Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GSNO Synthesis
2.3. Preparation of PL/AL Hydrogel
2.4. Incorporation of GSNO into PL/AL Hydrogel
2.5. Measurement of Gelation Temperature
2.6. Rheological Analyses of Hydrogels
2.7. Thermal Analyses of Hydrogels
2.8. In Vitro NO Release
2.9. Cytotoxicity of Hydrogels
2.10. In Vitro Antibacterial Activity
2.11. In Vivo Wound-Healing Activity
2.12. Histological Analysis
2.13. Reduction of Bacterial Burden in Wounds
2.14. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of GSNO-PL/AL Hydrogel
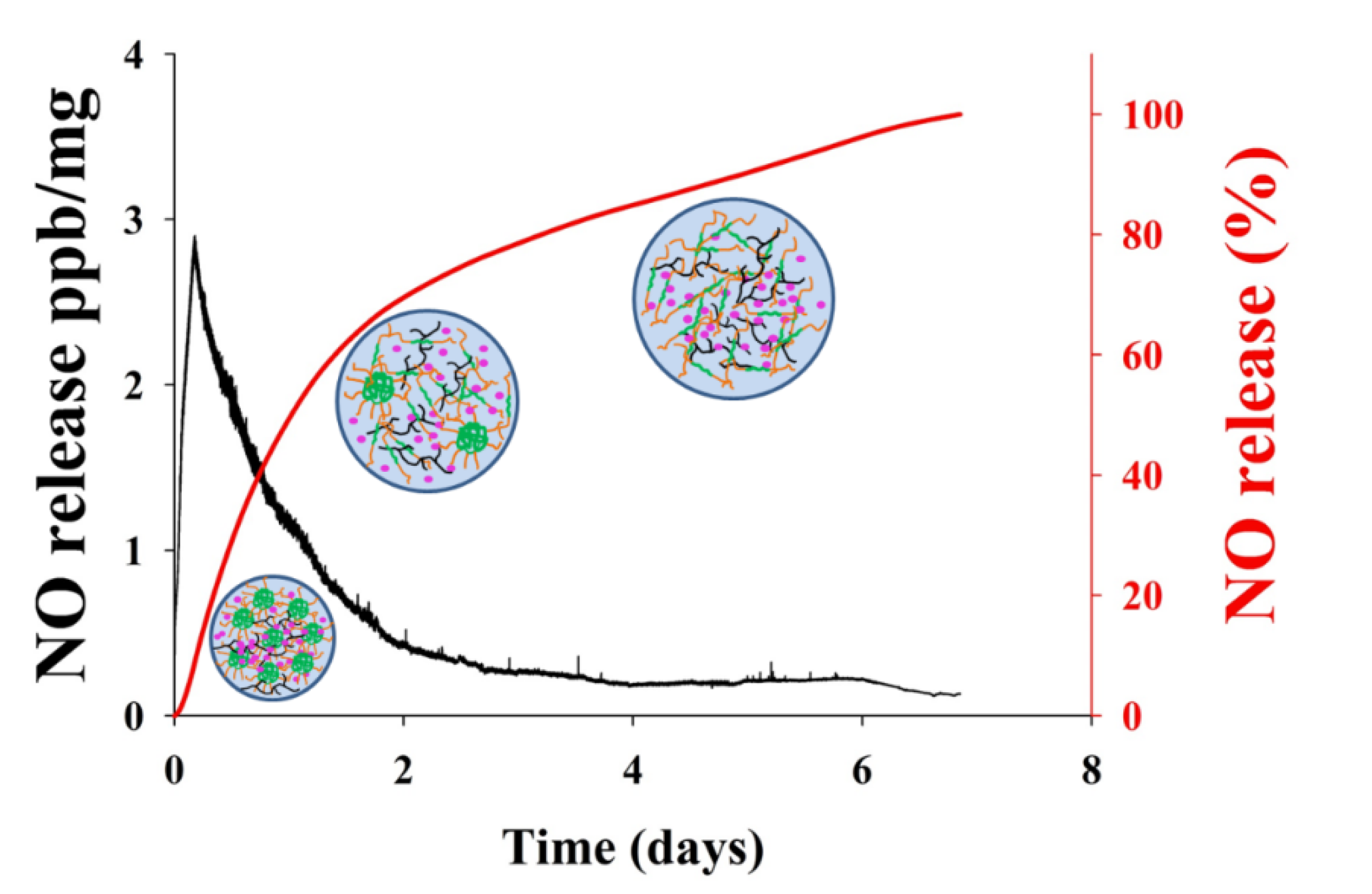
3.2. In Vitro NO Release
3.3. In Vitro Cytotoxicity Study
3.4. In Vitro Antibacterial Activity of GSNO-PL/AL Hydrogel
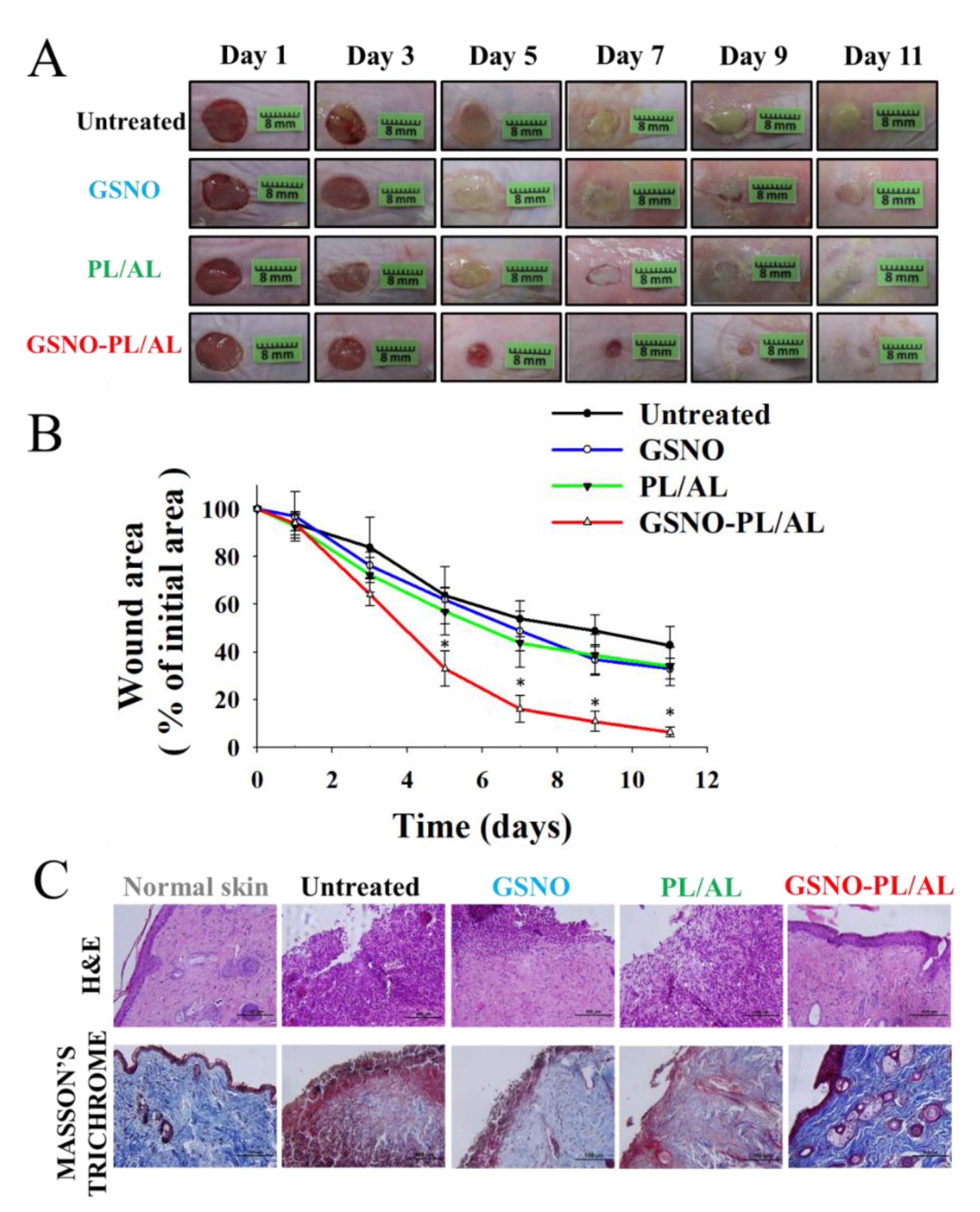
3.5. In Vivo Wound Healing Activity
3.6. Reduction of Bacterial Burden in Wounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scatena, R.; Bottoni, P.; Pontoglio, A.; Giardina, B. Pharmacological modulation of nitric oxide release: New pharmacological perspectives, potential benefits and risks. Curr. Med. Chem. 2010, 17, 61–73. [Google Scholar] [PubMed]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. NO and angiogenesis. Atheroscler. Suppl. 2003, 4, 53–60. [Google Scholar] [CrossRef]
- Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar]
- Ignarro, L.J. Nitric Oxide: Biology and Pathobiology; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Hill, B.G.; Dranka, B.P.; Bailey, S.M.; Lancaster, J.R.; Darley-Usmar, V.M. What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010, 285, 19699–19704. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [PubMed]
- Efron, D.T.; Most, D.; Barbul, A. Role of nitric oxide in wound healing. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 197–204. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.-L.; Yoo, J.-W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C 2019, 103, 109741. [Google Scholar] [CrossRef]
- Nurhasni, H.; Cao, J.; Choi, M.; Kim, I.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Nitric oxide-releasing poly (lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity. Int. J. Nanomed. 2015, 10, 3065–3080. [Google Scholar]
