The Silent Syndrome of Long COVID and Gaps in Scientific Knowledge: A Narrative Review
Abstract
:1. Introduction
2. Long COVID—Post-COVID-19 Syndrome
2.1. History and Case Definition
- In symptomatic individuals infected with SARS-CoV-2, symptoms are present for more than 2 weeks in patients with mild disease, more than 4 weeks in patients with moderate/severe disease, and more than 6 weeks in patients with critical illness.
- In asymptomatic individuals infected with SARS-CoV-2, the symptoms of LC persist 2 weeks after a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test; the symptoms of LC persist 1 week after a positive antibody status; the symptoms of LC persist 2 weeks after a positive chest computed tomography or chest X-ray; LC symptoms begin 2 weeks after contact with suspected or positive cases of COVID-19; and in any doubtful cases [21].
2.2. Possible Pathophysiological Mechanisms
2.2.1. Tissue Damage
2.2.2. Lungs
2.2.3. Brain
2.2.4. Heart
2.2.5. Thrombotic Events
2.2.6. Other Neurological Organs and Systems
2.2.7. Gastrointestinal Organs and Systems
2.2.8. Other Organs and Systems
2.3. Inflammation and Immune Response
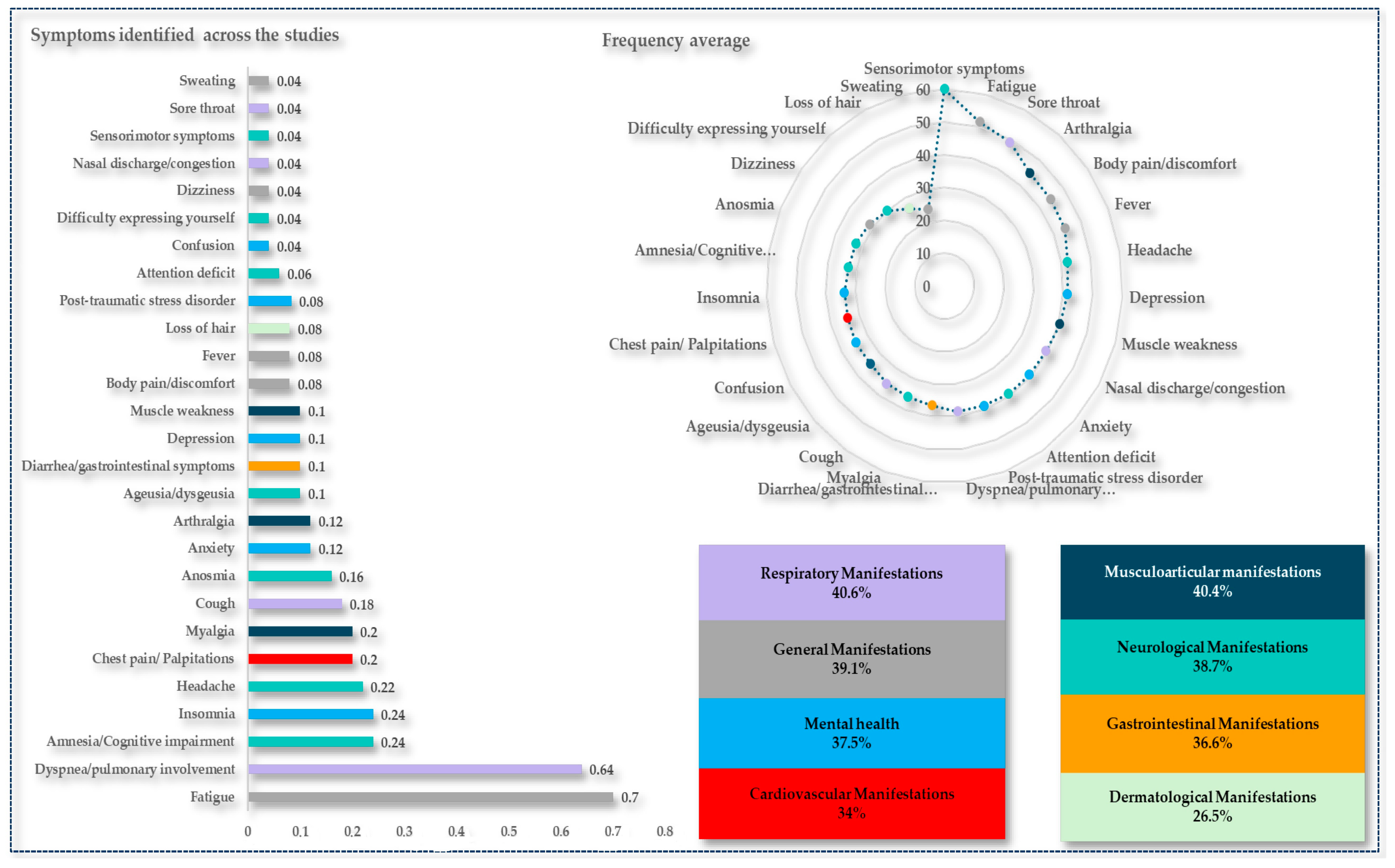
2.4. Clinical Manifestations
2.5. Epidemiology
2.5.1. Long Covid Prevalences Worldwide
2.5.2. Long Covid Symptoms Prevalences
2.6. Risk Factors
2.7. Association with Cellular, Molecular, and Genetic Factors
3. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zhang, H.; Zhang, W. SARS-CoV-2 Variants, Immune Escape, and Countermeasures. Front. Med. 2022, 16, 196–207. [Google Scholar] [CrossRef]
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the Pandemic: Perspectives on the Future Trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of Post-Acute COVID-19 Syndrome Symptoms at Different Follow-up Periods: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention—CDC. Long COVID—Household Pulse Survey—COVID-19. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 30 June 2023).
- Martimbianco, A.L.C.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, Signs and Symptoms, and Criteria Adopted for Long COVID-19: A Systematic Review. Int. J. Clin. Pract. 2021, 75, e14357. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Isaaka, L.; Mumm, R.; Scheidt-Nave, C.; Heldt, K.; Schuster, A.; Abdulaziz, M.; Bcheraoui, C.E.; Hanefeld, J.; Agweyu, A. Prevalence and Risk Factors for Long COVID and Post-COVID-19 Condition in Africa: A Systematic Review. Lancet Glob. Health 2023, 11, e1713–e1724. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Nat. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA J. Am. Med. Assoc. 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Rose, E.B.; Shapiro, N.I.; Clark, F.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Callard, F.; Perego, E. How and Why Patients Made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://pubmed.ncbi.nlm.nih.gov/33555768/ (accessed on 13 July 2023).
- Centers for Disease Control and Prevention—CDC. Long COVID or Post-COVID Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 27 June 2023).
- World Health Organization. ICD-10 Version_2019. Available online: https://icd.who.int/browse10/2019/en#/U09.9 (accessed on 27 June 2023).
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of Post-Acute Covid-19 in Primary Care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- PubMed—National Center for Biotechnology Information. _Long COVID_Post-COVID-19_Condition Post-COVID-19_Post-COVID-19 Syndrome_COVID Long Haulers_Long-Term Symptoms COVID-19_Long-Term COVID-19—Search Results. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=%22long+COVID%22+or+%22post-COVID-19%22+or+%22condition+post-COVID-19%22+or+%22post-COVID-19+syndrome%22+or+%22COVID+long+haulers%22+or+%22long-term+symptoms+COVID-19%22+or+%22long-term+COVID-19%22 (accessed on 23 January 2024).
