers, H.; Knoops, S.; Opdenakker, G.; Van den Steen, P.E. CCR2 Is Dispensable for Disease Resolution but Required for the Restoration of Leukocyte Homeostasis Upon Experimental Malaria-Associated Acute Respiratory Distress Syndrome. Front. Immunol. 2021, 11, 628643. [Google Scholar] [CrossRef]
- Eiland, L.S. Respiratory Syncytial Virus: Diagnosis, Treatment and Prevention. J. Pediatr. Pharmacol. Ther. 2009, 14, 75–85. [Google Scholar] [CrossRef]
- Harker, J.; Bukreyev, A.; Collins, P.L.; Wang, B.; Openshaw, P.J.M.; Tregoning, J.S. Virally Delivered Cytokines Alter the Immune Response to Future Lung Infections. J. Virol. 2007, 81, 13105–13111. [Google Scholar] [CrossRef]
- Weijerman, M.E.; Brand, P.L.; van Furth, M.A.; Broers, C.J.; Gemke, R.J. Recurrent wheeze in children with Down syndrome: Is it asthma?: Is wheeze in Down syndrome asthma? Acta Paediatr. 2011, 100, 194–197. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.Z.M.; de França Barros, J.; de Azevedo, M.; de Godoy, J.R.P.; Arena, R.; Cipriano, G. Bone mineral density and respiratory muscle strength in male individuals with mental retardation (with and without Down Syndrome). Res. Dev. Disabil. 2010, 31, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Bem, R.A.; Domachowske, J.B.; Rosenberg, H.F. Animal models of human respiratory syncytial virus disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L148–L156. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.A.; You, D.; Honnegowda, S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert. Rev. Anti-Infect. Ther. 2010, 8, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Sebina, I.; Phipps, S. The Contribution of Neutrophils to the Pathogenesis of RSV Bronchiolitis. Viruses 2020, 12, 808. [Google Scholar] [CrossRef]
- Kirsebom, F.; Michalaki, C.; Agueda-Oyarzabal, M.; Johansson, C. Neutrophils do not impact viral load or the peak of disease severity during RSV infection. Sci. Rep. 2020, 10, 1110. [Google Scholar] [CrossRef]
- Openshaw, P.J.M.; Tregoning, J.S. Immune Responses and Disease Enhancement during Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2005, 18, 541–555. [Google Scholar] [CrossRef]
- Bueno, S.M.; González, P.A.; Cautivo, K.M.; Mora, J.E.; Leiva, E.D.; Tobar, H.E.; Fennelly, G.J.; Eugenin, E.A.; Jacobs, W.R.; Riedel, C.A.; et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci. USA 2008, 105, 20822–20827. [Google Scholar] [CrossRef]
- Stark, J.M.; McDowell, S.A.; Koenigsknecht, V.; Prows, D.R.; Leikauf, J.E.; Le Vine, A.M.; Leikauf, G.D. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J. Med. Virol. 2002, 67, 92–100. [Google Scholar] [CrossRef]
- Stokes, K.L.; Chi, M.H.; Sakamoto, K.; Newcomb, D.C.; Currier, M.G.; Huckabee, M.M.; Lee, S.; Goleniewska, K.; Pretto, C.; Williams, J.V.; et al. Differential Pathogenesis of Respiratory Syncytial Virus Clinical Isolates in BALB/c Mice. J. Virol. 2011, 85, 5782–5793. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Moore, M.L.; Rudd, B.D.; Berlin, A.A.; Collins, R.D.; Olson, S.J.; Ho, S.B.; Peebles, R.S. Differential Immune Responses and Pulmonary Pathophysiology Are Induced by Two Different Strains of Respiratory Syncytial Virus. Am. J. Pathol. 2006, 169, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.L.; Chi, M.H.; Luongo, C.; Lukacs, N.W.; Polosukhin, V.V.; Huckabee, M.M.; Newcomb, D.C.; Buchholz, U.J.; Crowe, J.E.; Goleniewska, K.; et al. A Chimeric A2 Strain of Respiratory Syncytial Virus (RSV) with the Fusion Protein of RSV Strain Line 19 Exhibits Enhanced Viral Load, Mucus, and Airway Dysfunction. J. Virol. 2009, 83, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Piatti, G.; Allegra, L.; Ambrosetti, U.; De Santi, M.M. Nasal Ciliary Function and Ultrastructure in Down Syndrome. Laryngoscope 2001, 111, 1227–1230. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tielemans, B.; De Herdt, L.; Pollenus, E.; Vanhulle, E.; Seldeslachts, L.; Marain, F.; Belmans, F.; Ahookhosh, K.; Vanoirbeek, J.; Vermeire, K.; et al. A Multimodal Imaging-Supported Down Syndrome Mouse Model of RSV Infection. Viruses 2023, 15, 993. https://doi.org/10.3390/v15040993
Tielemans B, De Herdt L, Pollenus E, Vanhulle E, Seldeslachts L, Marain F, Belmans F, Ahookhosh K, Vanoirbeek J, Vermeire K, et al. A Multimodal Imaging-Supported Down Syndrome Mouse Model of RSV Infection. Viruses. 2023; 15(4):993. https://doi.org/10.3390/v15040993
Chicago/Turabian StyleTielemans, Birger, Lander De Herdt, Emilie Pollenus, Emiel Vanhulle, Laura Seldeslachts, Fopke Marain, Flore Belmans, Kaveh Ahookhosh, Jeroen Vanoirbeek, Kurt Vermeire, and et al. 2023. "A Multimodal Imaging-Supported Down Syndrome Mouse Model of RSV Infection" Viruses 15, no. 4: 993. https://doi.org/10.3390/v15040993
APA StyleTielemans, B., De Herdt, L., Pollenus, E., Vanhulle, E., Seldeslachts, L., Marain, F., Belmans, F., Ahookhosh, K., Vanoirbeek, J., Vermeire, K., Van den Steen, P. E., & Vande Velde, G. (2023). A Multimodal Imaging-Supported Down Syndrome Mouse Model of RSV Infection. Viruses, 15(4), 993. https://doi.org/10.3390/v15040993