- Miller, M.; Megson, I. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007, 151, 305–321. [Google Scholar]
- Wang, P.G.; Cai, T.B.; Taniguchi, N. Nitric Oxide Donors: For. Pharmaceutical and Biological Applications; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Forman, H.J. Glutathione—From antioxidant to post-translational modifier. Arch. Biochem. Biophys. 2016, 595, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Hornyák, I.; Marosi, K.; Kiss, L.; Gróf, P.; Lacza, Z. Increased stability of S-nitrosothiol solutions via pH modulations. Free Radic. Res. 2012, 46, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; De Oliveira, M.G. Poly (vinyl alcohol) and poly (vinyl pyrrolidone) blended films for local nitric oxide release. Biomaterials 2004, 25, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Yoo, J.-W. In vitro and in vivo evaluation of a novel nitric oxide-releasing ointment for the treatment of methicillin-resistant Staphylococcus aureus-infected wounds. J. Pharm. Investig. 2020. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.-W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Kim, J.; Lee, J.; Hasan, N.; Cao, J.; Naeem, M.; Lee, E.H.; Shin, J.H.; Jung, Y.; Lee, B.-L. S-Nitrosoglutathione loaded poly (lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds. Eur. J. Pharm. Biopharm. 2018, 132, 94–102. [Google Scholar] [CrossRef]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Ahn, H.-J.; Song, K.-W.; Yoo, J.-W. In Situ Hydrogel-Forming/Nitric Oxide-Releasing Wound Dressing for Enhanced Antibacterial Activity and Healing in Mice with Infected Wounds. Pharmaceutics 2019, 11, 496. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, D.; Kim, H.; Kim, J.; Hlaing, S.P.; Hasan, N.; Cao, J.; Yoo, J.-W. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly (Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics 2020, 12, 618. [Google Scholar] [CrossRef]
- Field, C.K.; Kerstein, M.D. Overview of wound healing in a moist environment. Am. J. Surg. 1994, 167, S2–S6. [Google Scholar] [CrossRef]
