Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Preparation of Powders
2.4. Estimation of Drug Content
2.5. Minimal Inhibitory Concentration (MIC) against M. Tuberculosis
2.6. Morphology and Particle Size
2.7. Thermal Analysis
2.7.1. Differential Scanning Calorimetry
2.7.2. Hot-Stage Microscopy
2.8. Crystallinity
2.9. Drug-Excipient Interaction
2.10. In Vitro Aerosolization
2.11. Cytotoxicity
2.12. Stability
2.13. Statistical Analysis
3. Results and Discussion
3.1. Process Yield and Drug Content
3.2. Minimal Inhibitory Concentration (MIC)
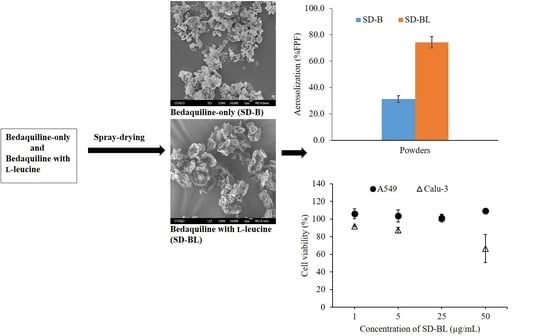
3.3. Morphology and Particle Size
3.4. Crystallinity
3.5. Thermal Analysis
3.6. Drug-Excipient Interaction Study
3.7. Aerosolization
3.8. Stability
3.9. Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Tuberculosis Report 2018. World Health Organization: Geneva, Switzerland. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 29 October 2018).
- Goldman, R.C.; Plumley, K.V.; Laughon, B.E. The evolution of extensively drug resistant tuberculosis (XDR-TB): History, status and issues for global control. Infect. Disord. Drug Targets 2007, 7, 73–91. [Google Scholar] [CrossRef]
- Rawal, T.; Butani, S. Combating tuberculosis infection: A forbidding challenge. Indian J. Pharm. Sci. 2016, 78, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.; Laessig, K. FDA approval of bedaquiline—the benefit–risk balance for drug-resistant tuberculosis. N. Eng. J. Med. 2014, 371, 689–691. [Google Scholar] [CrossRef]
- Huitric, E.; Verhasselt, P.; Andries, K.; Hoffner, S.E. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob. Agents Chemother. 2007, 51, 4202–4204. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Andries, K.; Lounis, N.; Chauffour, A.; Truffot-Pernot, C.; Jarlier, V.; Veziris, N. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Field, S.K. Bedaquiline for the treatment of multidrug-resistant tuberculosis: Great promise or disappointment? Ther. Adv. Chronic Dis. 2015, 6, 170–184. [Google Scholar] [CrossRef]
- Diacon, A.H.; Pym, A.; Grobusch, M.; Patientia, R.; Rustomjee, R.; Page-Shipp, L.; Pistorius, C.; Krause, R.; Bogoshi, M.; Churchyard, G.; et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Eng. J. Med. 2009, 360, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; Bantubani, N.; Narasimooloo, R.; De Marez, T.; Van Heeswijk, R.; et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: Long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef]
- Diacon, A.H.; Pym, A.; Grobusch, M.P.; de Los Rios, J.M.; Gotuzzo, E.; Vasilyeva, I.; Leimane, V.; Andries, K.; Bakare, N.; De Marez, T.; et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Eng. J. Med. 2014, 371, 723–732. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Guglielmetti, L.; Hewison, C.; Bakare, N.; Bastard, M.; Caumes, E.; Fréchet-Jachym, M.; Robert, J.; Veziris, N.; Khachatryan, N.; et al. Outcomes of Bedaquiline Treatment in Patients with Multidrug-Resistant Tuberculosis. Emerg. Infect. Dis. 2019, 25, 936–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.; Mudaly, V.; Voget, J.; Naledi, T.; Maartens, G.; Cohen, K. Adverse drug reactions in South African patients receiving bedaquiline-containing tuberculosis treatment: An evaluation of spontaneously reported cases. BMC Infect. Dis. 2019, 19, 544. [Google Scholar] [CrossRef]
- Sacks, L.V.; Pendle, S.; Orlovic, D.; Andre, M.; Popara, M.; Moore, G.; Thonell, L.; Hurwitz, S. Adjunctive salvage therapy with inhaled aminoglycosides for patients with persistent smear-positive pulmonary tuberculosis. Clin. Infect. Dis. 2001, 32, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Le Cocguic, Y.; Ferroni, A.; Clairicia, M.; Barthe, J.; Delaunay, J.P.; Brousse, V.; Lenoir, G. Nebulized antibiotics in cystic fibrosis. Pediatric Drugs 2002, 4, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, L.; Muttil, P.; Fallon, J.K.; Kabadi, M.; Gerety, R.; Hickey, A.J. Pharmacokinetics of sequential doses of capreomycin powder for inhalation in guinea pigs. Antimicrob. Agents Chemother. 2012, 56, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, L.; Sung, J.; Ibrahim, M.; Elbert, K.; Edwards, D.; Hickey, A. Pharmacokinetics of inhaled rifampicin porous particles for tuberculosis treatment: Insight into rifampicin absorption from the lungs of guinea pigs. Mol. Pharm. 2015, 12, 2642–2650. [Google Scholar] [CrossRef]
