Gellan Gum Promotes the Differentiation of Enterocytes from Human Induced Pluripotent Stem Cells
Abstract
1. Introduction
2. Materials and Methods
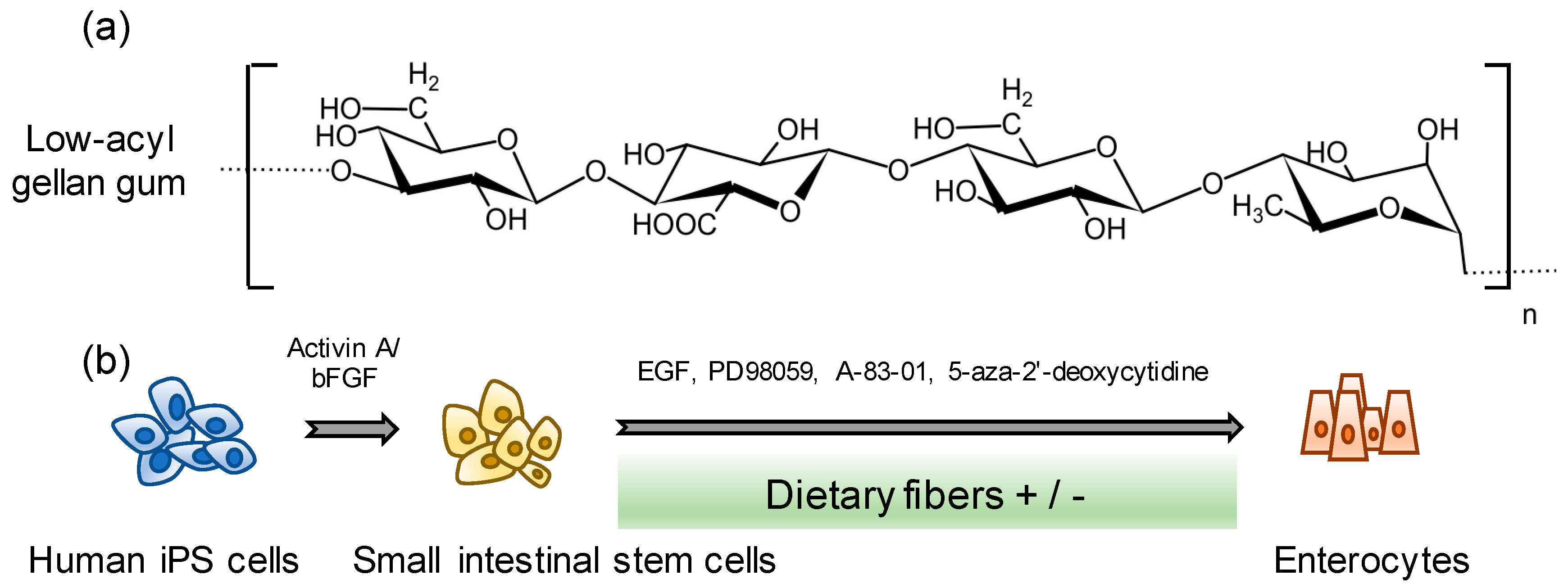
2.1. Human iPS Cells
2.2. Human iPS Cell-Derived Enterocyte Differentiation
2.3. Real-Time RT-PCR
2.4. Immunofluorescence Staining
2.5. Uptake Assay
2.6. Drug-Metabolizing Enzyme Activities
2.7. Cell Cycle Analysis
2.8. β-Galactosidase Assay
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Effects of GG on iPS Cell-Derived Enterocyte Differentiation
3.2. Activities of the Drug-Metabolizing Enzymes and Peptide Uptake Transporter
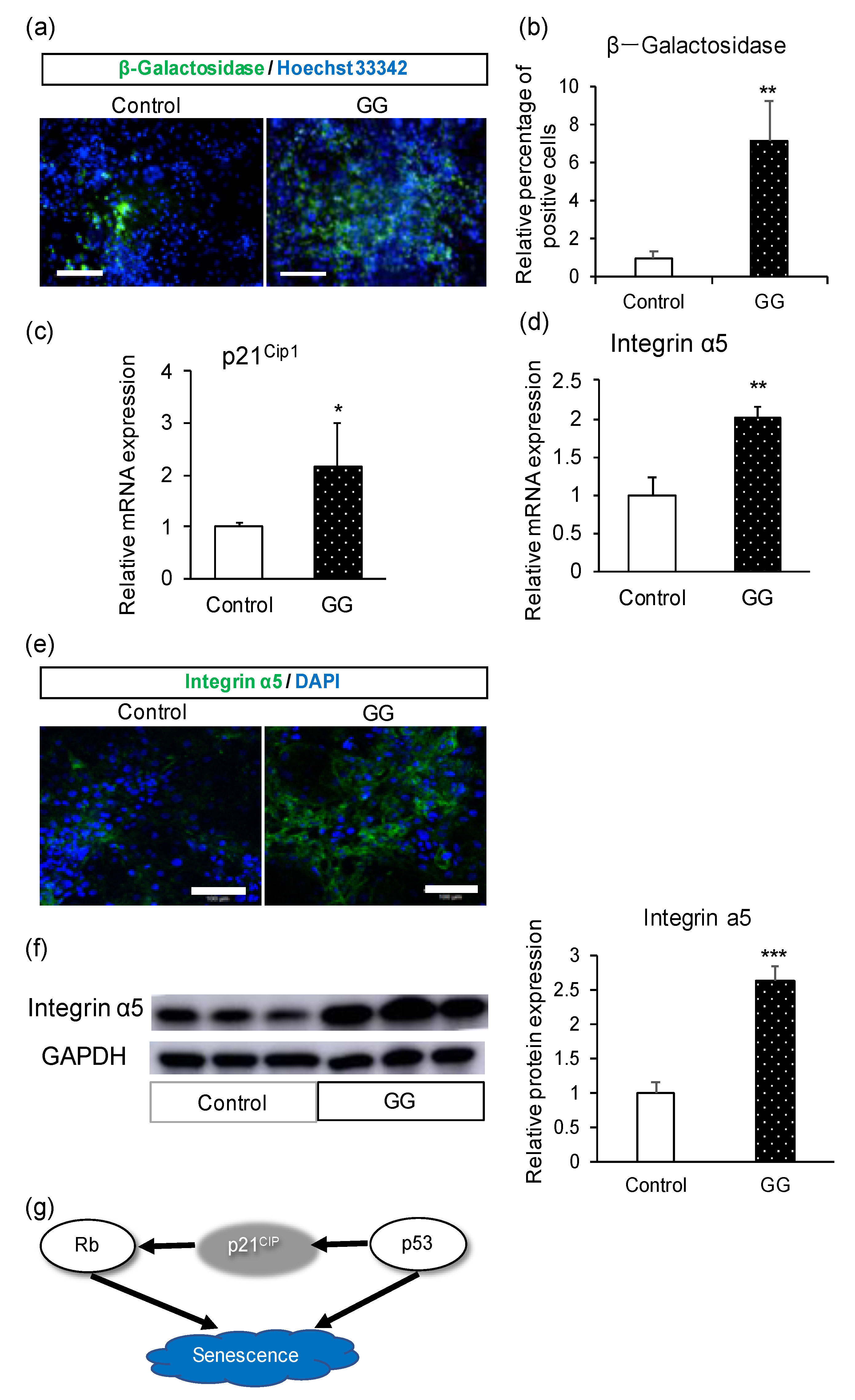
3.3. Analyses of Cellular Senescence and Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates 3 of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef]
- Adams, C.P.; Brantner, V.V. Estimating the cost of new drug development: Is it really 802 million dollars? Health Aff. (Millwood) 2006, 25, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.S.; Zhang, Q.Y. The small intestine as a xenobiotic-metabolizing organ. Drug Metab. Dispos. 2003, 31, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sugiyama, Y. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur. J. Pharm. Sci. 2000, 12, 3–12. [Google Scholar] [CrossRef]
- McGinnity, D.F.; Soars, M.G.; Urbanowicz, R.A.; Riley, J.R. Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab. Dispos. 2004, 32, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Schmiedlin-Ren, P.; Thummel, K.E.; Fisher, J.M.; Paine, M.F.; Lown, K.S.; Watkins, P.B. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1alpha,25-dihydroxyvitamin D3. Mol. Pharmacol. 1997, 51, 741–754. [Google Scholar] [CrossRef]
- Lennernäs, H.; Palm, K.; Fagerholm, U.; Artursson, P. Comparison between active and passive drug transport in human intestinal epithelial (caco-2) cells in vitro and human jejunum in vivo. Int. J. Pharm. 1996, 127, 103–107. [Google Scholar] [CrossRef]
- Vaessen, S.F.; van Lipzig, M.M.; Pieters, R.H.; Krul, C.A.; Wortelboer, H.M.; Steeg, E.V. Regional expression levels of drug transporters and metabolizing enzymes along 3 the pig and human intestinal tract and comparison with Caco-2 cells. Drug Metab. Dispos. 2017, 45, 353–360. [Google Scholar] [CrossRef]