- Korting, H.; Schöllmann, C.; White, R. Management of minor acute cutaneous wounds: Importance of wound healing in a moist environment. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 130–137. [Google Scholar] [CrossRef]
- Ousey, K.; Cutting, K.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Salehi, M.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Ebrahimi-Barough, S. Accelerating healing of excisional wound with alginate hydrogel containing naringenin in rat model. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Maftouhian, S.; Aliabadi, A.; Hassanzadeh-Khayyat, M.; Mohammadpour, F.; Khameneh, B.; Hadizadeh, F. Casein-based hydrogel carrying insulin: Preparation, in vitro evaluation and in vivo assessment. J. Pharm. Investig. 2019, 49, 635–641. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid)(PLGA) copolymers for the design of drug delivery systems. J. Pharm. Investig. 2019, 49, 443–458. [Google Scholar] [CrossRef]
- Gou, M.; Li, X.; Dai, M.; Gong, C.; Wang, X.; Xie, Y.; Deng, H.; Chen, L.; Zhao, X.; Qian, Z. A novel injectable local hydrophobic drug delivery system: Biodegradable nanoparticles in thermo-sensitive hydrogel. Int. J. Pharm. 2008, 359, 228–233. [Google Scholar] [CrossRef]
- Wagh, V.D.; Inamdar, B.; Samanta, M. Polymers used in ocular dosage form and drug delivery systems. Asian J. Pharm. 2008, 2. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar]
- Yap, L.-S.; Yang, M.-C. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloids Surf. B Biointerfaces 2016, 146, 204–211. [Google Scholar] [CrossRef]
- Abdi, S.I.H.; Choi, J.Y.; Lee, J.S.; Lim, H.J.; Lee, C.; Kim, J.; Chung, H.Y.; Lim, J.O. In vivo study of a blended hydrogel composed of pluronic F-127-alginate-hyaluronic acid for its cell injection application. Tissue Eng. Regen. Med. 2012, 9, 1–9. [Google Scholar] [CrossRef]
