Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Manufacturing and Side Chain Modification
2.1.1. 3D Printing Technique
2.1.2. PLA PVTP Modification
2.2. Material Structure Characterisation
2.2.1. FT Infra-Red Spectroscopy
2.2.2. Contact Angle Measurement
2.2.3. Scanning Electron Microscopy
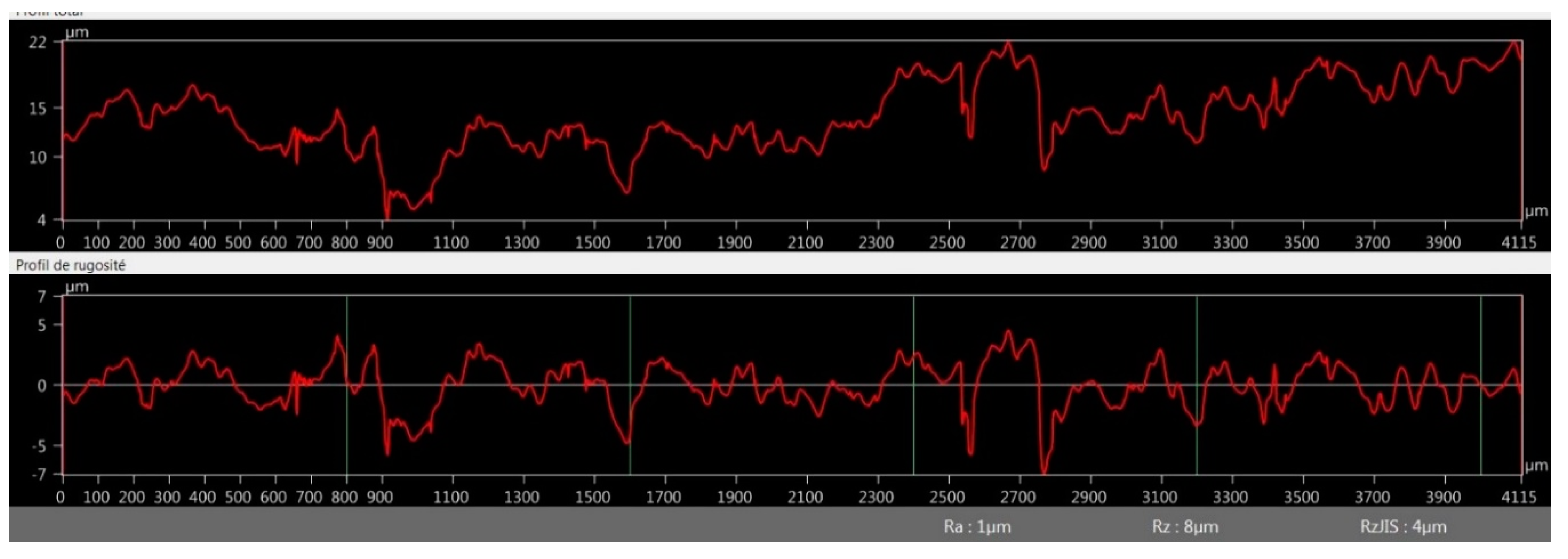
2.2.4. Surface Roughness Measurement
2.2.5. Positron Annihilation Lifetime Spectrometry
2.3. Cytotoxicity Experiments
2.3.1. Cytotoxicity PLA-Based PVTPs Sterilization
2.3.2. Cell Culture
2.3.3. MTT Cell Viability Test
2.3.4. Biofilm Formation
2.4. Statistical Analysis
3. Results
3.1. Implant Manufacturing and Side Chain Modification
3.2. Material Structure Characterization
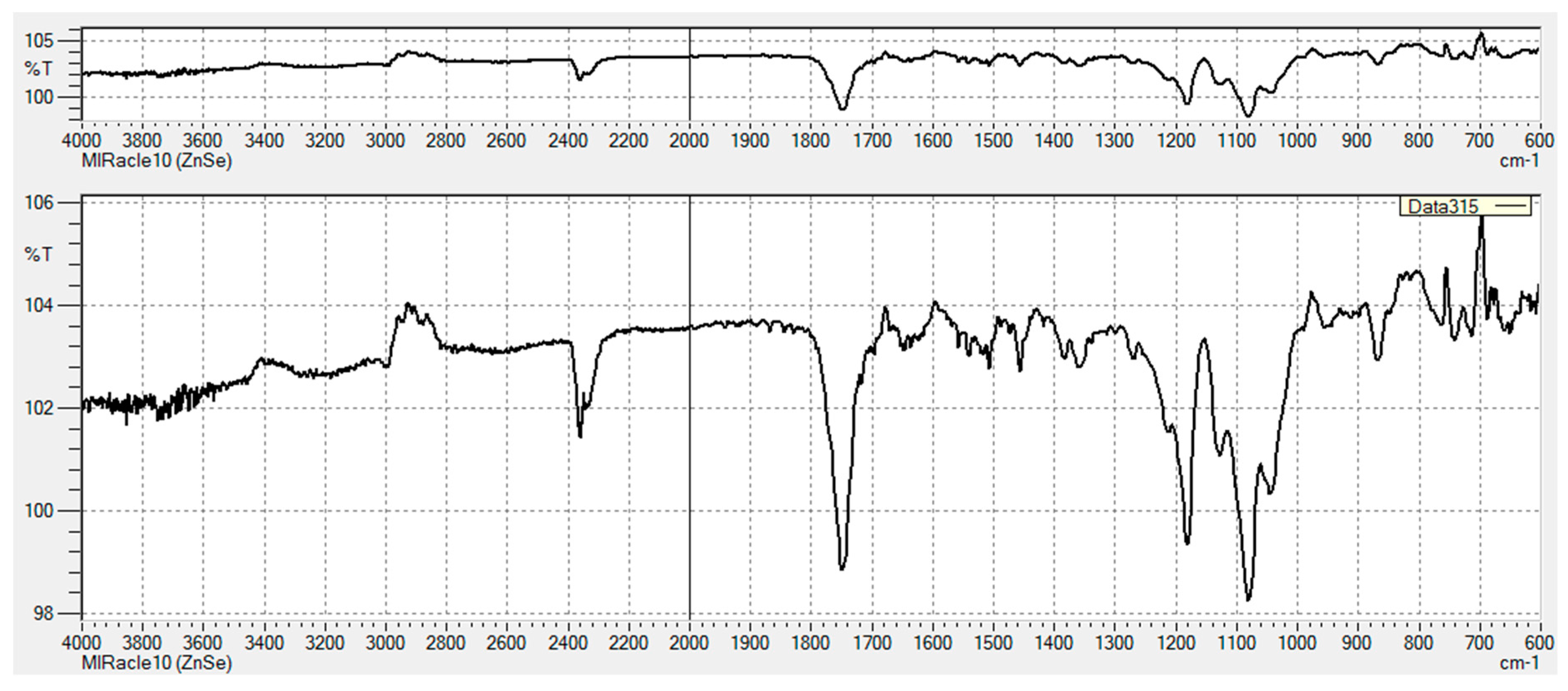
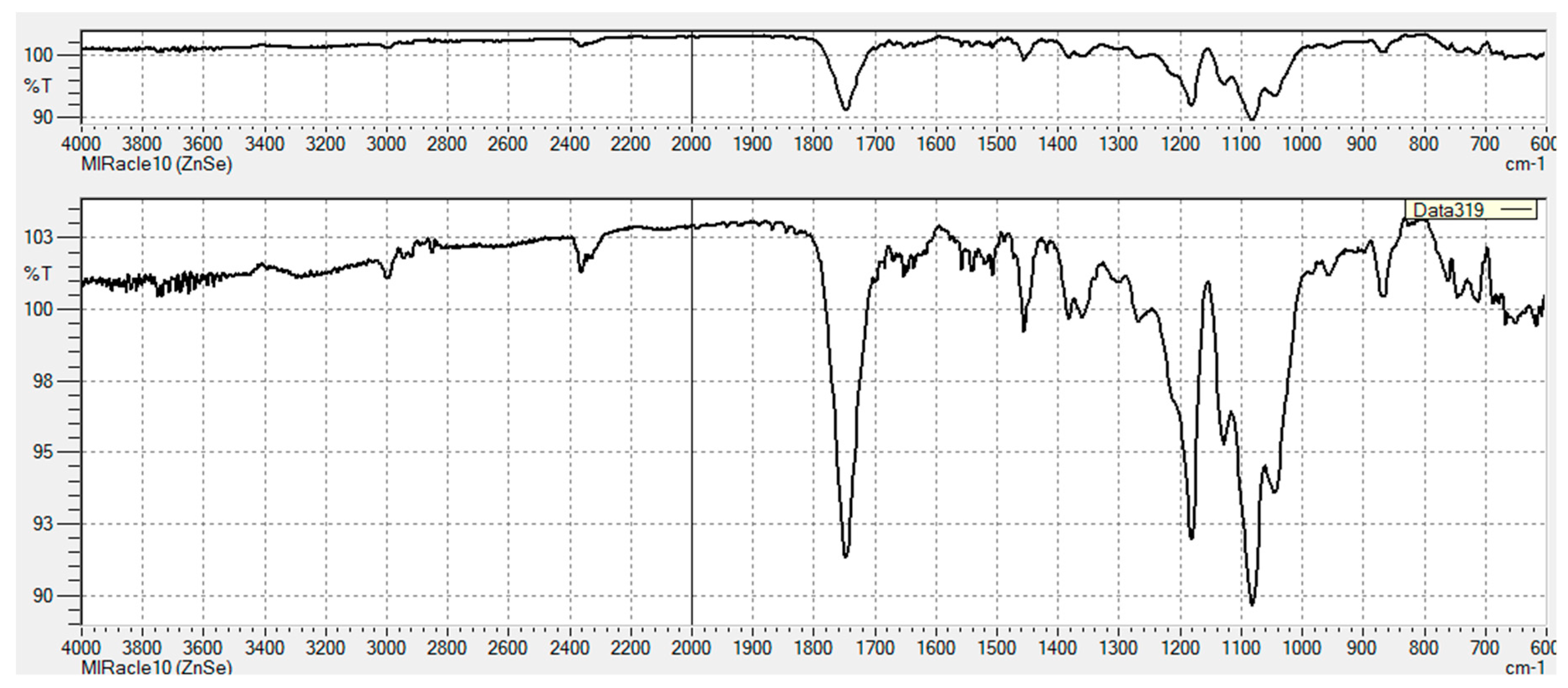
3.2.1. FT-IR Characterization of Chemical Modifications
3.2.2. Contact Angle Measurement
3.2.3. Scanning Electron Microscopy
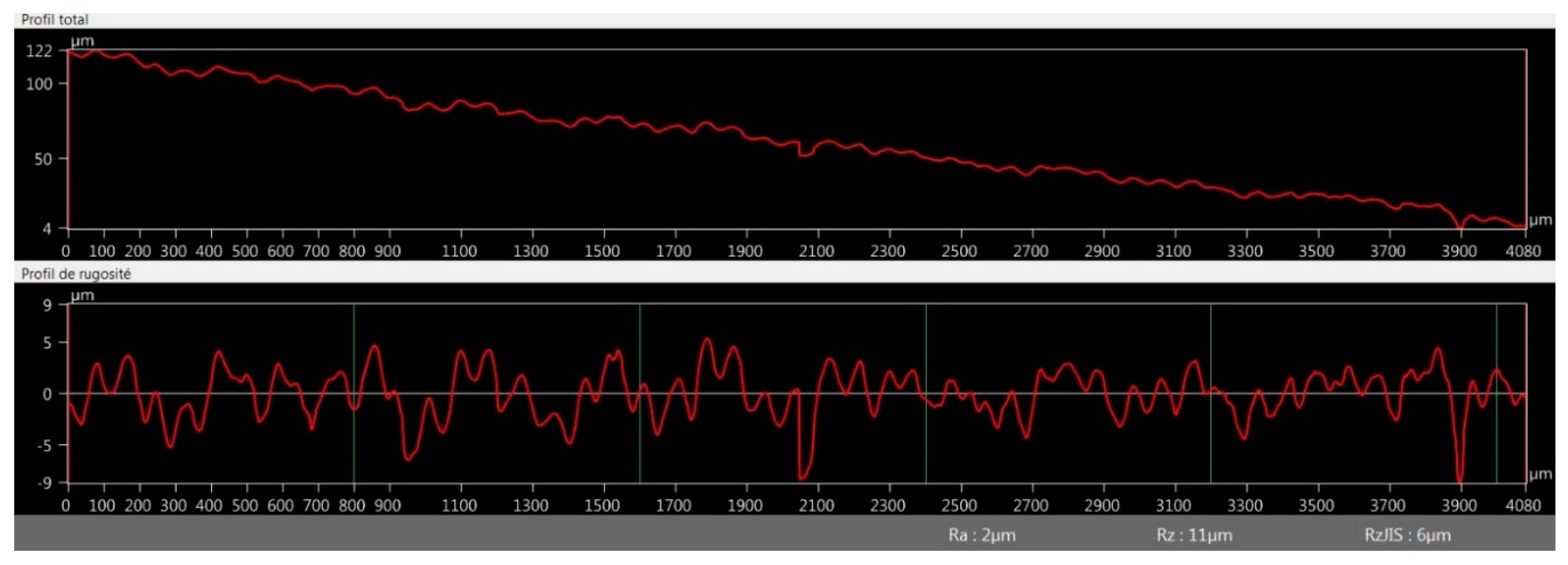
3.2.4. Optical Microscopy Roughness Measurement
3.2.5. Positron Annihilation Lifetime Spectrometry
3.3. Cytocompatibility Experiments
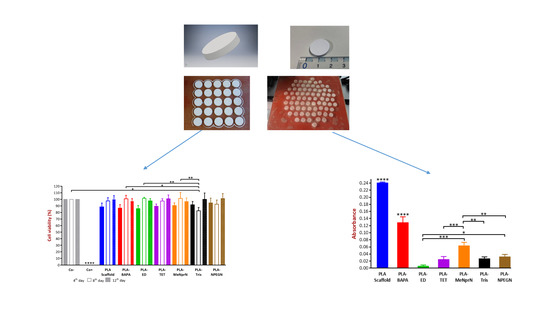
3.3.1. MTT Cell Viability Test
3.3.2. Biofilm Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A





Appendix B

References
- Li, Z.; Jia, S.; Xiong, Z.; Long, Q.; Yan, S.; Hao, F.; Liu, J.; Yuan, Z. 3D-printed scaffolds with calcified layer for osteochondral tissue engineering. J. Biosci. Bioeng. 2018, 126, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Boetker, J.; Water, J.J.; Aho, J.; Arnfast, L.; Bohr, A.; Rantanen, J. Modifying release characteristics from 3D printed drug-eluting products. Eur. J. Pharm. Sci. 2016, 90, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, L. Three-dimensional modelling and three-dimensional printing in pediatric and congenital cardiac surgery. Transl. Pediatr. 2018, 7, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, N.; Serrano, C.; Van Den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Aldaadaa, A.; Owji, N.; Knowles, J. Three-dimensional Printing in Maxillofacial Surgery: Hype versus Reality. