Twin Screw Granulation: Effects of Properties of Primary Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Powder
2.1.2. Granulator
2.2. Method
2.2.1. Morphology of Powders
2.2.2. Compressibility Factor for Powders
- σ1 = major principal stress
- σ2 = minor principal stress
- ρ1 = powder bulk density at σ1
- ρ2 = powder bulk density at σ2
2.2.3. Preparation of Granules
2.2.4. Size Analysis of Granules
2.2.5. Structural Analysis of Granules
2.2.6. Tableting
2.2.7. Analysis of Tablets
3. Results
3.1. Morphology of Powder
3.2. Compressibility Factor for Powders
3.3. Size of Granules
3.3.1. Lactose
3.3.2. Mannitol
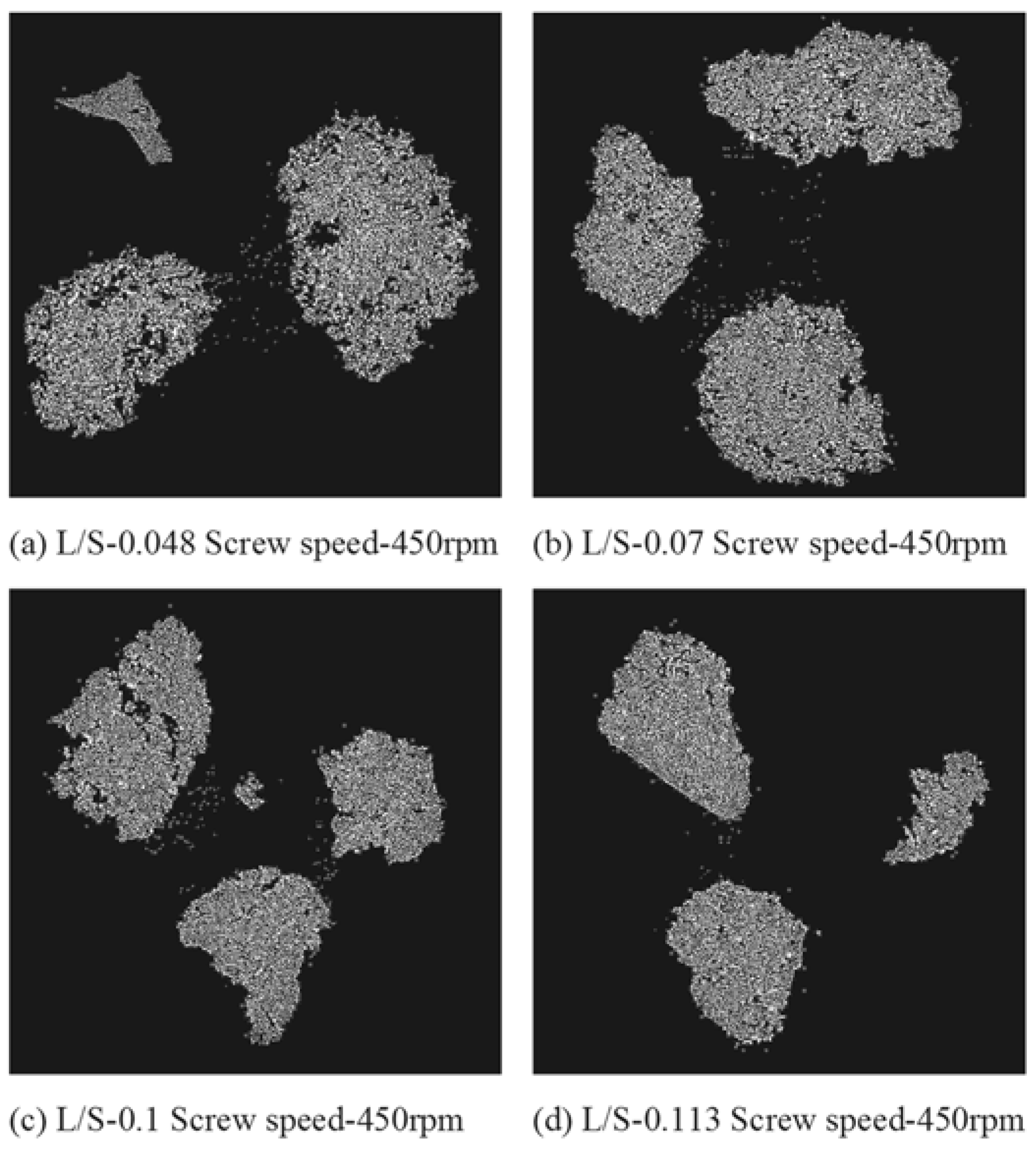
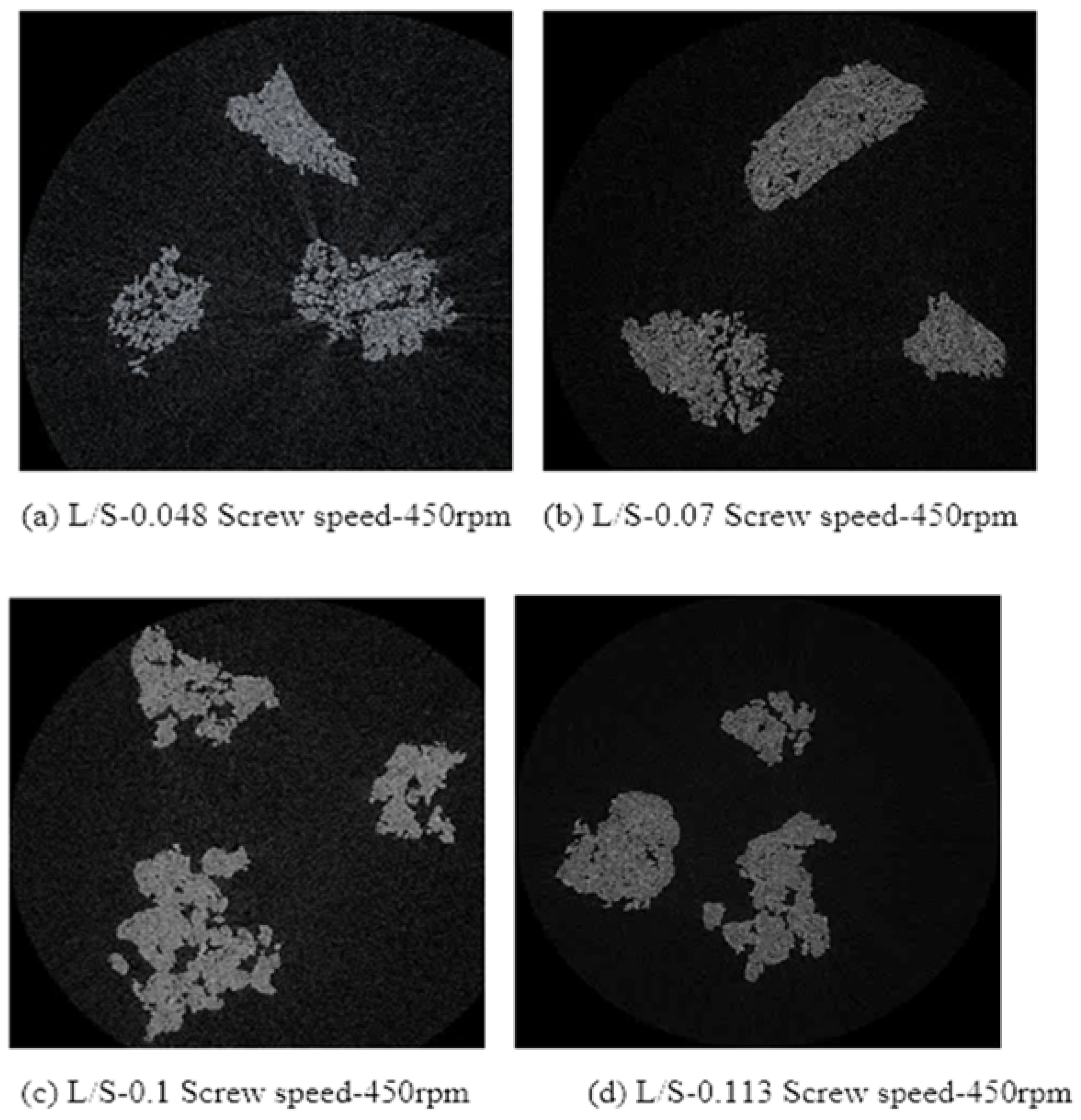
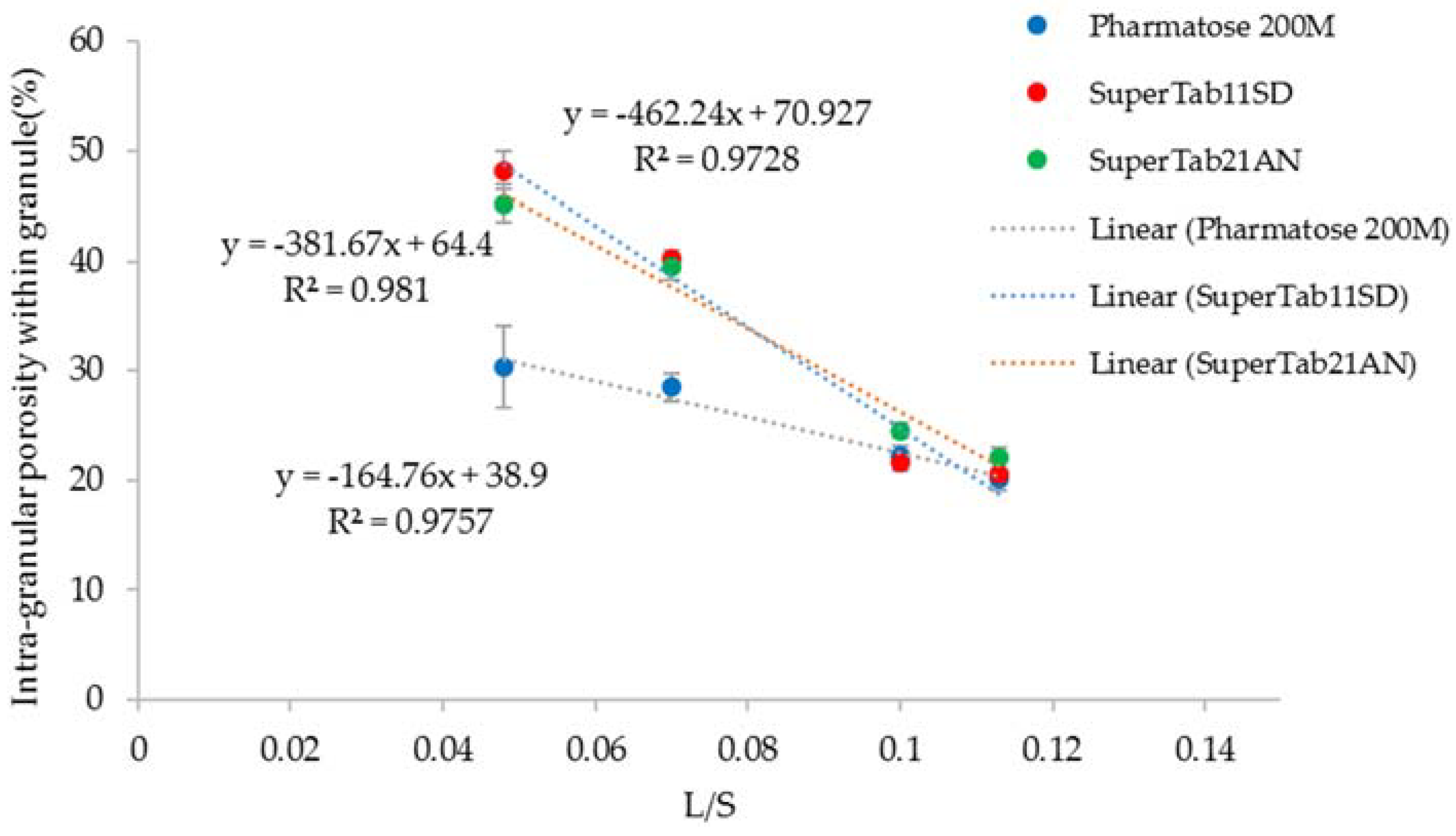
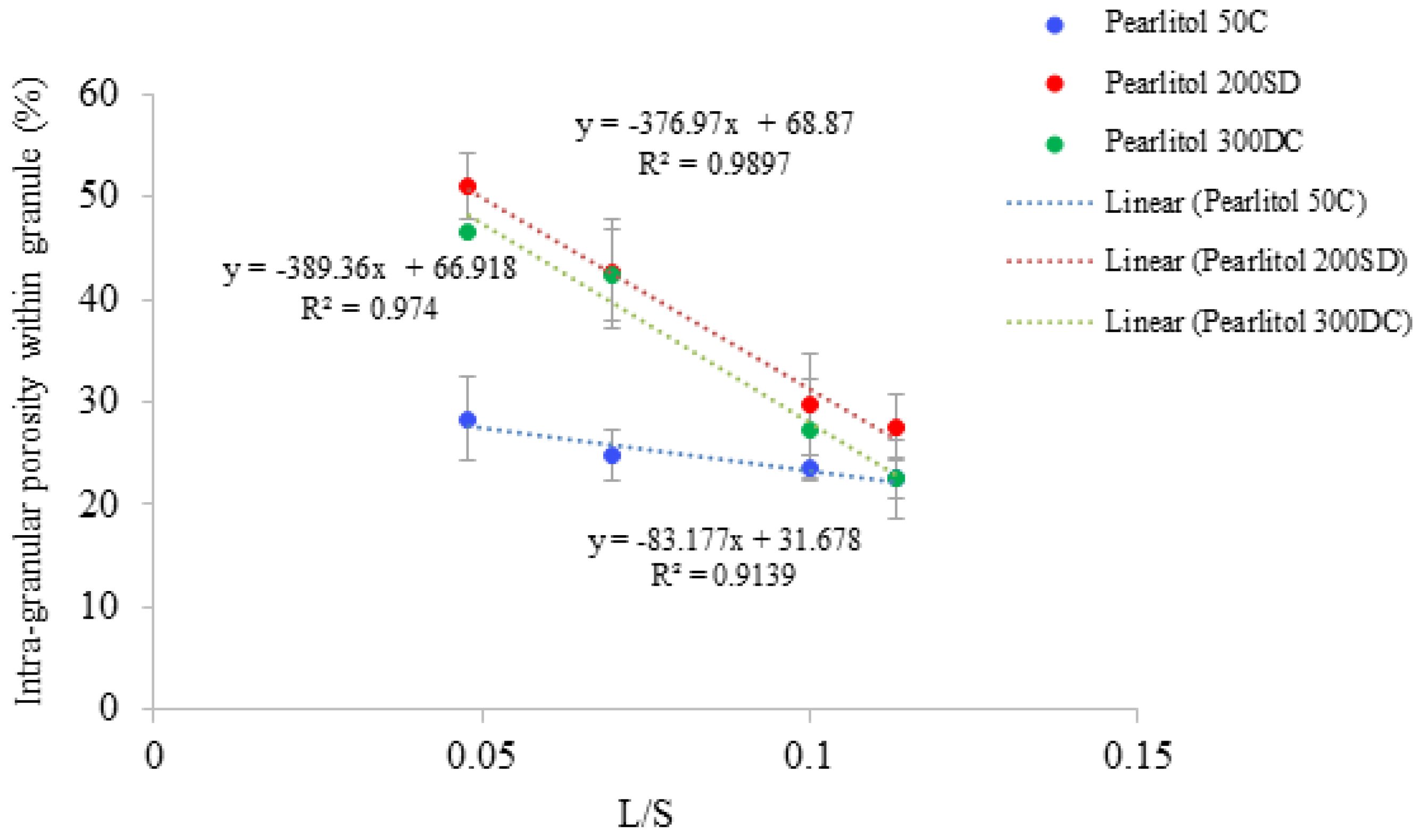
3.4. Structure of Granules
3.5. Tensile Strength of Tablets
3.5.1. Pharmatose 200M
3.5.2. SuperTab11SD
3.5.3. SuperTab21AN
3.5.4. Pearlitol 50C
3.5.5. Pearlitol 200SD
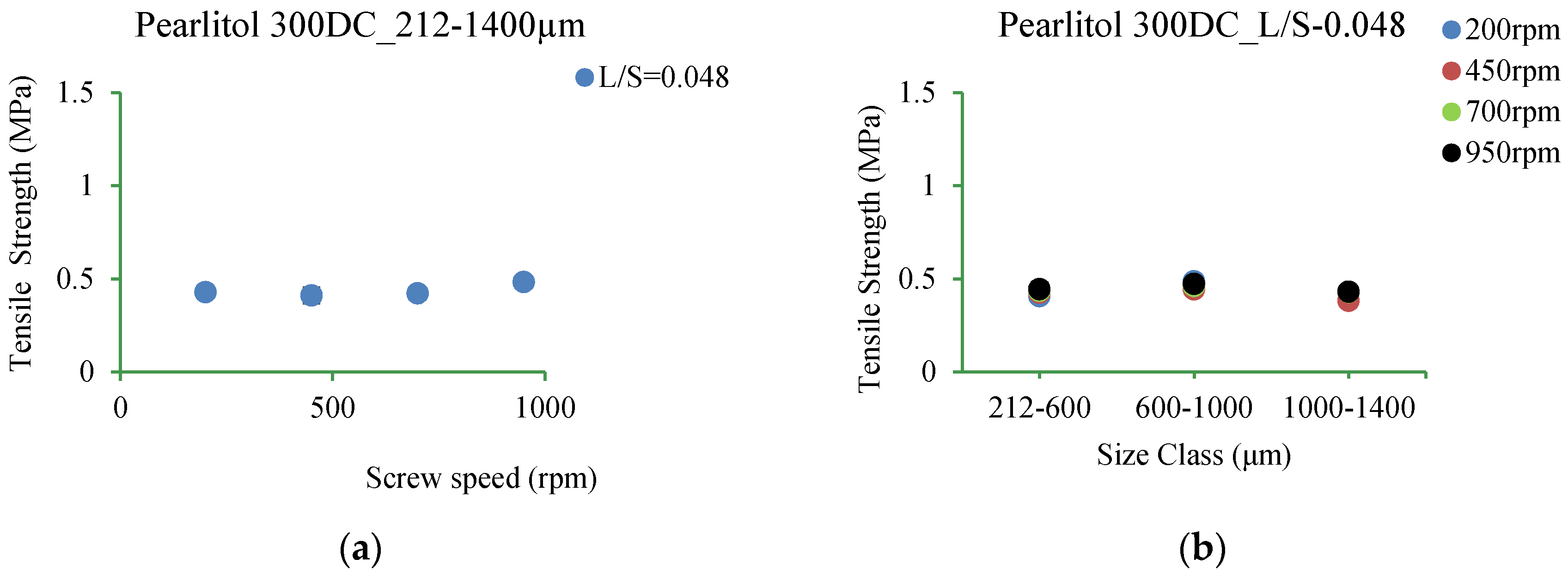