- Takenaka, T.; Harada, N.; Kuze, J.; Chiba, M.; Iwao, T.; Matsunaga, T. Human small 6 intestinal epithelial cells differentiated from adult intestinal stem cells as a novel system for predicting oral drug absorption in humans. Drug Metab. Dispos. 2014, 42, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Iwao, T.; Nakamura, K.; Sasaki, T.; Takahashi, S.; Kamada, N.; Matsubara, T.; Gonzalez, F.J.; Akutsu, H.; Miyagawa, Y.; et al. An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metab. Pharmacokinet. 2014, 29, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Iwao, T.; Kodama, N.; Kondo, Y.; Kabeya, T.; Nakamura, K.; Horikawa, T.; Niwa, T.; Kurose, K.; Matsunaga, T. Generation of enterocyte-like cells with pharmacokinetic functions from human induced pluripotent stem cells using small-molecule compounds. Drug Metab. Dispos. 2015, 43, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Iwao, T.; Toyota, M.; Miyagawa, Y.; Okita, H.; Kiyokawa, N.; Akutsu, H.; Umezawa, A.; Nagata, K.; Matsunaga, T. Differentiation of human induced pluripotent stem cells into functional enterocyte-like cells using a simple method. Drug Metab. Pharmacokinet. 2014, 29, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, S.; Shiraki, N.; Kume, K.; Kume, S. Wnt and Notch signals guide embryonic stem cell differentiation into the intestinal lineages. Stem Cells 2013, 31, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef]
- Seidner, D.L.; Lashner, B.A.; Brzezinski, A.; Banks, P.L.C.; Goldblum, J.; Fiocchi, C.; Katz, J.; Lichtenstein, G.R.; Anton, P.A.; Kam, L.Y.; et al. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: A randomized, controlled trial. Clin. Gastroenterol. Hepatol. 2005, 3, 358–369. [Google Scholar] [CrossRef]
- Cencetti, C.; Bellini, D.; Pavesio, A.; Senigaglia, D.; Passariello, C.; Virga, A.; Matricardi, P. Preparation and characterization of antimicrobial wound dressings based on silver, gellan, PVA and borax. Carbohydr. Polym. 2012, 90, 1362–1370. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.-A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regeneration in vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Goyal, R.; Tripathi, S.K.; Tyagi, S.; Ram, K.R.; Ansari, K.M.; Shukla, Y.; Chowdhuri, D.K.; Kumar, P.; Gupta, K.C. Gellan gum blended PEI nanocomposites as gene delivery agents: Evidences from in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2011, 79, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Cooke, M.J.; Tam, R.Y.; Sousa, N.; Salgado, A.J.; Reis, R.L.; Shoichet, M.S. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomater, 2012; 33, 6345–6354. [Google Scholar]