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kitson, P.J.; Glatzel, S.; Cronin, L. The digital code driven autonomous synthesis of ibuprofen automated in a 3D-printer-based robot. Beilstein J. Org. Chem. 2016, 12, 2776–2783. [Google Scholar] [CrossRef] [Green Version]
- Glatzel, S.; Hezwani, M.; Kitson, P.J.; Gromski, P.S.; Schürer, S.; Cronin, L. A PorTable 3D Printer System for the Diagnosis and Treatment of Multidrug-Resistant Bacteria. Chem 2016, 1, 494–504. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104–125. [Google Scholar] [CrossRef]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components—Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Patrick, P.S.; Page, K.; Powell, M.J.; Lythgoe, M.F.; Miodownik, M.A.; Parkin, I.P.; Carmalt, C.J.; Kalber, T.L.; Bear, J.C. Chemically Treated 3D Printed Polymer Scaffolds for Biomineral Formation. ACS Omega 2018, 3, 4342–4351. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.J.; Thiré, R.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alfredo, N.; Dorronsoro, A.; Cortajarena, A.L.; Rodríguez-Hernández, J. Antimicrobial 3D Porous Scaffolds Prepared by Additive Manufacturing and Breath Figures. ACS Appl. Mater. Interfaces 2017, 9, 37454–37462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pach, K.; Filipecki, J.; Golis, E.; Yousef, E.S.; Boyko, V. Measurements of Defect Structures by Positron Annihilation Lifetime Spectroscopy of the Tellurite Glass 70TeO2-5XO-10P2O5-10ZnO-5PbF2 (X = Mg, Bi2, Ti) Doped with Ions of the Rare Earth Element Er3. Nanoscale Res. Lett. 2017, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.J.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.J.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of ImplanTable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Pizzoferrato, A.; Ciapetti, G.; Stea, S.; Cenni, E.; Arciola, C.R.; Granchi, D.; Savarino, L. Cell culture methods for testing biocompatibility. Clin. Mater. 1994, 15, 173–190. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Galdiero, M.; Larocca, F.; Iovene, M.R.; Francesca, M.; Pieretti, G.; D’Oriano, V.; Franci, G.; Ferraro, G.; D’Andrea, F.; Nicoletti, G.F. Microbial Evaluation in Capsular Contracture of Breast Implants. Plast. Reconstr. Surg. 2018, 141, 23–30. [Google Scholar] [CrossRef]
- Salari, S.; Sadat Seddighi, N.; Ghasemi Nejad Almani, P. Evaluation of biofilm formation ability in different Candida strains and anti-biofilm effects of Fe3O4-NPs compared with Fluconazole: An in vitro study. J. Mycol. Med. 2018, 28, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; Garland, M.J.; McInnes, F.J.; Donnelly, R.F.; El-Khordagui, L.K.; Wilson, C.G. Microneedle/nanoencapsulation-mediated transdermal delivery: Mechanistic insights. Eur. J. Pharm. Biopharm. 2014, 86, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Tauran, Y.; Tarhan, M.C.; Mollet, L.; Gerves, J.B.; Fujit, H.; Collard, D.; Perret, F.; Desbrosses, M.; Leon, D. Elucidating the mechanism of the considerable mechanical stiffening of DNA induced by the couple Zn2+/Calix[4]arene-1,3-O-diphosphorous acid. Sci. Rep. 2018, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.; Noel, S.; Liberelle, B.; El Ayoubi, R.; Ajji, A.; De Crescenzo, G. Fabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineering. Biomatter 2016, 6, e1231276. [Google Scholar] [CrossRef] [Green Version]
- Yildirimer, L.; Seifalian, A.M.; Butler, P.E. Surface and mechanical analysis of explanted Poly Implant Prosthèse silicone breast implants. Br. J. Surg. 2013, 100, 761–767. [Google Scholar] [CrossRef]
- Tham, C.Y.; Abdul Hamid, Z.A.; Ahmad, Z.; Ismail, H. Surface Modification of Poly(lactic acid) (PLA) via Alkaline Hydrolysis Degradation. Adv. Mater. Res. 2014, 970, 324–327. [Google Scholar] [CrossRef]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA laminated composite for implantable application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Gortemaker, A.; Van’t Hof, M.A.; Jansen, J.A.; Creugers, N.H.J. Implant surface roughness and bone