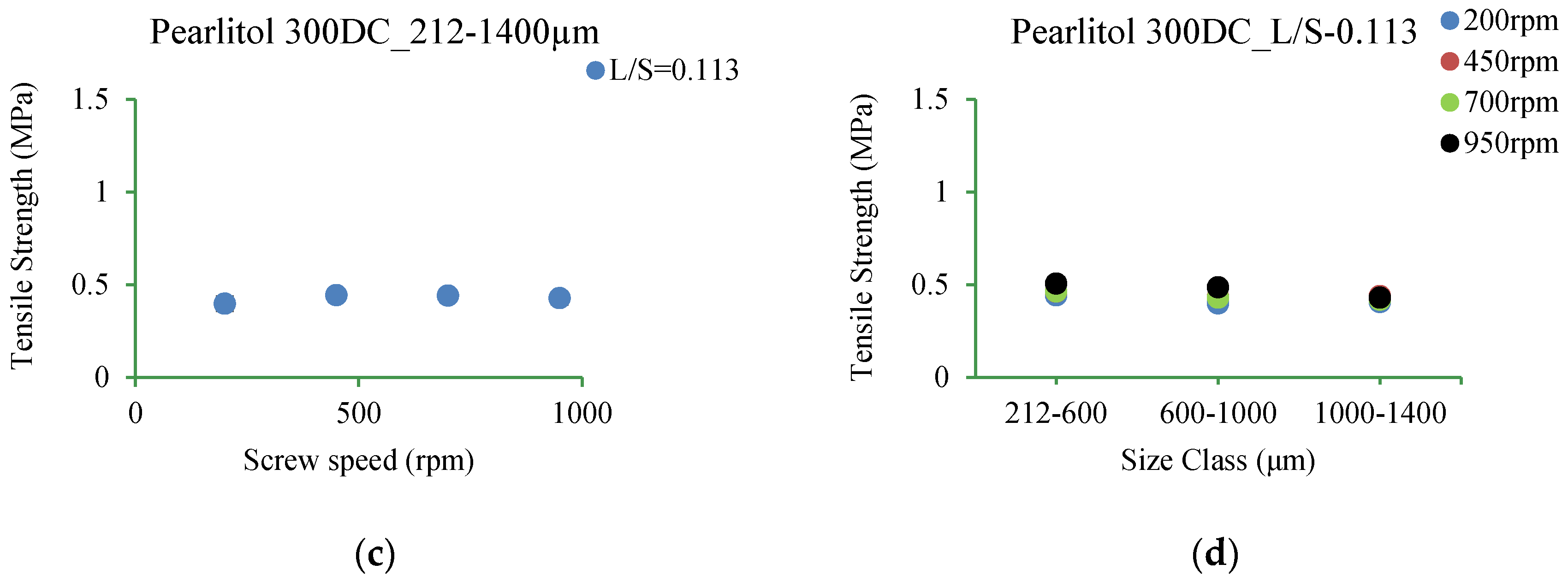
3.5.6. Pearlitol 300DC
4. Discussion
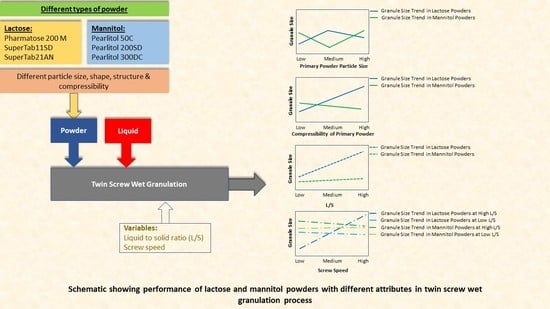
4.1. Size of Granules
4.1.1. Lactose
Effect of Varying L/S
Effect of Varying Screw Speed
4.1.2. Mannitol
Effect of Varying L/S
Effect of Varying Screw Speed
4.2. Structure of Granules
4.3. Tensile Strength of Tablets
4.3.1. Pharmatose 200M
4.3.2. SuperTab11SD
4.3.3. SuperTab21AN
4.3.4. Pearlitol 50C
4.3.5. Pearlitol 200SD
4.3.6. Pearlitol 300DC
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Huang, W.; Shi, Y.; Wang, C.; Yu, K.; Sun, F.; Li, Y. Using spray-dried lactose monohydrate in wet granulation method for a low-dose oral formulation of a paliperidone derivative. Powder Technol. 2013, 246, 379–394. [Google Scholar] [CrossRef]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Keleb, E.I.; Vermeire, A.; Vervaet, C.; Remon, J.P. Single-step granulation/tabletting of different grades of lactose: A comparison with high shear granulation and compression. Eur. J. Pharm. Biopharm. 