- Patole, V.C.; Pandit, A.P. Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: In vitro and in vivo characterization. J. Pharm. Investig. 2017, 48, 257–267. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Lima, B.d.A.; Do Nascimento, M.H.; Lombello, C.B.; Brocchi, M.; Seabra, A.B. Biocompatible and Antibacterial Nitric Oxide-Releasing Pluronic F-127/Chitosan Hydrogel for Topical Applications. Polymers 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Tirnaksiz, F.; Robinson, J. Rheological, mucoadhesive and release properties of pluronic F-127 gel and pluronic F-127/polycarbophil mixed gel systems. Die Pharm. 2005, 60, 518–523. [Google Scholar]
- Yergoz, F.; Hastar, N.; Cimenci, C.E.; Ozkan, A.D.; Tekinay, T.; Guler, M.O.; Tekinay, A.B. Heparin mimetic peptide nanofiber gel promotes regeneration of full thickness burn injury. Biomaterials 2017, 134, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; Ghahremankhani, A.A.; Moghadam, S.H.; Malekshahi, M.R.; Atyabi, F.; Dinarvand, R. In situ gel forming systems of poloxamer 407 and hydroxypropyl cellulose or hydroxypropyl methyl cellulose mixtures for controlled delivery of vancomycin. J. Appl. Polym. Sci. 2008, 109, 2369–2374. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Vercelino, R.; Cunha, T.M.; Ferreira, E.S.; Cunha, F.Q.; Ferreira, S.H.; de Oliveira, M.G. Skin vasodilation and analgesic effect of a topical nitric oxide-releasing hydrogel. J. Mater. Sci. Mater. Electron. 2013, 24, 2157–2169. [Google Scholar] [CrossRef]
- Pradines, B.; Djabourov, M.; Vauthier, C.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. Gelation and micellization behaviors of pluronic® F127 hydrogel containing poly (isobutylcyanoacrylate) nanoparticles specifically designed for mucosal application. Colloids Surf. B Biointerfaces 2015, 135, 669–676. [Google Scholar] [CrossRef]
- Zhang, M.; Djabourov, M.; Bourgaux, C.; Bouchemal, K. Nanostructured fluids from pluronic® mixtures. Int. J. Pharm. 2013, 454, 599–610. [Google Scholar] [CrossRef]
- Trong, L.C.P.; Djabourov, M.; Ponton, A. Mechanisms of micellization and rheology of PEO–PPO–PEO triblock copolymers with various architectures. J. Colloid Interface Sci. 2008, 328, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Shishido, S.M.; Seabra, A.B.; Loh, W.; de Oliveira, M.G. Thermal and photochemical nitric oxide release from S-nitrosothiols incorporated in Pluronic F127 gel: Potential uses for local and controlled nitric oxide release. Biomaterials 2003, 24, 3543–3553. [Google Scholar] [CrossRef]