healing: A systematic review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Kazsoki, A.; Szabó, P.; Süvegh, K.; Vörös, T.; Zelkó, R. Macro- and microstructural tracking of ageing-related changes of papaverine hydrochloride-loaded electrospun nanofibrous buccal sheets. J. Pharm. Biomed. Anal. 2017, 143, 62–67. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Kuiper, R.V.; De Bruin, A.; Van Nostrum, C.F.; Vermonden, T.; Dhert, W.J.A.; Hennink, W.E. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials 2012, 33, 4309–4318. [Google Scholar] [CrossRef]
- Nemes, D.; Ujhelyi, Z.; Arany, P.; Peto, A.; Feher, P.; Varadi, J.; Fenyvesi, F.; Vecsernyes, M.; Bacskay, I. Biocompatibility investigation of different pharmaceutical excipients used in liquid dosage forms. Pharmazie 2018, 73, 16–18. [Google Scholar] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-ribot, J.L. A simple and reproducible 96 well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2009, 3, 1494–1500. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, e2437. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Pranovich, A.; Uppstu, P.; Wang, X.; Kronlund, D.; Hemming, J.; Öblom, H.; Moritz, N.; Preis, M.; Sandler, N.; et al. Novel biorenewable composite of wood polysaccharide and polylactic acid for three dimensional printing. Carbohydr. Polym. 2018, 187, 51–58. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Shiraishi, R.; Hirayama, N. Cytotoxicity associated with prolonged room temperature storage of serum and proposed methods for reduction of cytotoxicity. J. Virol. Methods 2015, 225, 16–22. [Google Scholar] [CrossRef]
- Kinnari, T.J.; Soininen, A.; Esteban, J.; Zamora, N.; Alakoski, E.; Kouri, V.P.; Lappalainen, R.; Konttinen, Y.T.; Gomez-Barrena, E.; Tiainen, V.M. Adhesion of staphylococcal and Caco-2 cells on diamond-like carbon polymer hybrid coating. J. Biomed. Mater. Res. Part A 2008, 86, 760–768. [Google Scholar] [CrossRef]