2004, 58, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lerk, C.F. Consolidation and Compaction of Lactose. Drug Dev. Ind. Pharm. 1993, 19, 2359–2398. [Google Scholar] [CrossRef]
- Kibbe, A.H. Handbook of Pharmaceutical Excipients; American Pharmaceutical Association: Washington, DC, USA, 2000. [Google Scholar]
- Westermarck, S.; Juppo, A.M.; Kervinen, L.; Yliruusi, J. Pore structure and surface area of mannitol powder, granules and tablets determined with mercury porosimetry and nitrogen adsorption. Eur. J. Pharm. Biopharm. 1998, 46, 61–68. [Google Scholar] [CrossRef]
- Wade, A.; Weller, P.J. Handbook of Pharmaceutical Excipients; American Pharmaceutical association: Washington, DC, USA; Pharmaceutical Press: London, UK, 1994. [Google Scholar]
- Debord, B.; Lefebvre, C.; Guyot-Hermann, A.M.; Hubert, J.; Bouché, R.; Cuyot, J.C. Study of Different Crystalline forms of Mannitol: Comparative Behaviour under Compression. Drug Dev. Ind. Pharm. 1987, 13, 1533–1546. [Google Scholar] [CrossRef]
- Gabbott, I.P.; Al Husban, F.; Reynolds, G.K. The combined effect of wet granulation process parameters and dried granule moisture content on tablet quality attributes. Eur. J. Pharm. Biopharm. 2016, 106, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Alvarez–Nunez, F.A.; Rinella, J.V., Jr.; Magnusson, L.-E.; Sueda, K. Roller Compaction, Granulation and Capsule Product Dissolution of Drug Formulations Containing a Lactose or Mannitol Filler, Starch, and Talc. AAPS PharmSciTech 2008, 9, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schæfer, T.; Mathiesen, C. Melt pelletization in a high shear mixer. IX. Effects of binder particle size. Int. J. Pharm. 1996, 139, 139–148. [Google Scholar]
- Hapgood, K.P.; Litster, J.D.; Smith, R. Nucleation regime map for liquid bound granules. AIChE J. 2003, 49, 350–361. [Google Scholar] [CrossRef]