- Ceravolo, M.; Arienti, C.; de Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.; Patrini, M.; Negrini, S. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 Rapid Living Systematic Review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef]
- Raveendran, A.V. Long COVID-19: Challenges in the Diagnosis and Proposed Diagnostic Criteria. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 145–146. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and Risk Factors for Long COVID in Non-Hospitalized Adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Long COVID or Post-COVID-19 Syndrome: Putative Pathophysiology, Risk Factors, and Treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Seessle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Lledó, G.M.; Sellares, J.; Brotons, C.; Sans, M.; Antón, J.D.; Blanco, J.; Bassat, Q.; Sarukhan, A.; Miró, J.M.; de Sanjosé, S.; et al. Post-Acute COVID-19 Syndrome: A New Tsunami Requiring a Universal Case Definition. Clin. Microbiol. Infect. 2022, 28, 315–318. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge Symptoms and Rehabilitation Needs in Survivors of COVID-19 Infection: A Cross-Sectional Evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Á Steig, B.; Gaini, S.; Strøm, M.; Weihe, P. Long COVID in the Faroe Islands: A Longitudinal Study among Nonhospitalized Patients. Clin. Infect. Dis. 2021, 73, E4058–E4063. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Huang, J.; Wong, Y.Y.; Wong, G.L.H.; Yip, T.C.F.; Chan, R.N.Y.; Chau, S.W.H.; Ng, S.C.; Wing, Y.K.; Chan, F.K.L. Epidemiology, Symptomatology, and Risk Factors for Long COVID Symptoms: Population-Based, Multicenter Study. JMIR Public Health Surveill. 2023, 9, e42315. [Google Scholar] [CrossRef]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McConald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months after COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Cheallaigh, C.N.; et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Pinto Pereira, S.M.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and Mental Health 3 Months after SARS-CoV-2 Infection (Long COVID) among Adolescents in England (CLoCk): A National Matched Cohort Study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef]
- Nabavi, N. Long COVID: How to Define It and How to Manage It. BMJ 2020, 370, m3489. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus Infections and Immune Responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, Y.; Song, W.; Li, Q.; Xie, H.; Xu, Q.; Jia, J.; Li, L.; Mao, H.; Zhou, X.; et al. Follow-up Study of the Pulmonary Function and Related Physiological Characteristics of COVID-19 Survivors Three Months after Recovery. eClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Van Den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; Van Hees, H.W.H.; Van Helvoort, H.; Van Den Boogaard, M.; Van Der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months after Recovery from Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, E1089–E1098. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical Sequelae among Patients with COVID-19 Four Months after Hospital Discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef] [PubMed]
- Truffaut, L.; Demey, L.; Bruyneel, A.V.; Roman, A.; Alard, S.; De Vos, N.; Bruyneel, M. Post-Discharge Critical COVID-19 Lung Function Related to Severity of Radiologic Lung Involvement at Admission. Respir. Res. 2021, 22, 29. [Google Scholar] [CrossRef]
- Crameri, G.A.G.; Bielecki, M.; Züst, R.; Buehrer, T.W.; Stanga, Z.; Deuel, J.W. Reduced Maximal Aerobic Capacity after COVID-19 in Young Adult Recruits, Switzerland, May 2020. Eurosurveillance 2020, 25, 2001542. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Wang, Y.; Lou, X.; Chen, S.; Deng, H.; Shi, L.; Xie, J.; Tang, D.; Zhao, J.; et al. Damaged Lung Gas Exchange Function of Discharged COVID-19 Patients Detected by Hyperpolarized Xe MRI. Sci. Adv. 2021, 7, eabc8180. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient Outcomes after Hospitalisation with COVID-19 and Implications for Follow-up: Results from a Prospective UK Cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-Based 3-Month Follow-up Study: A Brief Title: Cerebral Changes in COVID-19. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Rutkai, I.; Mayer, M.G.; Hellmers, L.M.; Ning, B.; Huang, Z.; Monjure, C.J.; Coyne, C.; Silvestri, R.; Golden, N.; Hensley, K.; et al. Neuropathology and Virus in Brain of SARS-CoV-2 Infected Non-Human Primates. Nat. Commun. 2022, 13, 1745. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a Predictor of Long-Term Cognitive Impairment in Survivors of Critical Illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Bejoy, P.; Prerona, M.; Yuti, K.; Pranav, R.; Sara, Z.; Haytham, S.; Amer, H. COVID-19 and Its Long-Term Impact on the Cardiovascular System. Expert Rev. Cardiovasc. Ther. 2023, 21, 211–218. [Google Scholar] [CrossRef]
- Nasab, E.M.; Aghajani, H.; Makoei, R.H.; Athari, S.S. COVID-19’s Immuno-Pathology and Cardiovascular Diseases. J. Investig. Med. 2023, 71, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Levett, J.Y.; Raparelli, V.; Mardigyan, V.; Eisenberg, M.J. Cardiovascular Pathophysiology, Epidemiology, and Treatment Considerations of Coronavirus Disease 2019 (COVID-19): A Review. CJC Open 2021, 3, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Chiriacò, M.; Emdin, M.; Taddei, S.; Vergaro, G. Statin Therapy in COVID-19 Infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Moody, W.E.; Liu, B.; Mahmoud-Elsayed, H.M.; Senior, J.; Lalla, S.S.; Khan-Kheil, A.M.; Brown, S.; Saif, A.; Moss, A.; Bradlow, W.M.; et al. Persisting Adverse Ventricular Remodeling in COVID-19 Survivors: A Longitudinal Echocardiographic Study. J. Am. Soc. Echocardiogr. 2021, 34, 562–566. [Google Scholar] [CrossRef]
- Song, Y.; Myers, R.; Mehl, F.; Murphy, L.; Brooks, B.; Wilson, J.M.; Kadl, A.; Woodfolk, J.; Zeichner, S.L. ACE-2-like Enzymatic Activity Is Associated with Immunoglobulin in COVID-19 Patients. mBio 2024, 15, e0054124. [Google Scholar] [CrossRef]
- Mauro, M.; Cegolon, L.; Bestiaco, N.; Zulian, E.; Larese Filon, F. Heart Rate Variability Modulation through Slow Paced Breathing in Healthcare Workers with Long-COVID: A Case-Control Study. Am. J. Med. 2024. [Google Scholar] [CrossRef]
- Patell, R.; Bogue, T.; Bindal, P.; Koshy, A.; Merrill, M.; Aird, W.C.; Bauer, K.A.; Zwicker, J.I. Incidence of Thrombosis and Hemorrhage in Hospitalized Cancer Patients with COVID-19. J. Thromb. Haemost. 2020, 18, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Cook, C.; Thomas, S.; Hall, A.; Hubbard, R.; Vallance, P. Risk of Deep Vein Thrombosis and Pulmonary Embolism after acute Infection in a Community Setting. Lancet J. 2006, 367, 1075–1079. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Romano, C.; Scarabelli, T.M.; Chen-Scarabelli, C.; Saravolatz, L.; Dioguardi, F.S. Serum Metabolic Profile in Patients with Long-COVID (PASC) Syndrome: Clinical Implications. Front. Med. 2021, 8, 714426. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 746021. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A Central Role for Amyloid Fibrin Microclots in Long COVID/PASC: Origins and Therapeutic Implications. Biochem. J. 2022, 479, 537–559. [Google Scholar] [CrossRef]