- Picheth, G.F.; Marini, T.C.; Taladriz-Blanco, P.; Shimamoto, G.G.; Dos Santos, G.J.; Meneau, F.; de Oliveira, M.G. Influence of Pluronic F127 microenvironments on the photochemical nitric oxide release from S-nitrosoglutathione. J. Colloid Interface Sci. 2019, 544, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Burgner, D.; Rockett, K.; Kwiatkowski, D. Nitric oxide and infectious diseases. Arch. Dis. Child. 1999, 81, 185–188. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials 2014, 35, 1716–1724. [Google Scholar] [CrossRef]

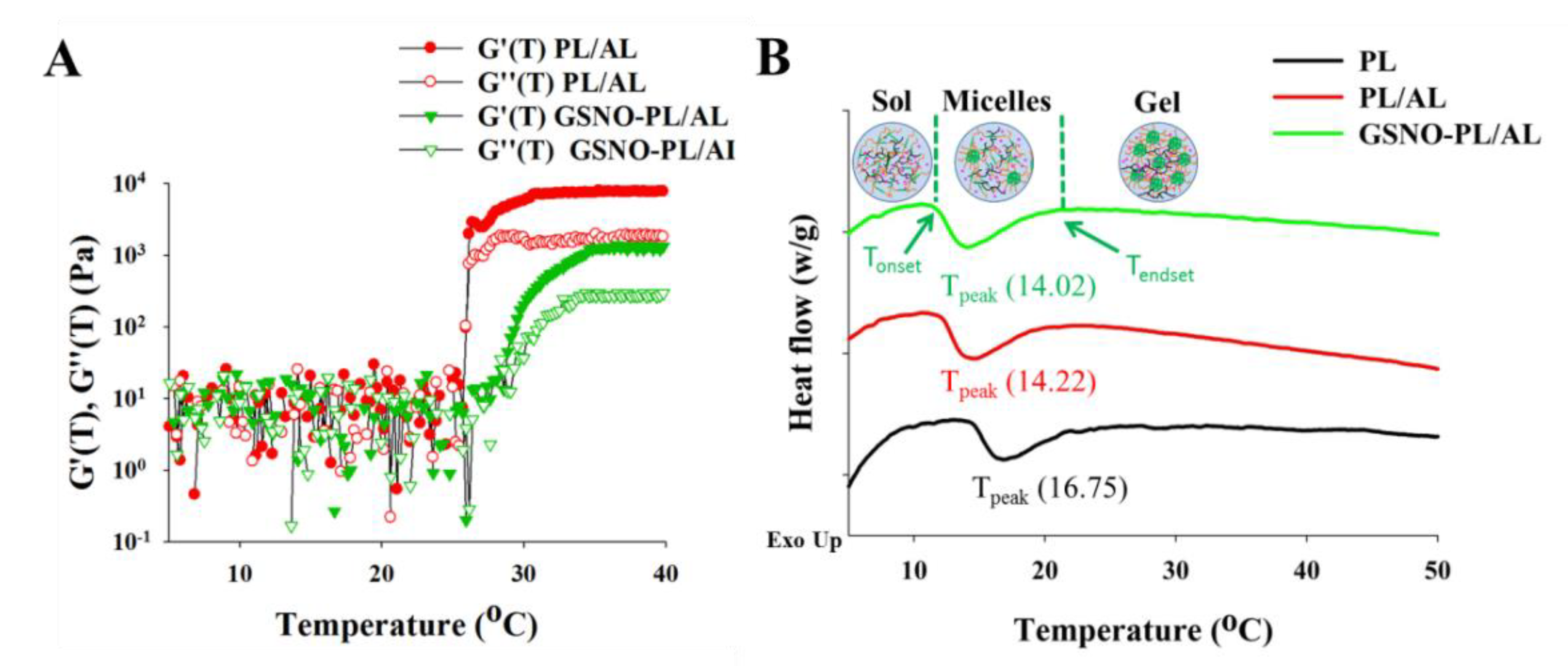



| Hydrogels | Tonset (°C) | Tpeak (°C) | Tendset (°C) | ∆H (j·g−1) | Tgel (°C) | Loading (%) |
|---|---|---|---|---|---|---|
| PL | 14.63 | 16.75 | 24.28 | 2.441 | 26.4 ± 0.2 | N.D. |
| PL/AL | 12.20 | 14.22 | 21.87 | 2.902 | 24.2 ± 0.3 | N.D. |
| GSNO-PL/AL | 11.76 | 14.02 | 21.21 | 3.042 | 23.4 ± 0.2 | 1.9 ± 0.2 |
| Material | [NO]max (ppb/mg) | t[NO]max (h) | t1/2 (h) | td (days) |
|---|---|---|---|---|
| GSNO-PL/AL | 2.88 | 5.82 | 24.51 | 7.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J.-W. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics 2020, 12, 926. https://doi.org/10.3390/pharmaceutics12100926
Cao J, Su M, Hasan N, Lee J, Kwak D, Kim DY, Kim K, Lee EH, Jung JH, Yoo J-W. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics. 2020; 12(10):926. https://doi.org/10.3390/pharmaceutics12100926
Chicago/Turabian StyleCao, Jiafu, Mingzhi Su, Nurhasni Hasan, Juho Lee, Dongmin Kwak, Dong Young Kim, Keonwoo Kim, Eun Hee Lee, Jee H. Jung, and Jin-Wook Yoo. 2020. "Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds" Pharmaceutics 12, no. 10: 926. https://doi.org/10.3390/pharmaceutics12100926
APA StyleCao, J., Su, M., Hasan, N., Lee, J., Kwak, D., Kim, D. Y., Kim, K., Lee, E. H., Jung, J. H., & Yoo, J.-W. (2020). Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics, 12(10), 926. https://doi.org/10.3390/pharmaceutics12100926