- Kayrak-Talay, D.; Litster, J.D. A priori performance prediction in pharmaceutical wet granulation: Testing the applicability of the nucleation regime map to a formulation with a broad size distribution and dry binder addition. Int. J. Pharm. 2011, 418, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.G.; Holm, P.; Schaefer, T. Mechanical properties of moist agglomerates in relation to granulation mechanisms part II. Effects of particle size distribution. Powder Technol. 1985, 44, 239–247. [Google Scholar] [CrossRef]
- Badawy, S.I.F.; Lee, T.J.; Menning, M.M. Effect of drug substance particle size on the characteristics of granulation manufactured in a high-shear mixer. AAPS PharmSciTech 2000, 1, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackaplow, M.B.; Rosen, L.A.; Michaels, J.N. Effect of primary particle size on granule growth and endpoint determination in high-shear wet granulation. Powder Technol. 2000, 108, 32–45. [Google Scholar] [CrossRef]
- Badawy, S.I.F.; Hussain, M.A. Effect of starting material particle size on its agglomeration behavior in high shear wet granulation. AAPS PharmSciTech 2004, 5, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hagrasy, A.S.; Hennenkamp, J.R.; Burke, M.D.; Cartwright, J.J.; Litster, J.D. Twin screw wet granulation: Influence of formulation parameters on granule properties and growth behavior. Powder Technol. 2013, 238, 108–115. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Bekaert, B.; Peeters, E.; de Beer, T.; Remon, J.P.; Vervaet, C. Improved tabletability after a polymorphic transition of delta-mannitol during twin screw granulation. Int. J. Pharm. 2016, 506, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, C.S.; Dhenge, R.M.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Roller compaction: Effect of relative humidity of lactose powder. Eur. J. Pharm. Biopharm. 2016, 106, 26–37. [Google Scholar] [CrossRef] [PubMed]
- DFE Pharma: Application notes–Anhydrous lactose. Available online: https://www.dfepharma.com/en/excipients/lactose/application-notes/ (accessed on 28 May 2018).