- Nicolai, L.; Kaiser, R.; Stark, K. Thromboinflammation in Long COVID—The Elusive Key to Postinfection Sequelae? J. Thromb. Haemost. 2023, 21, 2020–2031. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological Sequelae of Long-Haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Drury, H.A. Structural and Functional Analyses of Human Cerebral Cortex Using a Surface-Based Atlas. J. Neurosci. 1997, 17, 7079–7102. [Google Scholar] [CrossRef]
- Johansson, J.; Levi, R.; Jakobsson, M.; Gunnarsson, S.; Samuelsson, K. Multiprofessional Neurorehabilitation After COVID-19 Infection Should Include Assessment of Visual Function. Arch. Rehabil. Res. Clin. Transl. 2022, 4, 100184. [Google Scholar] [CrossRef] [PubMed]
- Noviello, D.; Costantino, A.; Muscatello, A.; Bandera, A.; Consonni, D.; Vecchi, M.; Basilisco, G. Functional Gastrointestinal and Somatoform Symptoms Five Months after SARS-CoV-2 Infection: A Controlled Cohort Study. Neurogastroenterol. Motil. 2022, 34, e14187. [Google Scholar] [CrossRef] [PubMed]
- OzkurtOzkurt, Z.; Ozkurt, Z.; Tanrıverdi, E.Ç. COVID-19: Gastrointestinal Manifestations, Liver Injury and Recommendations. World J. Clin. Cases 2022, 10, 1140–1163. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.; Chang, L. Gastrointestinal Symptoms in COVID-19_ the Long and the Short of It. Curr. Opin. Gastroenterol. 2022, 38, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin Reduction in Post-Acute Sequelae of Viral Infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef] [PubMed]
- Sikander, A.; Rana, S.V.; Prasad, K.K. Role of Serotonin in Gastrointestinal Motility and Irritable Bowel Syndrome. Clinica Chimica Acta 2009, 403, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.M.; Cook, E.H.; Blakely, R.D.; Sutcliffe, J.S.; Veenstra-Vanderweele, J. Long COVID-19 and Peripheral Serotonin: A Commentary and Reconsideration. J. Inflamm. Res. 2024, 17, 2169–2172. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Jansen, T.; Jacobs, L.; Bonder, M.J.; Kurilshikov, A.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.Y.; Zhang, F.; Liu, Q.; Li, A.Y.L.; Chung, A.C.K.; Cheung, C.P.; Tso, E.Y.K.; Fung, K.S.C.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Álvarez-Santacruz, C.; Tyrkalska, S.D.; Candel, S. The Microbiota in Long COVID. Int. J. Mol. Sci. 2024, 25, 1330. [Google Scholar] [CrossRef] [PubMed]
- Candel, S.; Tyrkalska, S.D.; Álvarez-Santacruz, C.; Mulero, V. The Nasopharyngeal Microbiome in COVID-19. Emerg. Microbes. Infect. 2023, 12, e2165970. [Google Scholar] [CrossRef] [PubMed]
- Boettler, T.; Marjot, T.; Newsome, P.N.; Mondelli, M.U.; Maticic, M.; Cordero, E.; Jalan, R.; Moreau, R.; Cornberg, M.; Berg, T. Impact of COVID-19 on the Care of Patients with Liver Disease: EASL-ESCMID Position Paper after 6 Months of the Pandemic. JHEP Rep. 2020, 2, 100169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Baldelli, L.; Marjot, T.; Barnes, E.; Barritt, A.S.; Webb, G.J.; Moon, A.M. SARS-CoV-2 Infection and Liver Disease: A Review of Pathogenesis and Outcomes. Gut Liver 2023, 17, 12–23. [Google Scholar] [CrossRef]
- Raina, R.; Mahajan, Z.A.; Vasistha, P.; Chakraborty, R.; Mukunda, K.; Tibrewal, A.; Neyra, J.A. Incidence and Outcomes of Acute Kidney Injury in COVID-19: A Systematic Review. Blood Purif. 2022, 51, 199–212. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence—NICE. Recommendations COVID-19 Rapid Guideline Managing COVID-19 Guidance. Available online: https://www.nice.org.uk/guidance/ng191/chapter/Recommendations (accessed on 13 July 2023).
- Bornstein, S.R.; Rubino, F.; Ludwig, B.; Rietzsch, H.; Schwarz, P.E.H.; Rodionov, R.N.; Khunti, K.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; et al. Consequences of the COVID-19 Pandemic for Patients with Metabolic Diseases. Nat. Metab. 2021, 3, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5. [Google Scholar] [CrossRef]
- Yang, J.K.; Lin, S.S.; Ji, X.J.; Guo, L.M. Binding of SARS Coronavirus to Its Receptor Damages Islets and Causes Acute Diabetes. Acta. Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef]
- Freeman, E.E.; McMahon, D.E.; Lipoff, J.B.; Rosenbach, M.; Kovarik, C.; Desai, S.R.; Harp, J.; Takeshita, J.; French, L.E.; Lim, H.W.; et al. The Spectrum of COVID-19–Associated Dermatologic Manifestations: An International Registry of 716 Patients from 31 Countries. J. Am. Acad. Dermatol. 2020, 83, 1118–1129. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Chernyshov, P.V.; Tomas Aragones, L.; Bewley, A.; Svensson, A.; Manolache, L.; Marron, S.; Suru, A.; Sampogna, F.; Salek, M.S.; et al. Methods to Improve Quality of Life, beyond Medicines. Position Statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Abadías-Granado, I.; Navarro-Bielsa, A.; Morales-Callaghan, A.; Roc, L.; Suso-Estívalez, C.; Povar-Echeverría, M.; Gilaberte, Y. COVID-19-Associated Cutaneous Manifestations: Does Human Herpesvirus 6 Play an Aetiological Role? Br. J. Dermatol. 2021, 184, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Leerunyakul, K.; Kositkuljorn, C. Cutaneous Manifestations in COVID-19: Lessons Learned from Current Evidence. J. Am. Acad. Dermatol. 2020, 83, 57–60. [Google Scholar] [CrossRef]
- Abrantes, T.F.; Artounian, K.A.; Falsey, R.; Simão, J.C.L.; Vañó-Galván, S.; Ferreira, S.B.; Davis, T.L.; Ridenour, W.; Goren, A.; Tosti, A.; et al. Time of Onset and Duration of Post-COVID-19 Acute Telogen Effluvium. J. Am. Acad. Dermatol. 2021, 85, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Cai, L.; Cheng, Z.S.; Cheng, H.; Deng, T.; Fan, Y.P.; Fang, C.; Huang, D.; Huang, L.Q.; Huang, Q.; et al. A Rapid Advice Guideline for the Diagnosis and Treatment of 2019 Novel Coronavirus (2019-NCoV) Infected Pneumonia (Standard Version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Du, L.; Ju, T.W.; Prabakaran, P.; Lau, C.C.Y.; Lu, L.; Liu, Q.; Wang, L.; Feng, Y.; Wang, Y.; et al. Exceptionally Potent Neutralization of Middle East Respiratory Syndrome Coronavirus by Human Monoclonal Antibodies. J. Virol. 2014, 88, 7796–7805. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.C.; Humbert, M.; Buggert, M. The Known Unknowns of T Cell Immunity to COVID-19. Sci. Immunol. 2020, 5, 8063. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.; Lee, C.; Chow, W.; Lee, A.; Tam, A.; Fong, C.; Law, C.; Leung, E.; To, K.; Tan, K.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2020, 106, e926–e935. [Google Scholar] [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic Autoantibodies in Serum from Patients Hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, 3876. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Remsik, J.; Wilcox, J.A.; Babady, N.E.; McMillen, T.A.; Vachha, B.A.; Halpern, N.A.; Dhawan, V.; Rosenblum, M.; Iacobuzio-Donahue, C.A.; Avila, E.K.; et al. Inflammatory Leptomeningeal Cytokines Mediate COVID-19 Neurologic Symptoms in Cancer Patients. Cancer Cell 2021, 39, 276–283.e3. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-las-peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial Dysfunction in Long COVID: Mechanisms, Consequences, and Potential Therapeutic Approaches. Geroscience 2024, 1–20. [Google Scholar] [CrossRef]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle Abnormalities Worsen after Post-Exertional Malaise in Long COVID. Nat. Commun. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.L.; van der Schaaf, M.; van den Borst, B.; Wiersinga, W.J.; van Vugt, M.; Weijs, P.J.M.; Murray, A.J.; et al. Skeletal Muscle Alterations in Patients with Acute COVID-19 and Post-Acute Sequelae of COVID-19. J. Cachexia. Sarcopenia Muscle 2022, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Braillard, O.; Chappuis, F.; Nehme, M.; Braillard, O.; Vetter, P.; Courvoisier, D.S.; Assal, F.; Lador, F.; Benzakour, L.; et al. The Chronification of Post-COVID Condition Associated with Neurocognitive Symptoms, Functional Impairment and Increased Healthcare Utilization. Sci. Rep. 2022, 12, 14505. [Google Scholar] [CrossRef]
- Sansone, D.; Tassinari, A.; Valentinotti, R.; Kontogiannis, D.; Ronchese, F.; Centonze, S.; Maggiore, A.; Cegolon, L.; Filon, F.L. Persistence of Symptoms 15 Months since COVID-19 Diagnosis: Prevalence, Risk Factors and Residual Work Ability. Life 2023, 13, 97. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health Outcomes in People 2 Years after Surviving Hospitalisation with COVID-19: A Longitudinal Cohort Study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abd-Elrahman, N.M.; Bakheet, T.M. Persistence of Symptoms after Improvement of Acute COVID19 Infection, a Longitudinal Study. J. Med. Virol. 2021, 93, 5942–5946. [Google Scholar] [CrossRef]
- Aly, M.A.E.G.; Saber, H.G. Long COVID and Chronic Fatigue Syndrome: A Survey of Elderly Female Survivors in Egypt. Int. J. Clin. Pract. 2021, 75, e14886. [Google Scholar] [CrossRef] [PubMed]
- Arjun, M.C.; Singh, A.K.; Roy, P.; Ravichandran, M.; Mandal, S.; Pal, D.; Das, K.; Gajjala, A.; Venkateshan, M.; Mishra, B.; et al. Long COVID Following Omicron Wave in Eastern India—A Retrospective Cohort Study. J. Med. Virol. 2023, 95, e28214. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female Gender Is Associated with Long COVID Syndrome: A Prospective Cohort Study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Lavelle, B.; Miller, J.; Jimenez, M.; Lim, P.H.; Orban, Z.S.; Clark, J.R.; Tomar, R.; Ludwig, A.; Ali, S.T.; et al. Multidisciplinary Center Care for Long COVID Syndrome–A Retrospective Cohort Study. Am. J. Med. 2023. [Google Scholar] [CrossRef]
- Brandão Neto, D.; Fornazieri, M.A.; Dib, C.; Di Francesco, R.C.; Doty, R.L.; Voegels, R.L.; de Pinna, F.R. Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol. Head Neck Surg. 2021, 164, 512–518. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of Adults with Noncritical COVID-19 Two Months after Symptom Onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Daitch, V.; Yelin, D.; Awwad, M.; Guaraldi, G.; Milić, J.; Mussini, C.; Falcone, M.; Tiseo, G.; Carrozzi, L.; Pistelli, F.; et al. Characteristics of Long-COVID among Older Adults: A Cross-Sectional Study. Int. J. Infect. Dis. 2022, 125, 287–293. [Google Scholar] [CrossRef]
- D’cruz, R.F.; Waller, M.D.; Perrin, F.; Periselneris, J.; Norton, S.; Smith, L.J.; Patrick, T.; Walder, D.; Heitmann, A.; Lee, K.; et al. Chest Radiography Is a Poor Predictor of Respiratory Symptoms and Functional Impairment in Survivors of Severe COVID-19 Pneumonia. ERJ Open Res. 2021, 7, 00655–02020. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan Impairment in Low-Risk Individuals with Post-COVID-19 Syndrome: A Prospective, Community-Based Study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Dennis, A.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Eichert, N.; Mouchti, S.; Pansini, M.; Roca-Fernandez, A.; Thomaides-Brears, H.; et al. Multi-Organ Impairment and Long COVID: A 1-Year Prospective, Longitudinal Cohort Study. J. R. Soc. Med. 2023, 116, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.; Mudara, C.; Vika, C.; Blumberg, L.; Mayet, N.; Cohen, C.; Tempia, S.; Parker, A.; Nel, J.; Perumal, R.; et al. Post-COVID-19 Condition 3 Months after Hospitalisation with SARS-CoV-2 in South Africa: A Prospective Cohort Study. Lancet Glob. Health 2022, 10, e1247–e1256. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Peyser, N.D.; Lin, F.; Knight, S.J.; Djibo, A.; Khatib, R.; Kitzman, H.; O’Brien, E.; Williams, N.; et al. Factors Associated with Long COVID Symptoms in an Online Cohort Study. Open Forum Infect. Dis. 2023, 10, ofad047. [Google Scholar] [CrossRef] [PubMed]
- Elmazny, A.; Magdy, R.; Hussein, M.; Elsebaie, E.H.; Ali, S.H.; Abdel Fattah, A.M.; Hassan, M.; Yassin, A.; Mahfouz, N.A.; Elsayed, R.M.; et al. Neuropsychiatric Post-Acute Sequelae of COVID-19: Prevalence, Severity, and Impact of Vaccination. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1349–1358. [Google Scholar] [CrossRef]
- El Otmani, H.; Nabili, S.; Berrada, M.; Bellakhdar, S.; El Moutawakil, B.; Abdoh Rafai, M. Prevalence, Characteristics and Risk Factors in a Moroccan Cohort of Long-COVID-19. Neurol. Sci. 2022, 43, 5175–5180. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-Discharge Persistent Symptoms and Health-Related Quality of Life after Hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Galal, I.; Hussein, A.A.R.M.; Amin, M.T.; Saad, M.M.; Zayan, H.E.E.; Abdelsayed, M.Z.; Moustafa, M.M.; Ezzat, A.R.; Helmy, R.E.D.; Abd_Elaal, H.K.; et al. Determinants of Persistent Post-COVID-19 Symptoms: Value of a Novel COVID-19 Symptom Score. Egypt. J. Bronchol. 2021, 15, 10. [Google Scholar] [CrossRef]
- Jassat, W.; Mudara, C.; Vika, C.; Welch, R.; Arendse, T.; Dryden, M.; Blumberg, L.; Mayet, N.; Tempia, S.; Parker, A.; et al. A Cohort Study of Post-COVID-19 Condition across the Beta, Delta, and Omicron Waves in South Africa: 6-Month Follow-up of Hospitalized and Nonhospitalized Participants. Int. J. Infect. Dis. 2023, 128, 102–111. [Google Scholar] [CrossRef]
- Khalaf, M.; Alboraie, M.; Abdel-Gawad, M.; Abdelmalek, M.; Abu-Elfatth, A.; Abdelhamed, W.; Zaghloul, M.; Eldeeb, R.; Abdeltwab, D.; Abdelghani, M.; et al. Prevalence and Predictors of Persistent Symptoms After Clearance of SARS-CoV-2 Infection: A Multicenter Study from Egypt. Infect. Drug Resist. 2022, 15, 2575–2587. [Google Scholar] [CrossRef]
- Kozak, R.; Armstrong, S.M.; Salvant, E.; Ritzker, C.; Feld, J.; Biondi, M.J.; Tsui, H. Recognition of Long-COVID-19 Patients in a Canadian Tertiary Hospital Setting: A Retrospective Analysis of Their Clinical and Laboratory Characteristics. Pathogens 2021, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, Lung Function and CT Findings 3 Months after Hospital Admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef]