- Das, S.; Tucker, I.; Stewart, P. Inhaled dry powder formulations for treating tuberculosis. Curr. Drug Deliv. 2015, 12, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Masters, K. Spray Drying Handbook, 5th ed.; Longman Scientific & Technical: Harlow, UK, 1991. [Google Scholar]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Chew, N.Y.; Shekunov, B.Y.; Tong, H.H.; Chow, A.H.; Savage, C.; Wu, J.; Chan, H.K. Effect of amino acids on the dispersion of disodium cromoglycate powders. J. Pharm. Sci. 2005, 94, 2289–2300. [Google Scholar] [CrossRef]
- Li, H.Y.; Seville, P.C.; Williamson, I.J.; Birchall, J.C. The use of amino acids to enhance the aerosolisation of spray-dried powders for pulmonary gene therapy. J. Gene Med. 2005, 7, 343–353. [Google Scholar] [CrossRef]
- Seville, P.C.; Learoyd, T.P.; Li, H.Y.; Williamson, I.J.; Birchall, J.C. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Sou, T.; Orlando, L.; McIntosh, M.P.; Kaminskas, L.M.; Morton, D.A. Investigating the interactions of amino acid components on a mannitol-based spray-dried powder formulation for pulmonary delivery: A design of experiment approach. Int. J. Pharm. 2011, 421, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Boraey, M.A.; Hoe, S.; Sharif, H.; Miller, D.P.; Lechuga-Ballesteros, D.; Vehring, R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol. 2013, 236, 171–178. [Google Scholar] [CrossRef]
- Sou, T.; McIntosh, M.P.; Kaminskas, L.M.; Prankerd, R.J.; Morton, D.A. Designing a multicomponent spray-dried formulation platform for pulmonary delivery of biomacromolecules: The effect of polymers on the formation of an amorphous matrix for glassy state stabilization of biomacromolecules. Dry. Technol. 2013, 31, 1451–1458. [Google Scholar] [CrossRef]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.A.M.; Sinha, S.; Tucker, I.G.; Doyle, C.; Das, S.C. Dry powder formulation of kanamycin with enhanced aerosolization efficiency for drug-resistant tuberculosis. Int. J. Pharm. 2017, 528, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Patel, S.; Butani, S. Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur. J. Pharm. Sci. 2018, 124, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J.J.G.C.F.W.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Tucker, I.G.; Das, S.C. High dose dry powder inhalers to overcome the challenges of tuberculosis treatment. Int. J. Pharm. 2018, 550, 398–417. [Google Scholar] [CrossRef]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. Phospholipid-based pyrazinamide spray-dried inhalable powders for treating tuberculosis. Int. J. Pharm. 2016, 506, 174–183. [Google Scholar] [CrossRef]
- Eedara, B.B.; Rangnekar, B.; Doyle, C.; Cavallaro, A.; Das, S.C. The influence of surface active l-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the improvement of aerosolization of pyrazinamide and moxifloxacin co-spray dried powders. Int. J. Pharm. 2018, 542, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.A.M.; Tucker, I.G.; Doyle, C.S.; Denman, J.A.; Sinha, S.; Das, S.C. Co-spray drying of hygroscopic kanamycin with the hydrophobic drug rifampicin to improve the aerosolization of kanamycin powder for treating respiratory infections. Int. J. Pharm. 2018, 541, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.A.M.; Sinha, S.; Tucker, I.G.; Das, S.C. Carrier-free combination dry powder inhaler formulation of ethionamide and moxifloxacin for treating drug-resistant tuberculosis. Drug Devel. Ind. Pharm. 2019, 45, 1–11. [Google Scholar] [CrossRef]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q.T. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q.T. Physico-chemical properties, aerosolization and dissolution of co-spray dried azithromycin particles with l-leucine for inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef] [PubMed]
- Jong, T.; Li, J.; Morton, D.A.; Zhou, Q.T.; Larson, I. Investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J. Pharm. Sci. 2016, 105, 1156–1163. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Methodology. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 6 November 1996. [Google Scholar]