- Braunstein, E.M.; Qiao, X.T.; Madison, B.; Pinson, K.; Dunbar, L.; Gumucio, D.L. Villin: A marker for development of the epithelial pyloric border. Dev. Dyn. 2002, 224, 90–102. [Google Scholar] [CrossRef]
- Maunoury, R.; Robine, S.; Pringault, E.; Huet, C.; Guénet, J.L.; Gaillard, J.A.; Louvard, D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988, 7, 3321–3329. [Google Scholar] [CrossRef]
- Choi, M.Y.; Romer, A.I.; Hu, M.; Lepourcelet, M.; Mechoor, A.; Yesilaltay, A.; Krieger, M.; Gray, P.A.; Shivdasani, R.A. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development 2006, 133, 4119–4129. [Google Scholar] [CrossRef]
- Sweetser, D.A.; Birkenmeier, E.; Klisak, I.; Zollman, S.; Sparkes, R.; Mohandas, T.; Lusis, A.; Gordon, J. The human and rodent intestinal fatty acid binding protein genes. Acomparative analysis of their structure, expression, and linkage relationships. J. Biol. Chem. 1987, 262, 16060–16071. [Google Scholar]
- Darmoul, D.; Voisin, T.; Couvineau, A.; Rouyer-Fessard, C.; Salomon, R.; Wang, Y.; Swallow, D.M.; Laburthe, M. Regional expression of epithelial dipeptidyl peptidase IV in the human intestines. Biochem. Biophys. Res. Commun. 1994, 203, 1224–1229. [Google Scholar] [CrossRef]
- Walters, J.R.; Howard, A.; Rumble, H.E.; Prathalingam, S.R.; Shaw-Smith, C.J.; Legon, S. Differences in expression of homeobox transcription factors in proximal and distal human small intestine. Gastroenterology 1997, 113, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Vrhovac, I.; Eror, D.B.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radovic, N.; Jadrijevic, S.; Aleksic, I.; Walles, T.; et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015, 467, 1881–1898. [Google Scholar] [CrossRef]
- Meier, Y.; Eloranta, J.J.; Darimont, J.; Ismair, M.G.; Hiller, C.; Fried, M.; Ublick, G.A.K.; Vavricka, S.R. Regional distribution of solute carrier mRNA expression along 18 the human intestinal tract. Drug Metab. Dispos. 2007, 35, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Mooij, M.G.; de Koning, B.E.; Lindenbergh-Kortleve, D.J.; Simons-Oosterhuis, Y.; van Groen, B.D.; Tibboel, D.; Samsom, J.N.; de Wildt, S.N. Human Intestinal PEPT1 Transporter Expression and Localization in Preterm and Term Infants. Drug Metab. Dispos. 2016, 44, 1014–1019. [Google Scholar] [CrossRef]