- Liang, L.; Yang, B.; Jiang, N.; Fu, W.; He, X.; Zhou, Y.; Ma, W.L.; Wang, X. Three-Month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. J. Korean Med. Sci. 2020, 35, e418. [Google Scholar] [CrossRef]
- Marra, A.R.; Sampaio, V.S.; Ozahata, M.C.; Lopes, R.; Brito, A.F.; Bragatte, M.; Kalil, J.; Miraglia, J.L.; Malheiro, D.T.; Guozhang, Y.; et al. Risk Factors for Long Coronavirus Disease 2019 (Long COVID) among Healthcare Personnel, Brazil, 2020–2022. Infect. Control. Hosp. Epidemiol. 2023, 44, 1972–1978. [Google Scholar] [CrossRef]
- Mendelsohn, A.S.; Nath, N.; De Sá, A.; Von Pressentin, K.B. Two Months Follow-up of Patients with Non-Critical COVID-19 in Cape Town, South Africa. S. Afr. Fam. Pract. 2022, 64. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long COVID Outcomes at One Year after Mild SARS-CoV-2 Infection: Nationwide Cohort Study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-Acute COVID-19 Syndrome. Incidence and Risk Factors: A Mediterranean Cohort Study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Perlis, R.H.; Santillana, M.; Ognyanova, K.; Safarpour, A.; Lunz Trujillo, K.; Simonson, M.D.; Green, J.; Quintana, A.; Druckman, J.; Baum, M.A.; et al. Prevalence and Correlates of Long COVID Symptoms Among US Adults. JAMA Netw. Open 2022, 5, E2238804. [Google Scholar] [CrossRef] [PubMed]
- van Gassel, R.J.J.; Bels, J.L.M.; Raafs, A.; van Bussel, B.C.T.; van de Poll, M.C.G.; Simons, S.O.; van der Meer, L.W.L.; Gietema, H.A.; Posthuma, R.; van Santen, S. High Prevalence of Pulmonary Sequelae at 3 Months after Hospital Discharge in Mechanically Ventilated of COVID-19. Am. J. Respir. Crit. Care Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Shah, A.S.; Wong, A.W.; Hague, C.J.; Murphy, D.T.; Johnston, J.C.; Ryerson, C.J.; Carlsten, C. A Prospective Study of 12-Week Respiratory Outcomes in COVID-19-Related Hospitalisations. Thorax 2021, 76, 402–404. [Google Scholar] [CrossRef]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary Recovery after COVID-19: An Observational Prospective Multicentre Trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Benatti, S.V.; Casati, M.; Binda, F.; Zuglian, G.; Imeri, G.; Conti, C.; Biffi, A.M.; Spada, M.S.; Bondi, E.; et al. Surviving COVID-19 in Bergamo Province: A Post-Acute Outpatient Re-Evaluation. Epidemiol. Infect. 2021, 149, e32. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.W.; Shah, A.S.; Johnston, J.C.; Carlsten, C.; Ryerson, C.J. Patient-Reported Outcome Measures after COVID-19: A Prospective Cohort Study. Eur. Respir. J. 2020, 56, 2003276. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent Neurocognitive Deficits after Recovery from Mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef] [PubMed]
- Kinge, C.W.; Hanekom, S.; Lupton-Smith, A.; Akpan, F.; Mothibi, E.; Maotoe, T.; Lebatie, F.; Majuba, P.; Sanne, I.; Chasela, C. Persistent Symptoms among Frontline Health Workers Post-Acute COVID-19 Infection. Int. J. Environ. Res. Public Health 2022, 19, 5933. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical Sequelae of COVID-19 Survivors in Wuhan, China: A Single-Centre Longitudinal Study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zulu, J.E.; Banda, D.; Hines, J.Z.; Luchembe, M.; Sivile, S.; Siwingwa, M.; Kampamba, D.; Zyambo, K.D.; Chirwa, R.; Chirwa, L.; et al. Two-Month Follow-up of Persons with SARS-CoV-2 Infection-Zambia, September 2020: A Cohort Study. Pan Afr. Med. J. 2022, 41, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.N.; Paranhos, A.C.M.; da Silva, L.C.M.; Xavier, S.S.; Silva, C.C.; da Silva, R.; de Vasconcelos, L.A.; Peixoto, I.V.P.; Panzetti, T.M.N.; Tavares, P.R.; et al. Effect of Long COVID-19 Syndrome on Health-Related Quality of Life: A Cross-Sectional Study. Front. Psychol. 2024, 15, 1394068. [Google Scholar] [CrossRef] [PubMed]
- Román-Montes, C.M.; Flores-Soto, Y.; Guaracha-Basañez, G.A.; Tamez-Torres, K.M.; Sifuentes-Osornio, J.; González-Lara, M.F.; de León, A.P. Post-COVID-19 Syndrome and Quality of Life Impairment in Severe COVID-19 Mexican Patients. Front Public Health 2023, 11, 1155951. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-Acute COVID-19 Syndrome (PCS) and Health-Related Quality of Life (HRQoL)—A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Elnadi, H.; Al-Mustapha, A.I.; Odetokun, I.A.; Adeyemi Anjorin, A.; Mosbah, R.; Fasina, F.O.; Razouqi, Y.; Sherrif Awiagah, K.; Nyandwi, B.; Mhgoob, Z.E.; et al. Prevalence of Post COVID-19 Condition among Healthcare: Self-Reported Online Survey in Four African, December 2021–January 2022. COVID 2023, 3, 1663–1676. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The Prevalence and Long-Term Health Effects of Long COVID among Hospitalised and Non-Hospitalised Populations: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 2021, 325, 2015. [Google Scholar] [CrossRef]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Persistent Symptoms 1.5-6 Months after COVID-19 in Non-Hospitalised Subjects: A Population-Based Cohort Study. Thorax 2021, 76, 405–407. [Google Scholar] [CrossRef]
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual Dimorphism in COVID-19: Potential Clinical and Public Health Implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of Post-COVID-19 Symptoms in Hospitalized and Non-Hospitalized COVID-19 Survivors: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-Term and Long-Term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-Month, 6-Month, 9-Month, and 12-Month Respiratory Outcomes in Patients Following COVID-19-Related Hospitalisation: A Prospective Study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Yelin, D.; Margalit, I.; Yahav, D.; Runold, M.; Bruchfeld, J. Long COVID-19—It’s Not over Until? Clin. Microbiol. Infect. 2021, 27, 506–508. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in Children and Adolescents: A Systematic Review and Meta-Analyses. Nat. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Bonifácio, L.; Csizmar, V.; Barbosa-Júnior, F.; Pereira, A.; Koenigkam-Santos, M.; Wada, D.; Gaspar, G.; Carvalho, F.; Bollela, V.; Santana, R.; et al. Long-Term Symptoms among COVID-19 Survivors in Prospective Cohort Study, Brazil. Emerg. Infect. Dis. CDC 2022, 28, 730–733. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, D.A.P.; Gomes, S.V.C.; Filgueiras, P.S.; Corsini, C.A.; Almeida, N.B.F.; Silva, R.A.; Medeiros, M.I.V.A.R.C.; Vilela, R.V.R.; Fernandes, G.R.; Grenfell, R.F.Q. Long COVID-19 Syndrome: A 14-Months Longitudinal Study during the Two First Epidemic Peaks in Southeast Brazil. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 1007–1014. [Google Scholar] [CrossRef]
- Rede de Pesquisa Solidária—FIOCRUZ e USP BoletimPPS_44_12Jan2023. Available online: https://jornal.usp.br/wp-content/uploads/2023/01/BoletimPPS_44_12Jan2023.pdf (accessed on 4 July 2023).