- DFE Pharma: Application notes—Spray-dried lactose. Available online: https://www.dfepharma.com/en/excipients/lactose/application-notes/ (accessed on 28 May 2018).
- Paul, S.; Chang, S.-Y.; Dun, J.; Sun, W.-J.; Wang, K.; Tajarobi, P.; Boissier, C.; Sun, C.C. Comparative analyses of flow and compaction properties of diverse mannitol and lactose grades. Int. J. Pharm. 2018, 546, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Littringer, E.M.; Noisternig, M.F.; Mescher, A.; Schroettner, H.; Walzel, P.; Griesser, U.J.; Urbanetz, N.A. The morphology and various densities of spray dried mannitol. Powder Technol. 2013, 246, 193–200. [Google Scholar] [CrossRef]
- Hulse, W.L.; Forbes, R.T.; Bonner, M.C.; Getrost, M. The characterization and comparison of spray-dried mannitol samples. Drug Dev. Ind. Pharm. 2009, 35, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Sarraguça, M.C.; Fonteyne, M.; Vercruysse, J.; de Leersnyder, F.; Vanhoorne, V.; Bostijn, N.; Verstraeten, M.; Vervaet, C.; Remon, J.P.; et al. Multivariate statistical process control of a continuous pharmaceutical twin-screw granulation and fluid bed drying process. Int. J. Pharm. 2017, 528, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Mangwandi, C.; JiangTao, L.; Albadarin, A.B.; Dhenge, R.M.; Walker, G.M. High shear granulation of binary mixtures: Effect of powder composition on granule properties. Powder Technol. 2015, 270, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Omar, C.S.; Dhenge, R.M.; Osborne, J.D.; Althaus, T.O.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Roller compaction: Effect of morphology and amorphous content of lactose powder on product quality. Int. J. Pharm. 2015, 496, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Littringer, E.M.; Paus, R.; Mescher, A.; Schroettner, H.; Walzel, P.; Urbanetz, N.A. The morphology of spray dried mannitol particles—The vital importance of droplet size. Powder Technol. 2013, 239, 162–174. [Google Scholar] [CrossRef]
- Mitra, B.; Hilden, J.; Litster, J.D. Effects of the granule composition on the compaction behavior of deformable dry granules. Powder Technol. 2016, 291, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Lute, S.V.; Dhenge, R.M.; Hounslow, M.J.; Salman, A.D. Twin screw granulation: Understanding the mechanism of granule formation along the barrel length. Chem. Eng. Res. Design 2016, 110, 43–53. [Google Scholar] [CrossRef]
- Iveson, S.M.; Litster, J.D.; Hapgood, K.; Ennis, B.J. Nucleation, growth and breakage phenomena in agitated wet granulation processes: A review. Powder Technol. 2001, 117, 3–39. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Vanbillemont, B.; Vercruysse, J.; de Leersnyder, F.; Gomes, P.; Beer, T.D.; Remon, J.P.; Vervaet, C. Development of a controlled release formulation by continuous twin screw granulation: Influence of process and formulation parameters. Int. J. Pharm. 2016, 505, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.F.; Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Binder delivery. Int. J. Pharm. 2015, 487, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Burggraeve, A.; Fonteyne, M.; Cappuyns, P.; Delaet, U.; van Assche, I.; de Beer, T.; Remon, J.P.; Vervaet, C. Impact of screw configuration on the particle size distribution of granules produced by twin screw granulation. Int. J. Pharm. 2015, 479, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alakarjula, M.; Vanhoorne, V.; Toiviainen, M.; de Leersnyder, F.; Vercruysse, J.; Juuti, M.; Ketolainen, J.; Vervaet, C.; Remon, J.P.; et al. Linking granulation performance with residence time and granulation liquid distributions in twin-screw granulation: An experimental investigation. Eur. J. Pharm. Sci. 2016, 90, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329. [Google Scholar] [CrossRef]