- Natori, T.; Fujiyoshi, M.; Uchida, M.; Abe, N.; Kanaki, T.; Fukumoto, Y.; Ishii, I. Growth arrest of vascular smooth muscle cells in suspension culture using low-acyl gellan gum. In Vitro Cell Dev. Biol. Anim. 2017, 53, 191–198. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Wei, W.; Sedivy, J.M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 1997, 277, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef]
- Puzan, M.; Hosic, S.; Ghio, C.; Koppes, A. Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci. Rep. 2018, 8, 6313. [Google Scholar] [CrossRef] [PubMed]
- Delon, L.C.; Guo, Z.; Oszmiana, A.; Chien, C.C.; Gibson, R.; Prestidge, C.; Thierry, B. A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials 2019, 225, 119521. [Google Scholar] [CrossRef]
- Paine, M.F.; Hart, H.L.; Ludington, S.S.; Haining, R.L.; Rettie, A.E.; Zeldin, D.C. The human intestinal cytochrome P450 “pie”. Drug Metab. Dispos. 2006, 34, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Pan, L.; Yang, H.L.; Xia, P.; Yu, W.C.; Tang, W.Q.; Zhang, Y.X.; Chen, S.F.; Xue, Y.Z.; Wang, L.X. Integrin alpha5beta1 suppresses rBMSCs anoikis and promotes nitric oxide production. Biomed. Pharmacother. 2018, 99, 1–8. [Google Scholar] [CrossRef]
- Alanko, J.; Mai, A.; Jacquemet, G.; Schauer, K.; Kaukonen, R.; Saari, M.; Goud, B.; Ivaska, J. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 2015, 17, 1412–1421. [Google Scholar] [CrossRef]
- Di Fagagna, F.D.; Teo, S.H.; Jackson, S.P. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 2004, 18, 1781–1799. [Google Scholar] [CrossRef]
- Steegenga, W.T.; de Wit, N.J.; Boekschoten, M.V.; Ijssennagger, N.; Lute, C.; Keshtkar, S.; Bromhaar, M.M.; Kampman, E.; de Groot, L.C.; Muller, M. Structural, functional and molecular analysis of the effects of aging in the small intestine and colon of C57BL/6J mice. BMC Med. Genomics 2012, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Frazzoni, M.; Gatto, M.R.; Gasbarrini, G. Ageing and small-bowel morphometric study. Gerontology 1996, 32, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Warren, P.M.; Pepperman, M.A.; Montgomery, R.D. Age changes in small-intestinal mucosa. Lancet 1978, 2, 849–850. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst) 2016, 42, 63–71. [Google Scholar] [CrossRef]