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and Predictors of Acute and Chronic Post-COVID Syndrome: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Qasmieh, S.A.; Kulkarni, S.G.; Teasdale, C.A.; Jones, H.E.; McNairy, M.; Borrell, L.N.; Nash, D. The Epidemiology of Long Coronavirus Disease in US Adults. Clin. Infect. Dis. 2023, 76, 1636–1645. [Google Scholar] [CrossRef]
- World Health Organization. At Least 17 Million People in the WHO European Region Experienced Long COVID in the First Two Years of the Pandemic; Millions May Have to Live with It for Years to Come. Available online: https://www.who.int/europe/news/item/13-09-2022-at-least-17-million-people-in-the-who-european-region-experienced-long-covid-in-the-first-two-years-of-the-pandemic--millions-may-have-to-live-with-it-for-years-to-come (accessed on 23 July 2023).
- Cegolon, L.; Mauro, M.; Sansone, D.; Tassinari, A.; Gobba, F.M.; Modenese, A.; Casolari, L.; Liviero, F.; Pavanello, S.; Scapellato, M.L.; et al. A Multi-Center Study Investigating Long COVID-19 in Healthcare Workers from North-Eastern Italy: Prevalence, Risk Factors and the Impact of Pre-Existing Humoral Immunity—ORCHESTRA Project. Vaccines 2023, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of Long COVID Associated with Delta versus Omicron Variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Margarita, M.; et al. Impact of COVID-19 Vaccination on the Risk of Developing Long-COVID and on Existing Long-COVID Symptoms: A Systematic Review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of Long COVID Symptoms after COVID-19 Vaccination: Community Based Cohort Study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and Its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID Burden and Risk Factors in 10 UK Longitudinal Studies and Electronic Health Records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.J.; McCarthy, F.P.; Direk, K.; Gonzalez-Izquierdo, A.; Prieto-Merino, D.; Casas, J.P.; Chappell, L. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records a CALIBER Study. Circulation 2019, 140, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Abbas-Hanif, A.; Modi, N.; Majeed, A. Long Term Implications of COVID-19 in Pregnancy. BMJ 2022, 377, e071296. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.; Ayuk, P. Post-COVID-19 Condition and Pregnancy. Case Rep. Women’s Health 2022, 37, e00458. [Google Scholar] [CrossRef]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw. Open 2022, 5, e2215787. [Google Scholar] [CrossRef]
- Saini, G.; Aneja, R. Cancer as a Prospective Sequela of Long COVID-19. BioEssays 2021, 43, 2000331. [Google Scholar] [CrossRef] [PubMed]
- Jahankhani, K.; Ahangari, F.; Adcock, I.M.; Mortaz, E. Possible Cancer-Causing Capacity of COVID-19: Is SARS-CoV-2 an Oncogenic Agent? Biochimie 2023, 213, 130–138. [Google Scholar] [CrossRef]
- Smith, C.; Khanna, R. Immune-Based Therapeutic Approaches to Virus-Associated Cancers. Curr. Opin. Virol. 2018, 32, 24–29. [Google Scholar] [CrossRef]
- Passaro, A.; Bestvina, C.; Velez Velez, M.; Garassino, M.C.; Garon, E.; Peters, S. Severity of COVID-19 in Patients with Lung Cancer: Evidence and Challenges. J. Immunother. Cancer 2021, 9, e002266. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, R.; Crowder, S.; Sarma, K.P.; Arthur, A.E.; Pepino, M.Y. Taste and Smell Function in Head and Neck Cancer Survivors. Chem. Senses 2021, 46, bjab026. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Liu, Z.; Tang, L.; Li, L.; Gan, Q.; Shi, H.; Jiao, Q.; Guan, Y.; Xie, M.; He, X.; et al. Longitudinal Clinical and Radiographic Evaluation Reveals Interleukin-6 as an Indicator of Persistent Pulmonary Injury in COVID-19. Int. J. Med. Sci. 2021, 18, 29–41. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Ward, M.M. Validation of New Biomarkers in Systemic Autoimmune Diseases. Nat. Rev. Rheumatol. 2011, 7, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.F.; das Neves, P.F.M.; Lima, S.S.; Lopes, J.d.C.; Torres, M.K.d.S.; Vallinoto, I.M.V.C.; de Brito, M.T.F.M.; da Silva, A.L.S.; Leite, M.d.M.; da Costa, F.P.; et al. Cytokine Profiles Associated with Acute COVID-19 and Long COVID-19 Syndrome. Front. Cell Infect. Microbiol. 2022, 12, 922422. [Google Scholar] [CrossRef] [PubMed]
- das Neves, P.F.M.; Quaresma, J.A.S.; Queiroz, M.A.F.; Silva, C.C.; Maia, E.V.; Oliveira, J.S.d.S.; das Neves, C.M.A.; Mendonça, S.d.S.; Falcão, A.S.C.; Melo, G.S.; et al. Imbalance of Peripheral Temperature, Sympathovagal, and Cytokine Profile in Long COVID. Biology 2023, 12, 749. [Google Scholar] [CrossRef]
- Lammi, V.; Nakanishi, T.; Jones, S.E.; Andrews, S.J.; Karjalainen, J.; Cortés, B.; O’Brien, H.E.; Fulton-Howard, B.E.; Haapaniemi, H.H.; Schmidt, A.; et al. Genome-Wide Association Study of Long COVID. medRxiv 2023, 2023.06.29.23292056. [Google Scholar] [CrossRef]