- Bouffard, J.; Kaster, M.; Dumont, H. Influence of Process Variable and Physicochemical Properties on the Granulation Mechanism of Mannitol in a Fluid Bed Top Spray Granulator. Drug Dev. Ind. Pharm. 2005, 31, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Souihi, N.; Dumarey, M.; Wikström, H.; Tajarobi, P.; Fransson, M.; Svensson, O.; Josefson, M.; Trygg, J. A quality by design approach to investigate the effect of mannitol and dicalcium phosphate qualities on roll compaction. Int. J. Pharm. 2013, 447, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Dhenge, R.M.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effect of powder feed rate. Adv. Powder Technol. 2011, 22, 162–166. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effects of properties of granulation liquid. Powder Technol. 2012, 229, 126–136. [Google Scholar] [CrossRef]
- Vercruysse, J.; Díaz, D.C.; Peeters, E.; Fonteyne, M.; Delaet, U.; van Assche, I.; de Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, R.; Moll, K.-P.; Krumme, M.; Kleinebudde, P. Impact of fill-level in twin-screw granulation on critical quality attributes of granules and tablets. Eur. J. Pharm. Biopharm. 2017, 115, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Djuric, D.; Kleinebudde, P. Impact of screw elements on continuous granulation with a twin-screw extruder. J. Pharm. Sci. 2008, 97, 4934–4942. [Google Scholar] [CrossRef] [PubMed]
- Fonteyne, M.; Correia, A.; de Plecker, S.; Vercruysse, J.; Ilić, I.; Zhou, Q.; Vervaet, C.; Remon, J.P.; Onofre, F.; Bulone, V.; et al. Impact of microcrystalline cellulose material attributes: A case study on continuous twin screw granulation. Int. J. Pharm. 2015, 478, 705–717. [Google Scholar] [CrossRef] [PubMed]

















| Experiment | Powder Type | Powder Feed Rate (kg/h) | Screw Speed (rpm) | L/S | Liquid Binder |
|---|---|---|---|---|---|
| Effect of powder type | Pharmatose 200M | 2 | 200, 450, 700, 950 | 0.048, 0.07, 0.1, 0.113 | Distilled water |
| SuperTab11SD | |||||
| SuperTab21AN | |||||
| Pearlitol 50C | |||||
| Pearlitol 200SD | |||||
| Pearlitol 300DC |
| Powder Grade | Particle Size (μm) | ||
|---|---|---|---|
| d10 | d50 | d90 | |
| Pharmatose 200M | 9.3 | 42.1 | 110.0 |
| SuperTab11SD | 45.0 | 113.4 | 191.3 |
| SuperTab21AN | 27.5 | 172.3 | 330.0 |
| Pearlitol 50C | 10.1 | 33.9 | 114.9 |
| Pearlitol 200SD | 26.0 | 145.3 | 200.9 |
| Pearlitol 300DC | 58.3 | 249.9 | 385.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lute, S.V.; Dhenge, R.M.; Salman, A.D. Twin Screw Granulation: Effects of Properties of Primary Powders. Pharmaceutics 2018, 10, 68. https://doi.org/10.3390/pharmaceutics10020068
Lute SV, Dhenge RM, Salman AD. Twin Screw Granulation: Effects of Properties of Primary Powders. Pharmaceutics. 2018; 10(2):68. https://doi.org/10.3390/pharmaceutics10020068
Chicago/Turabian StyleLute, Sushma V., Ranjit M. Dhenge, and Agba D. Salman. 2018. "Twin Screw Granulation: Effects of Properties of Primary Powders" Pharmaceutics 10, no. 2: 68. https://doi.org/10.3390/pharmaceutics10020068
APA StyleLute, S. V., Dhenge, R. M., & Salman, A. D. (2018). Twin Screw Granulation: Effects of Properties of Primary Powders. Pharmaceutics, 10(2), 68. https://doi.org/10.3390/pharmaceutics10020068