- Alffenaar, J.W.C.; Bolhuis, M.; van Hateren, K.; Sturkenboom, M.; Akkerman, O.; de Lange, W.; Greijdanus, B.; van der Werf, T.; Touw, D. Determination of bedaquiline in human serum using liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 2015, 59, 5675–5680. [Google Scholar] [CrossRef]
- Santoso, K.T.; Menorca, A.; Cheung, C.Y.; Cook, G.M.; Stocker, B.L.; Timmer, M.S. The synthesis and evaluation of quinolinequinones as anti-mycobacterial agents. Bioorg. Med. Chem. 2019, 27, 3532–3545. [Google Scholar] [CrossRef]
- Bardarov, S.; Bardarov Jr, S.; Pavelka Jr, M.S.; Sambandamurthy, V.; Larsen, M.; Tufariello, J.; Chan, J.; Hatfull, G.; Jacobs Jr, W.R. Specialized transduction: An efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiol. 2002, 148, 3007–3017. [Google Scholar] [CrossRef]
- Sambandamurthy, V.K.; Wang, X.; Chen, B.; Russell, R.G.; Derrick, S.; Collins, F.M.; Morris, S.L.; Jacobs Jr, W.R. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 2002, 8, 1171–1174. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Tucker, I.G.; Doyle, C.S.; Denman, J.A.; Das, S.C. Manipulation of spray-drying conditions to develop dry powder particles with surfaces enriched in hydrophobic material to achieve high aerosolization of a hygroscopic drug. Int. J. Pharm. 2018, 543, 318–327. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.H.; Chan, H.K.; Price, R. A critical view on lactose based drug formulation and device studies for dry powder inhalation: Which are relevant and what interactions to expect? Adv. Drug Deliv. Rev. 2012, 64, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.G.; Duke, C.C.; Ong, H.X.; Chan, J.C.; Tyne, A.S.; Chan, H.K.; Britton, W.J.; Young, P.M.; Traini, D. A novel inhalable form of rifapentine. J. Pharm. Sci. 2014, 103, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.G.Y.; Chan, H.K.; Prestidge, C.A.; Denman, J.A.; Young, P.M.; Traini, D. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur. J. Pharm. Biopharm. 2013, 83, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Mueannoom, W.; Gaisford, S.; Kett, V.L. Investigation into the effect of varying l-leucine concentration on the product characteristics of spray-dried liposome powders. J. Pharm. Pharmacol. 2012, 64, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Howes, T.; Lecomte, D.; Bhandari, B.R. A glass transition temperature approach for the prediction of the surface stickiness of a drying droplet during spray drying. Powder Technol. 2005, 149, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Lopez, B.; Siqueira de Oliveira, R.; Pinhata, J.M.; Chimara, E.; Pacheco Ascencio, E.; Puyén Guerra, Z.M.; Wainmayer, I.; Simboli, N.; Del Granado, M.; Palomino, J.C.; et al. Bedaquiline and linezolid MIC distributions and epidemiological cut-off values for Mycobacterium tuberculosis in the Latin American region. J. Antimicrob. Chemother. 2018, 74, 373–379. [Google Scholar] [CrossRef]
- Bosquillon, C.; Rouxhet, P.G.; Ahimou, F.; Simon, D.; Culot, C.; Préat, V.; Vanbever, R. Aerosolization properties, surface composition and physical state of spray-dried in powders. J. Controlled Rel. 2004, 99, 357–367. [Google Scholar] [CrossRef]
- Kim, E.H.J.; Dong Chen, X.; Pearce, D. On the mechanisms of surface formation and the surface compositions of industrial milk powders. Dry. Technol. 2003, 21, 265–278. [Google Scholar] [CrossRef]
- Zeng, X.M.; Martin, G.P.; Marriott, C. The controlled delivery of drugs to the lung. Int. J. Pharm. 1995, 124, 149–164. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R.W.M. Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties. AAPS Pharmscitech. 2009, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Behara, S.R.B.; Morton, D.A.; Larson, I.; Stewart, P.J. Importance of particle size and shape on the tensile strength distribution and de-agglomeration of cohesive powders. Powder Technol. 2013, 249, 297–303. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Zhang, X.Y.; Wang, X.Z.; Chai, J.; Luo, H.Y.; Yang, Z.Q. Crystal forms of bedaquiline fumarate and preparation methods therefor. U.S. Patent 0265473A1, 20 September 2018. [Google Scholar]