- da Silva, R.; de Sarges, K.M.L.; Cantanhede, M.H.D.; da Costa, F.P.; dos Santos, E.F.; Rodrigues, F.B.B.; de Nazaré do Socorro de Almeida Viana, M.; de Meira Leite, M.; da Silva, A.L.S.; de Brito, M.T.M.; et al. Thrombophilia and Immune-Related Genetic Markers in Long COVID. Viruses 2023, 15, 885. [Google Scholar] [CrossRef]
- Queiroz, M.A.F.; dos Brito, W.R.S.; Pereira, K.A.S.; Pereira, L.M.S.; da Amoras, E.S.G.; Lima, S.S.; dos Santos, E.F.; da Costa, F.P.; de Sarges, K.M.L.; Cantanhede, M.H.D.; et al. Severe COVID-19 and Long COVID Are Associated with High Expression of STING, CGAS and IFN-α. Sci. Rep. 2024, 14, 4974. [Google Scholar] [CrossRef]

| Studies | Years | n | Ethnicity | Sex | Gravity | Time (Days) | Prevalence LC | Most Common Reported Symptoms | |
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | ||||||||
| [107] | 2021 | 172 | Africa | 65.7% | 34.3% | Mild 87%, Moderate 9%, Severe 4% | 240–300 | 65% | Fatigue (37.3%), Dyspnea (22%), Depression (22%) |
| [108] | 2021 | 115 | Africa | 100% | 0 | - | 45 | 77% | Sleep disturbance (63.5%), Fatigue (57.4%), Stress (56.5%), Sadness (47.8%), Cognitive dysfunction (25.2%), Recurrent falls (25.2%) |
| [109] | 2023 | 524 | Asia | 40.5% | 59.5% | Mild or moderate 96.4%, Severe 2.1% | 73 | 8.2% | Fatigue (34.9%), Cough (27.9%) |
| [42] | 2021 | 110 | Europe | 44% | 56% | Hospitalized 100% | 83 | 74% | Dyspnea (39%), Fatigue (39%), Insomnia (24%), Myalgia (22%) |
| [110] | 2022 | 377 | Europe | 81.7% | 18.3% | Hospitalized 100% | 102 | 69% | Fatigue (39.5%), Dyspnea (28.9%), Myalgias (21.2%), Brain fog (20.2%) |
| [111] | 2023 | 1802 | North America | 55.3% | 44.7% | Mild 81%, Hospitalized 19% | 240 | 55% | Cognitive impairment (51%), Change in lung function (44.9%) |
| [112] | 2021 | 655 | South America | 64.7% | 35.3% | Mild or moderate 92.4%, Severe 7.6% | 76 | 21.8% | Olfactory dysfunction (53.8%), Taste deficit (68.3%) |
| [11] | 2020 | 143 | Europe | 37% | 63% | Hospitalized 100% | 60 | 87% | Fatigue (53%), Dyspnea (43%), Arthralgias (27%), Chest pain (21.7%) |
| [113] | 2021 | 150 | Europe | 56% | 44% | Mild 100% | 60 | 66% | Asthenia (40%), Dyspnea (36.7%), Anosmia (23%), Ageusia (23%) |
| [114] | 2021 | 798 | Africa | 62.9% | 37.1% | Mild 74.3%, Moderate or severe 25.7% | 180 | 77.4% | Fatigue (42.2%), Shortness of breath (41.5%), Headaches (22.1%), Chest pain (20.3%) |
| [115] | 2022 | 2333 | Asia and Europe | 49.2% | 50.8% | Asymptomatic, Mild, Moderate 63%, Severe 37% | 46 | 67.2% | Fatigue (38%), Dyspnea (30%), Myalgia (21.1%), Memory impairment (20.5%) |
| [3] | 2021 | 3762 | Europe and North America | 78.9% | 21.1% | Mild 91.6%, Severe 8.4% | 210 | 55.9% | Fatigue (80%), Malaise (73.3%), Cognitive dysfunction (58.4%), Sensorimotor symptoms (55.7%), Headache (53.6%), Memory problems (51%), Insomnia (21%) |
| [116] | 2021 | 119 | Europe | 38% | 62% | Hospitalized 100% | 60 | 87% | Fatigue (68%), Sleep disorders (57%), Dyspnea (32%), Post-traumatic stress disorder (25%), Anxiety (22%) |
| [117] | 2021 | 201 | Europe | 71% | 29% | Mild 81.6%, Severe 18.4% | 140 | 99% | Fatigue (98%), Myalgia (86.7%), Dyspnea (87.1%), Headache (82.6%), Arthralgias (78.1%), Fever (75.1%), Cough (73. 6%), Chest pain (73.1%), Sore throat (71.1%), Diarrhea (59.2%), Pain (53.7%), Wheezing (48.3%), Muscle weakness (40.3%), Runny nose (33.8%) |
| [118] | 2023 | 536 | Europe | 73% | 27% | Mild 87%, Hospitalized 13% | 360 | 62% | Fatigue (64%), Myalgia (35%), Shortness of breath (47%), Headache (34%), Chest pain (38%) |
| [119] | 2022 | 1873 | South Africa | 51.3% | 48.8% | Mild 29%, Moderate 29%, Severe 42% | 120 | 66.7% | Fatigue (65.5%), headache (21.7%) |
| [120] | 2023 | 1480 | North America | 75.1% | 24.9% | Mild 98.53%, Hospitalized 1.47% | 360 | 32.2% | Fatigue (48.3%), Dyspnea (22.9%), Confusion (22.7%), Headache (21.6%), Anosmia (20.6%), Ageusia (20.6%) |
| [121] | 2023 | 1638 | Africa | 65.3% | 34.7% | Mild 83%, Moderate 11.8%, Severe 4.8% | 90 | 36.5% | Fatigue (24.6%), Mild depression (91.67%), Anxiety (93.25%) |
| [122] | 2022 | 118 | Africa | 71% | 29% | Mild 100% | 120 | 47.5% | Asthenia (25.3%) |
| [123] | 2020 | 120 | Europe | 37.5% | 62.5% | Hospitalized 100% | 110 | 55% | Fatigue (55%), Dyspnea (42%), Memory problems (34%), Sleep disorders (30.8%), Impaired concentration (28%), Hair loss (20%) |
| [124] | 2021 | 430 | Africa | 63.7% | 36.3% | Mild 83.3%%, Moderate 12%, Severe 4.7% | 176 | 86% | Decreased daily activities (57.0%), Nervousness or hopelessness (53.3%), Sleeping troubles (50.9%); Cough (29.3%), Dyspnea (29.1%); Chest pain (32.6%); Anorexia (42.6%), Gastritis (32.3%); Myalgia (60.0%), Arthralgia (57.2%) |
| [26] | 2021 | 100 | Europe | 46% | 54% | Moderate 70%, Severe 30% | 48 | 60–72% | Fatigue (72%), Dyspnea (65%), Stress (47%) |
| [37] | 2021 | 1733 | Asia | 48% | 52% | Hospitalized 100% | 186 | 76% | Fatigue (63%), Muscle weakness (63%), Pain (27%), Dyspnea (26%), Insomnia (26%), Anxiety (23%), Depression (23%), Hair loss (22%) |
| [125] | 2023 | 3700 | South Africa | 59% | 41% | Mild 14%, Moderate or Severe 86% | 180 | 38.5% | Fatigue (32.1%) |