| Gene Name | Sense (5′→3′) | Antisense (5′→3′) |
|---|---|---|
| Villin1 | AGCCAGATCACTGCTGAGGT | TGGACAGGTGTTCCTCCTTC |
| ISX | CAGGAAGGAAGGAAGAGCAA | TGGGTAGTGGGTAAAGTGGAA |
| FABP2 | TTGGAAGGTAGACCGGAGTG | AGGTCCCCCTGAGTTCAGTT |
| Occludin | CTGTGTGTTTCCAAGAGAAGTTAC | TGCATCATCAATTTCCTCCTGCAG |
| CDX2 | ACCTGTGCGAGTGGATGC | TCCTTTGCTCTTGCGGTTCT |
| DPP4 | CAAATTGAAGCAGCCAGACA | GGAGTTGGGAGACCCATGTA |
| CYP3A4 | CTGTGTGTTTCCAAGAGAAGTTAC | TGCATCATCAATTTCCTCCTGCAG |
| CYP2C9 | GACATGAACAACCCTCAGGACTTT | TGCTTGTCGTCTCTGTCCCA |
| CYP2C19 | GAACACCAAGAATCGATGGACA | TCAGCAGGAGAAGGAGAGCATA |
| P-gp | CCCATCATTGCAATAGCAGG | TGTTCAAACTTCTGCTCCTGA |
| SGLT1 | CAACATCGCCTATCCAACCT | TAAACAACCTTCCGGCAATC |
| PEPT1 | CACCTCCTTGAAGAAGATGGCA | GGGAAGACTGGAAGAGTTTTATCG |
| BCRP | AGATGGGTTTCCAAGCGTTCAT | CCAGTCCCAGTACGACTGTGACA |
| Integrin α5 | TGCAGTGTGAGGCTGTGTACA | GTGGCCACCTGACGCTCT |
| p21Cip1 | TGTCCGTCAGAACCCATGC | AAAGTCGAAGTTCCATCGCTC |
| HPRT | CTTTGCTTTCCTTGGTCAGG | TCAAGGGCATATCCTACAACA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Kabeya, T.; Ogawa, I.; Anno, S.; Hayashi, H.; Kanaki, T.; Hashita, T.; Iwao, T.; Matsunaga, T. Gellan Gum Promotes the Differentiation of Enterocytes from Human Induced Pluripotent Stem Cells. Pharmaceutics 2020, 12, 951. https://doi.org/10.3390/pharmaceutics12100951
Qiu S, Kabeya T, Ogawa I, Anno S, Hayashi H, Kanaki T, Hashita T, Iwao T, Matsunaga T. Gellan Gum Promotes the Differentiation of Enterocytes from Human Induced Pluripotent Stem Cells. Pharmaceutics. 2020; 12(10):951. https://doi.org/10.3390/pharmaceutics12100951
Chicago/Turabian StyleQiu, Shimeng, Tomoki Kabeya, Isamu Ogawa, Shiho Anno, Hisato Hayashi, Tatsuro Kanaki, Tadahiro Hashita, Takahiro Iwao, and Tamihide Matsunaga. 2020. "Gellan Gum Promotes the Differentiation of Enterocytes from Human Induced Pluripotent Stem Cells" Pharmaceutics 12, no. 10: 951. https://doi.org/10.3390/pharmaceutics12100951
APA StyleQiu, S., Kabeya, T., Ogawa, I., Anno, S., Hayashi, H., Kanaki, T., Hashita, T., Iwao, T., & Matsunaga, T. (2020). Gellan Gum Promotes the Differentiation of Enterocytes from Human Induced Pluripotent Stem Cells. Pharmaceutics, 12(10), 951. https://doi.org/10.3390/pharmaceutics12100951