- Chessa, D.; Ganau, G.; Spiga, L.; Bulla, A.; Mazzarello, V.; Campus, G.V.; Rubino, S. Staphylococcus aureus and Staphylococcus epidermidis Virulence Strains as Causative Agents of Persistent Infections in Breast Implants. PLoS ONE 2016, 11, e0146668. [Google Scholar] [CrossRef]
- ISO/EN10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity, 3rd ed.; International Organization of Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Finkel, J.S.; Mitchell, A.P. Genetic Control of Candida Albicans Biofilm Development. Natl. Reviiew Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U.; Weisman, J.; Ballard, D.; Wolford, D.; Pascual-Garrido, C.; Wolford, L.; Woodard, P.; Mills, D. 3D Printing Custom Bioactive and Absorbable Surgical Screws, Pins, and Bone Plates for Localized Drug Delivery. J. Funct. Biomater. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Bigam, C.G.; Yao, J.; Abildgaard, F.; Dyson, H.J.; Oldfield, E.; Markley, J.L.; Sykes, B.D. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR 1995, 6, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Song, F.; Ma, L.; Fan, J.; Chen, Q.; Zhang, L.; Li, B.Q. Wetting behaviors of a nano-droplet on a rough solid substrate under perpendicular electric field. Nanomaterials 2018, 8, 340. [Google Scholar] [CrossRef]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [Green Version]
- Holmes-Farley, S.R.; Reamey, R.H.; McCarthy, T.J.; Deutch, J.; Whitesides, G.M. Acid-base behavior of carboxylic acid groups covalently attached at the surface of polyethylene: The usefulness of contact angle in following the ionization of surface functionality. Langmuir 1985, 1, 725–740. [Google Scholar] [CrossRef]
- Sebe, I.; Szabó, B.; Zelkó, R. Positron Annihilation Lifetime Spectrometry (PALS) and its pharmaceutical applications. Acta Pharm. Hung. 2012, 82, 23–32. [Google Scholar]