| [126] | 2022 | 538 | Africa | 54.1% | 45.9 | Mild 61.3%, Moderate 31%, Severe 7.7% | 83 | 84.6% | Fatigue (59.1%), Sense of fever (46.5%), Anorexia (24.3%), Diarrhea (24.3%) Loss of taste (21.7%), Loss of smell (22.9%), Headache (21.4%), Cough (20.8), Dyspnea (21%) |
| [127] | 2021 | 223 | North America | 53% | 47% | Mild 71% Moderate or severe 29% | >90 | 28% | Chest Pain (24.2%), Fatigue/Weakness/Tiredness (50%), Hair Loss (35.5%), Anosmia (30%), Brain Fog/Cognitive Issues (22.6%), Anxiety (35%), Insomnia (22.6%) |
| [128] | 2021 | 103 | Europe | 48% | 52% | Hospitalized 100% | 120 | 24% | Dyspnea (52%) |
| [129] | 2020 | 76 | Asia | 72.4% | 27.6% | Hospitalized 100% | 120 | 42% | Chest pain (62%), Dyspnea (61%), Cough (60%), Fatigue (59%), Sputum (43%), Diarrhea (26%), Fever (20%) |
| [44] | 2020 | 66 | Asia | 46.3% | 53.7% | Mild 78.3%, Severe 21.7% | 120 | 55% | Memory loss (28.3%), Myalgia (25%) |
| [130] | 2023 | 7051 | South America | 74.1% | 25.9% | Mild 100% | 180 | 27.4% | Headache (53.4%), Myalgia (46.6%), Arthralgia (46.6%), Nasal congestion (45.1%), Fatigue (38%), Fever (36%), Dyspnea (35%), Cough (28%), Pain throat (27%) |
| [131] | 2022 | 174 | South Africa | 62.1% | 37.9% | Mild 98%, Moderate or Severe 2% | 60 | 60.3% | Fatigue (34.5%), dyspnea (20.1%) |
| [132] | 2023 | 1,913,234 | Asia | 50.6% | 49.4% | Mild 100% | 180–360 | 30% | Dyspnea (35.4%), Weakness (50.2%), Palpitations (22.1), Dizziness (42%), Arthralgia (39.6) |
| [133] | 2021 | 277 | Europe | 47.3% | 52.7% | Mild 34.3%, Severe 65.7% | 77 | 51% | Fatigue (35%), Dyspnea (35%), Anosmia (38.5%), Dysgeusia (38.5%), Myalgias (20%), Arthralgia (20%) |
| [134] | 2022 | 16,091 | North America | 62.6% | 37.4% | Mild 100% | 360 | 14.7% | Fatigue (52.2%), Confusion (45.7%), Loss of smell (43.7%), Brain fog (40.4%), Shortness of breath (39.7%), Headache (33.6%), Insomnia (30%), Anxiety (28.7%), Depression (23.3%), Dizziness (20.6%) |
| [27] | 2021 | 180 | Europe | 54% | 46% | Mild 95.6%, Severe 4.4% | 125 | 55% | Fatigue (28.9%), Anosmia (27.2%) |
| [135] | 2021 | 48 | Europe | 31.2% | 68.8% | Hospitalized 100% | 120 | 92% | Dyspnea (32.5%) |
| [24] | 2022 | 96 | Europe | 55.2% | 44.8% | Hospitalized 32.3% | 360 | 77.1% | Reduced exercise capacity (56.3%), Fatigue (53.1%), Dyspnea (37.5%), Concentration problems (39.6%), Difficulty finding words (32.3%), Insomnia (26%) |
| [136] | 2021 | 60 | North America | 32% | 68% | Hospitalized 100% | 120 | 58% | Dyspnea (20%), Cough (20%) |
| [137] | 2021 | 145 | Europe | 43% | 57% | Mild 25%, Severe 75% | 103 | 41% | Dyspnea (36%), Pain (24%), Night sweats (24%), Insomnia (22%) |
| [10] | 2021 | 4182 | Europe | 57% | 43% | Mild 86.1%, Severe 13.9% | 120 | 20.1% | Fatigue (97.7%), Headache (91.2%) |
| [30] | 2021 | 128 | Europe | 54% | 46% | Mild 44.5%, Severe 55.5% | 75 | 62% | Fatigue (52.3%) |
| [39] | 2021 | 22 | Europe | 27.3% | 72.7% | Hospitalized 100% | 120 | 55% | Dyspnea (48%) |
| [36] | 2021 | 124 | Europe | 40% | 60% | Mild 21.8%, Moderate 41%, Severe 37.1% | 120 | 99% | Fatigue (69%), Functional impairment (64%), Cognitive impairments (36%) |
| [138] | 2021 | 767 | Europe | 33% | 67% | Hospitalized 100% | 81 | 51.4% | Fatigue (51%), Dyspnea (51%), Post-traumatic stress (30.5%) |
| [139] | 2020 | 78 | North America | 36% | 64% | Hospitalized 100% | 120 | 76% | Dyspnea (50%), Cough (23%) |
| [140] | 2020 | 18 | Europe | 57.95 | 42.05% | Mild 33%, Severe 61% | 85 | 78% | Attention deficits (50%, Concentration deficits (44.4%), Memory deficits (44.4%), Difficulty finding words (27.8%) |
| [141] | 2022 | 62 | South Africa | 75.8% | 24.2% | Mild 88.7%, Moderate 6.5%, Severe 4.8% | 120 | 24.2% | Fatigue (42%), Anxiety (34%), Difficulty sleeping (31%), Brain fog (21%); Chest pain (24%); Muscle pain (21%) |
| [142] | 2021 | 538 | Asia | 54.5% | 45.5% | Hospitalized 100% | 97 | 49.6% | Hair loss (28.6%), Fatigue (28.3%), Sweating (23.6%), Post-activity polypnea (21.4%) |
| [35] | 2020 | 55 | Asia | 41.8% | 58.2% | Mild 7.3%, Moderate 85.5%, Severe 7.3% | 120 | 71% | Gastrointestinal symptoms (30.91%) |
| [143] | 2022 | 302 | Africa | 58.7% | 41.3% | Mild 100% | 120 | 17.4% | Headache (25.9%), Cough (37.0%), Chest pain (22.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.; Vallinoto, A.C.R.; dos Santos, E.J.M. The Silent Syndrome of Long COVID and Gaps in Scientific Knowledge: A Narrative Review. Viruses 2024, 16, 1256. https://doi.org/10.3390/v16081256
da Silva R, Vallinoto ACR, dos Santos EJM. The Silent Syndrome of Long COVID and Gaps in Scientific Knowledge: A Narrative Review. Viruses. 2024; 16(8):1256. https://doi.org/10.3390/v16081256
Chicago/Turabian Styleda Silva, Rosilene, Antonio Carlos Rosário Vallinoto, and Eduardo José Melo dos Santos. 2024. "The Silent Syndrome of Long COVID and Gaps in Scientific Knowledge: A Narrative Review" Viruses 16, no. 8: 1256. https://doi.org/10.3390/v16081256