- Li, L.; Sun, S.; Parumasivam, T.; Denman, J.A.; Gengenbach, T.; Tang, P.; Mao, S.; Chan, H.K. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016, 102, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A. Relationship between surface concentration of L-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef]
- Adi, H.; Traini, D.; Chan, H.K.; Young, P.M. The influence of drug morphology on aerosolisation efficiency of dry powder inhaler formulations. J. Pharm. Sci. 2008, 97, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.C.; Bennett, D.J.; Bartus, R.T.; Emerich, D.F. Pulmonary Delivery for Levodopa. U.S. Patent 9,155,699 B2, 13 October 2015. [Google Scholar]
- Zijlstra, G.S.; Hinrichs, W.L.; de Boer, A.H.; Frijlink, H.W. The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix. Eur. J. Pharm. Sci. 2004, 23, 139–149. [Google Scholar] [CrossRef]
- El-Sabawi, D.; Edge, S.; Price, R.; Young, P.M. Continued investigation into the influence of loaded dose on the performance of dry powder inhalers: Surface smoothing effects. Drug Dev. Ind. Pharm. 2006, 32, 1135–1138. [Google Scholar] [CrossRef]
- Chew, N.Y.; Tang, P.; Chan, H.K.; Raper, J.A. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm. Res. 2005, 22, 148–152. [Google Scholar] [CrossRef]
- Glover, W.; Chan, H.K.; Eberl, S.; Daviskas, E.; Verschuer, J. Effect of particle size of dry powder mannitol on the lung deposition in healthy volunteers. Int. J. Pharm. 2008, 349, 314–322. [Google Scholar] [CrossRef]
- Chan, J.G.Y.; Tyne, A.S.; Pang, A.; Chan, H.K.; Young, P.M.; Britton, W.J.; Duke, C.C.; Traini, D. A rifapentine-containing inhaled triple antibiotic formulation for rapid treatment of tubercular infection. Pharm. Res. 2014, 31, 1239–1253. [Google Scholar] [CrossRef]
- Yu, J.; Chan, H.K.; Gengenbach, T.; Denman, J.A. Protection of hydrophobic amino acids against moisture-induced deterioration in the aerosolization performance of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2017, 119, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Pezron, I.; Li, Y.; Mitra, A.K. Enhanced pulmonary delivery of insulin by lung lavage fluid and phospholipids. Int. J. Pharm. 2001, 217, 25–31. [Google Scholar] [CrossRef]
- Codrons, V.; Vanderbist, F.; Ucakar, B.; Préat, V.; Vanbever, R. Impact of formulation and methods of pulmonary delivery on absorption of parathyroid hormone (1–34) from rat lungs. J. Pharm. Sci. 2004, 93, 1241–1252. [Google Scholar] [CrossRef] [PubMed]









| Compound | MIC (µg/mL) |
|---|---|
| Bedaquiline | 0.1 |
| Bedaquiline with l-leucine | 0.1 |
| l-leucine | ND |
| Formulation | SD-B | SD-BL |
|---|---|---|
| Recovery (%) | 84.1 ± 0.6 | 91.4 ± 1.5 |
| Emitted dose, ED (%) | 80.8 ± 2.7 | 81.6 ± 3.2 |
| Fine particle fraction, FPF (%ED) | 31.3 ± 2.5 | 74.4 ± 4.3 |
| Fine particle fraction, FPF (%RD) | 25.3 ± 1.2 | 60.8 ± 5.8 |
| Mass median aerodynamic diameter, MMAD (µm) | 5.9 ± 0.1 | 2.4 ± 0.2 |
| Geometric standard deviation, GSD | 2.7 ± 0.1 | 2.0 ± 0.1 |
| Conditions | Initial | Desiccator | 75% RH |
|---|---|---|---|
| Recovery (%) | 91.4 ± 1.5 | 89.0 ± 4.0 | 102.0 ± 3.0 |
| ED (%) | 81.6 ± 3.2 | 70.0 ± 1.0 | 75.0 ± 1.0 |
| FPF (%) | 74.4 ± 4.3 | 72.0 ± 5.0 | 63.0 ± 7.0 |
| MMAD (µm) | 2.4 ± 0.2 | 2.9 ± 0.4 | 3.1 ± 0.5 |
| GSD | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momin, M.A.M.; Rangnekar, B.; Sinha, S.; Cheung, C.-Y.; Cook, G.M.; Das, S.C. Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies. Pharmaceutics 2019, 11, 502. https://doi.org/10.3390/pharmaceutics11100502
Momin MAM, Rangnekar B, Sinha S, Cheung C-Y, Cook GM, Das SC. Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies. Pharmaceutics. 2019; 11(10):502. https://doi.org/10.3390/pharmaceutics11100502
Chicago/Turabian StyleMomin, Mohammad A. M., Bhamini Rangnekar, Shubhra Sinha, Chen-Yi Cheung, Gregory M. Cook, and Shyamal C. Das. 2019. "Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies" Pharmaceutics 11, no. 10: 502. https://doi.org/10.3390/pharmaceutics11100502
APA StyleMomin, M. A. M., Rangnekar, B., Sinha, S., Cheung, C.-Y., Cook, G. M., & Das, S. C. (2019). Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies. Pharmaceutics, 11(10), 502. https://doi.org/10.3390/pharmaceutics11100502