- Wurm, M.C.; Möst, T.; Bergauer, B.; Rietzel, D.; Neukam, F.W.; Cifuentes, S.C.; von Wilmowsky, C. In-vitro evaluation of Polylactic acid (PLA) manufactured by fused deposition modeling. J. Biol. Eng. 2017, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Johnson, H.J.; Northup, S.J.; Darby, T.D. Biocompatibility test procedures for materials evaluation in vitro. II. Objective methods of toxicity assessment. J. Biomed. Mater. Res. 1984, 19, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, S.; Jarraya, M. Technical note: Comparison of the PrestoBlue and LDH release assays with the MTT assay for skin viability assessment. Cell Tissue Bank. 2015, 16, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]









| Printer | Lulzbot Mini | Lulzbot Taz 5 |
| Filament Type | PLA | PLA |
| Source | Maker Shop France | Maker Shop France |
| Filament Diameter; mm | 2.85 | 2.85 |
| Extruder Nozzle Diameter; µm | 350 | 350 |
| Infill Percentage | 100 | 100 |
| Extrusion Temperature (°C) | 215 | 215 |
| Bed Temperature (°C) | 60 | 60 |
| Layer Thickness µm | 50 | 75 |
| Print Speed mm/s | 50 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arany, P.; Róka, E.; Mollet, L.; Coleman, A.W.; Perret, F.; Kim, B.; Kovács, R.; Kazsoki, A.; Zelkó, R.; Gesztelyi, R.; et al. Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties. Pharmaceutics 2019, 11, 277. https://doi.org/10.3390/pharmaceutics11060277
Arany P, Róka E, Mollet L, Coleman AW, Perret F, Kim B, Kovács R, Kazsoki A, Zelkó R, Gesztelyi R, et al. Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties. Pharmaceutics. 2019; 11(6):277. https://doi.org/10.3390/pharmaceutics11060277
Chicago/Turabian StyleArany, Petra, Eszter Róka, Laurent Mollet, Anthony W. Coleman, Florent Perret, Beomjoon Kim, Renátó Kovács, Adrienn Kazsoki, Romána Zelkó, Rudolf Gesztelyi, and et al. 2019. "Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties" Pharmaceutics 11, no. 6: 277. https://doi.org/10.3390/pharmaceutics11060277
APA StyleArany, P., Róka, E., Mollet, L., Coleman, A. W., Perret, F., Kim, B., Kovács, R., Kazsoki, A., Zelkó, R., Gesztelyi, R., Ujhelyi, Z., Fehér, P., Váradi, J., Fenyvesi, F., Vecsernyés, M., & Bácskay, I. (2019). Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties. Pharmaceutics, 11(6), 277. https://doi.org/10.3390/pharmaceutics11060277